In Silico Study and Bioprospection of the Antibacterial and Antioxidant Effects of Flavone and Its Hydroxylated Derivatives
Abstract
:1. Introduction
2. Results
2.1. In Silico Tests—PASS Online
2.2. Antibacterial Activity
2.2.1. Determination of Minimum Inhibitory Concentration (MIC)
2.2.2. Determination of Minimum Bactericidal Concentration (MBC)
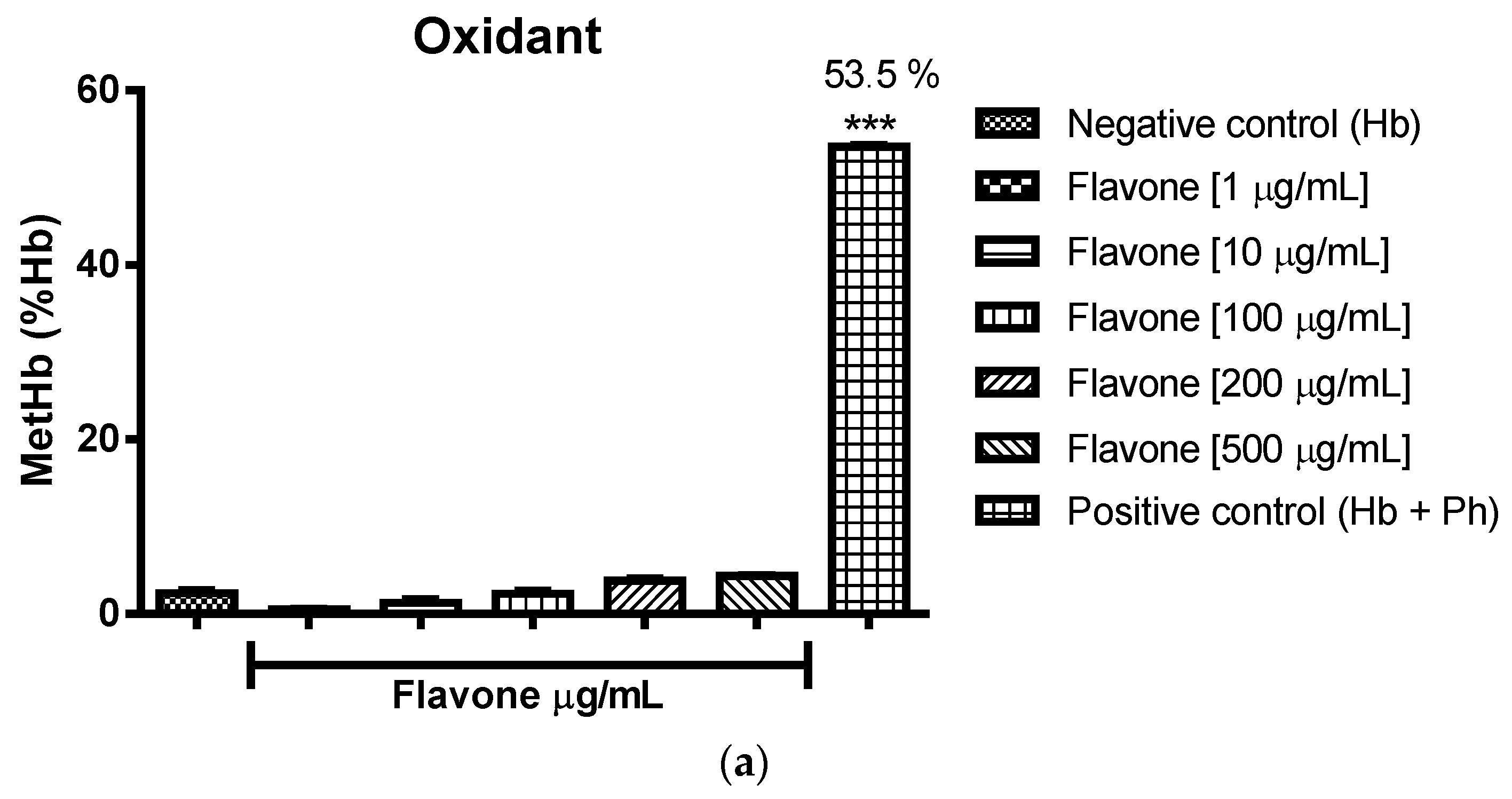
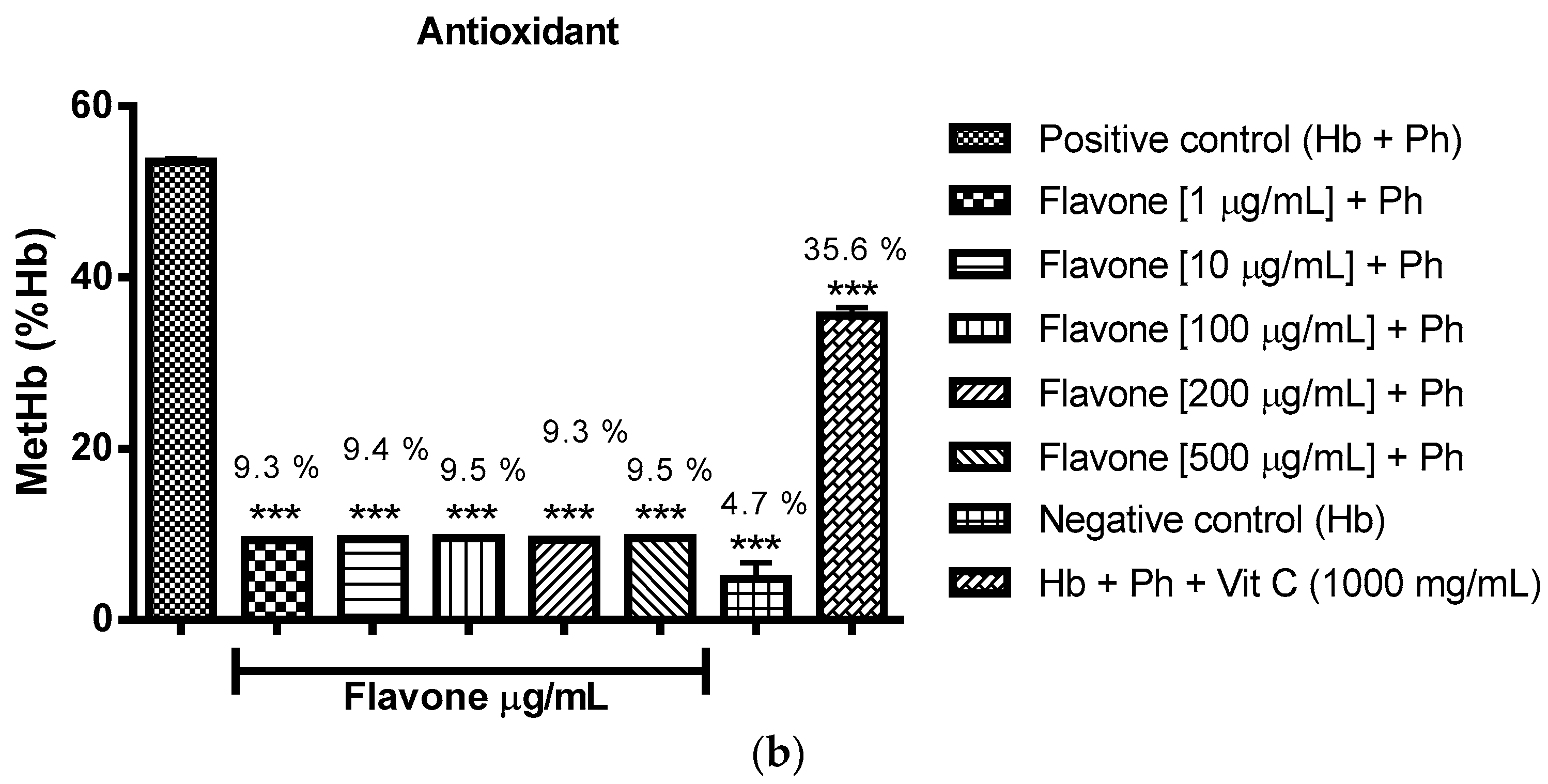
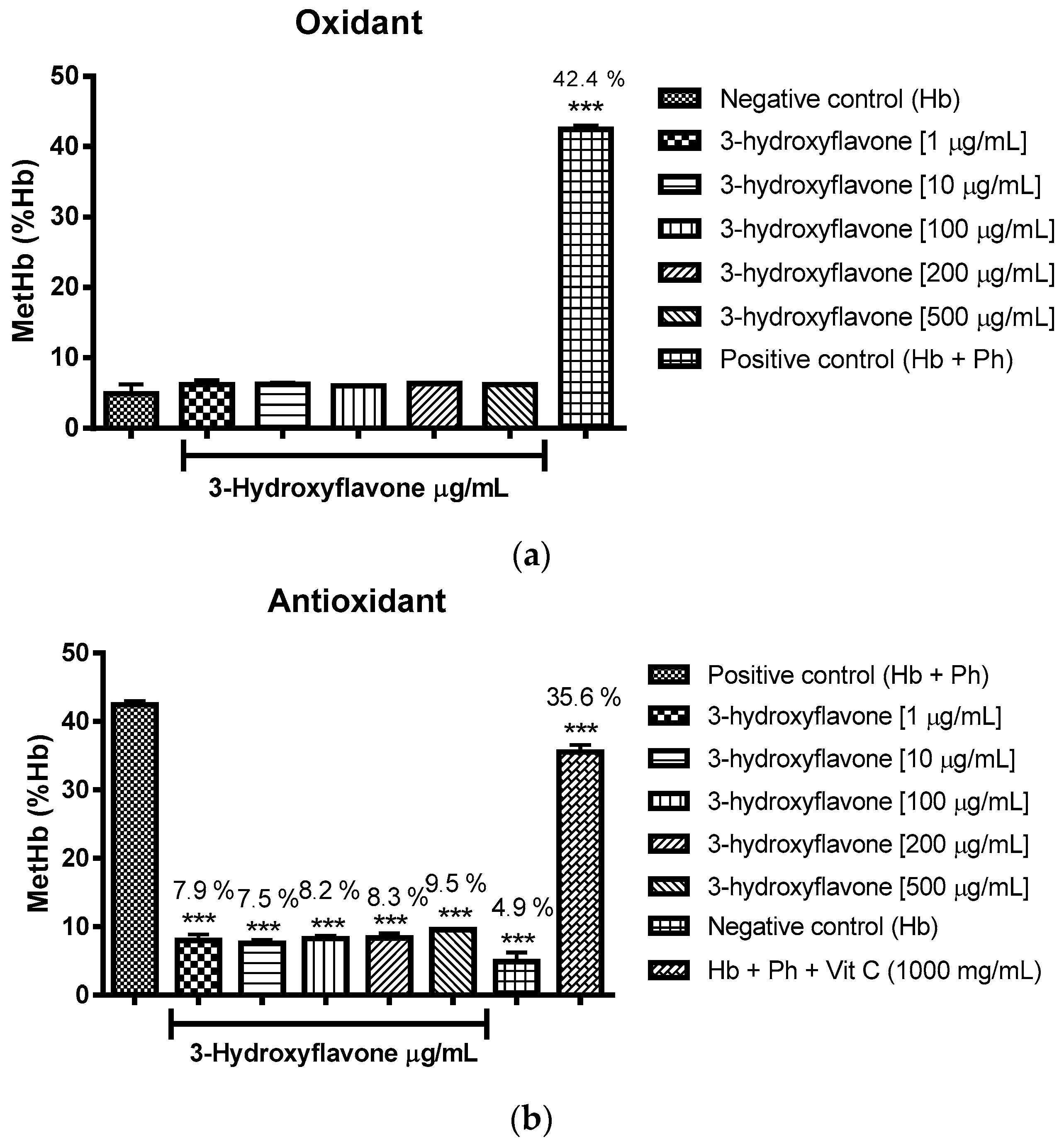
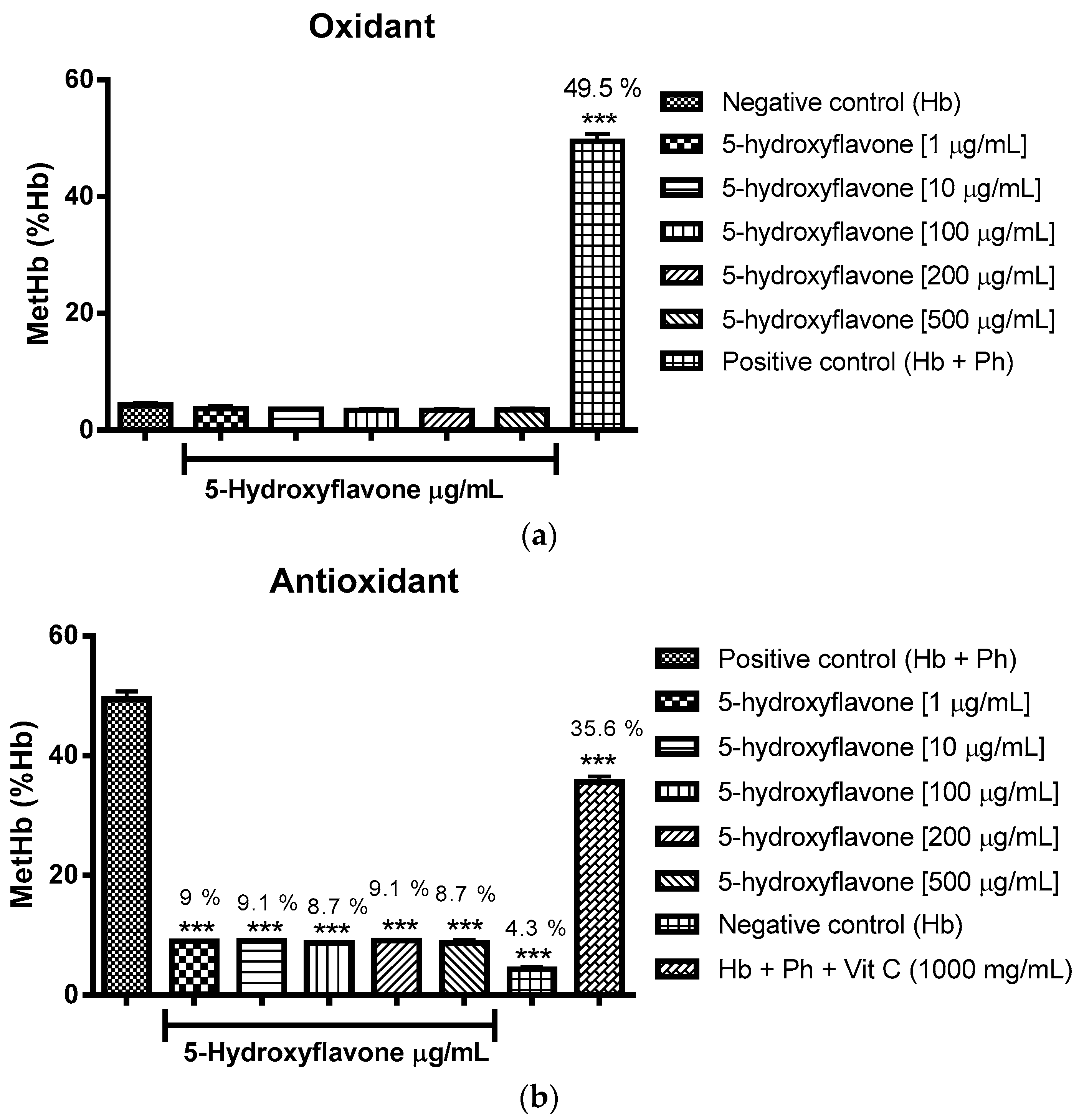
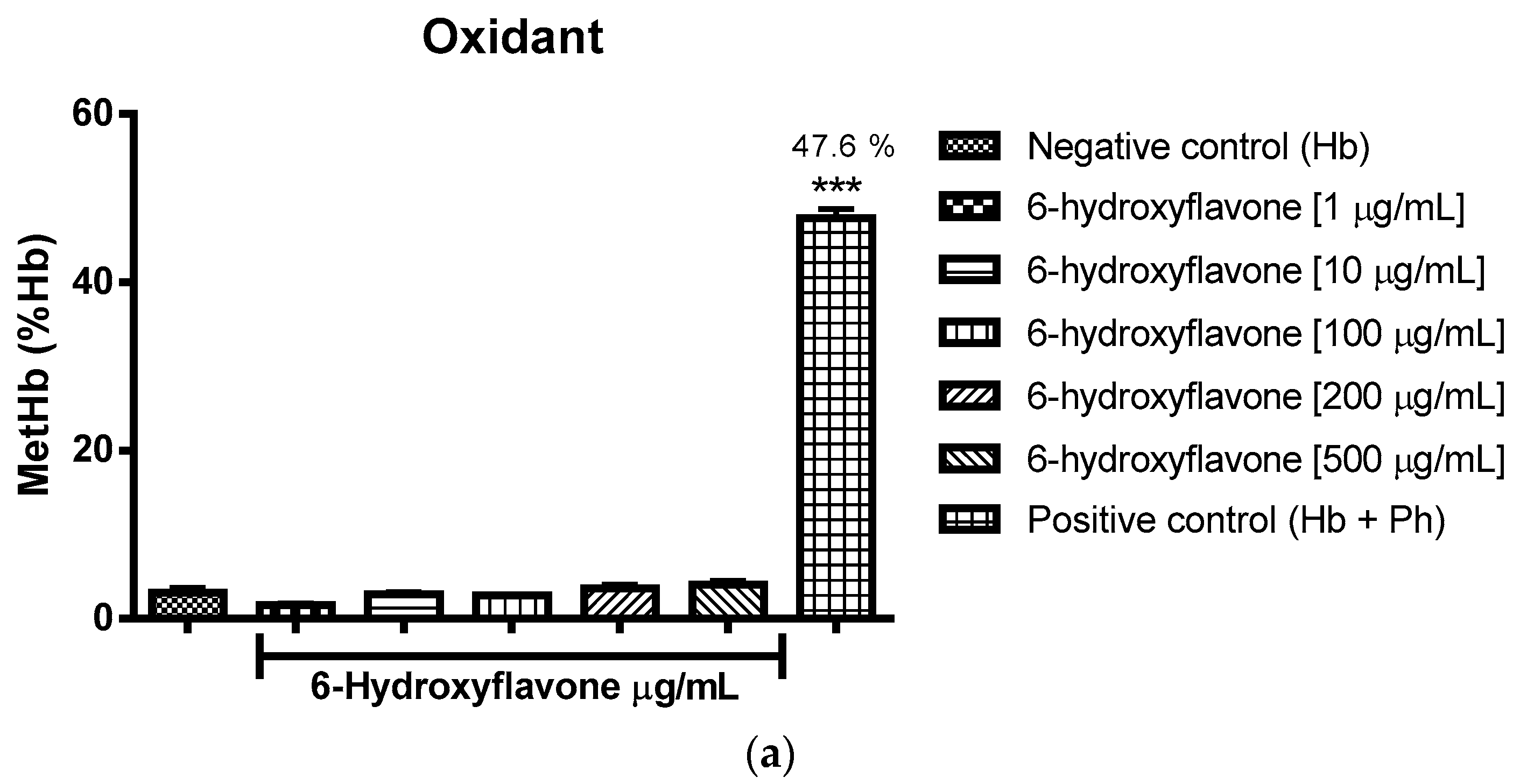
2.3. Oxidant and Antioxidant Activity Assay
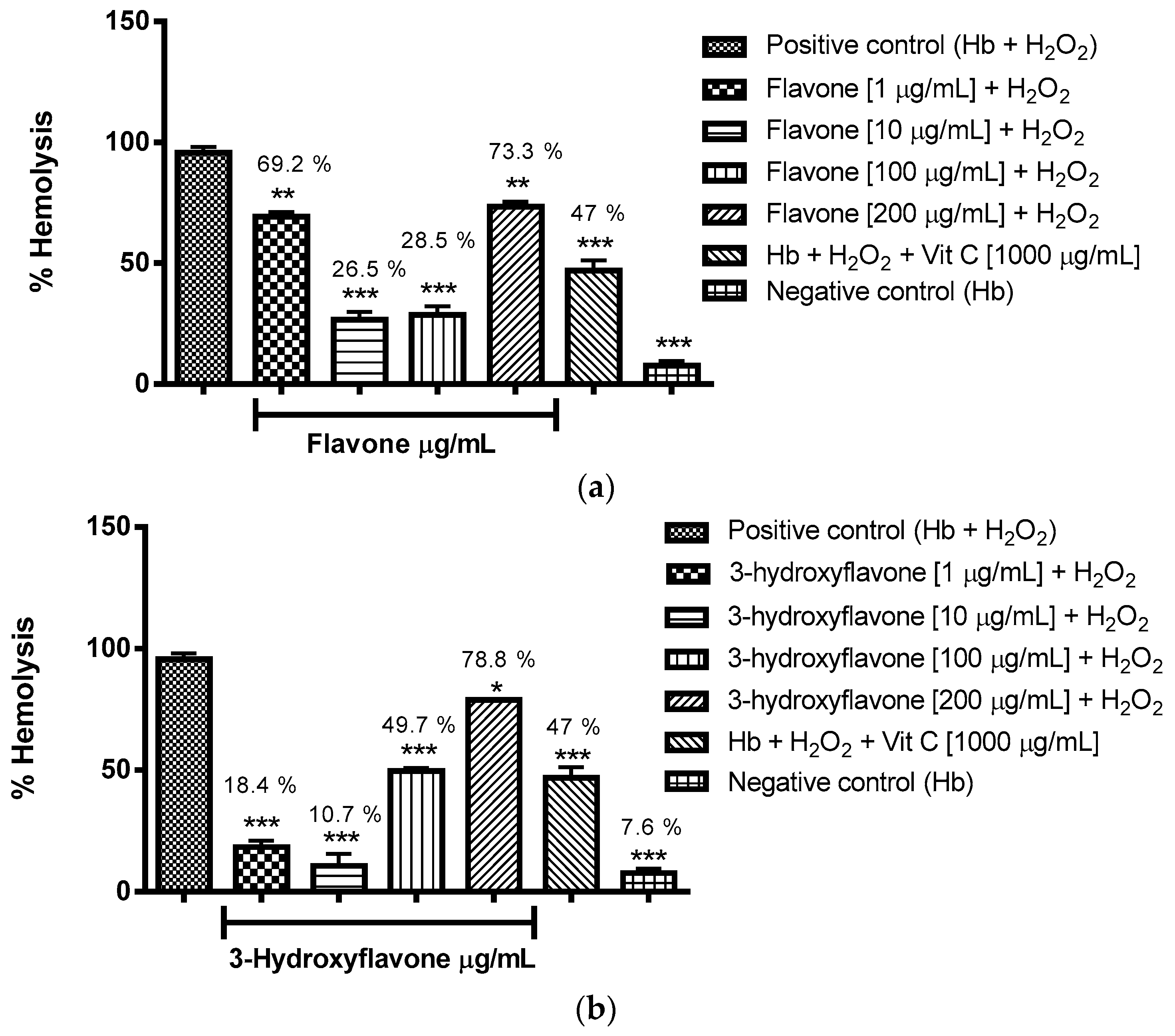
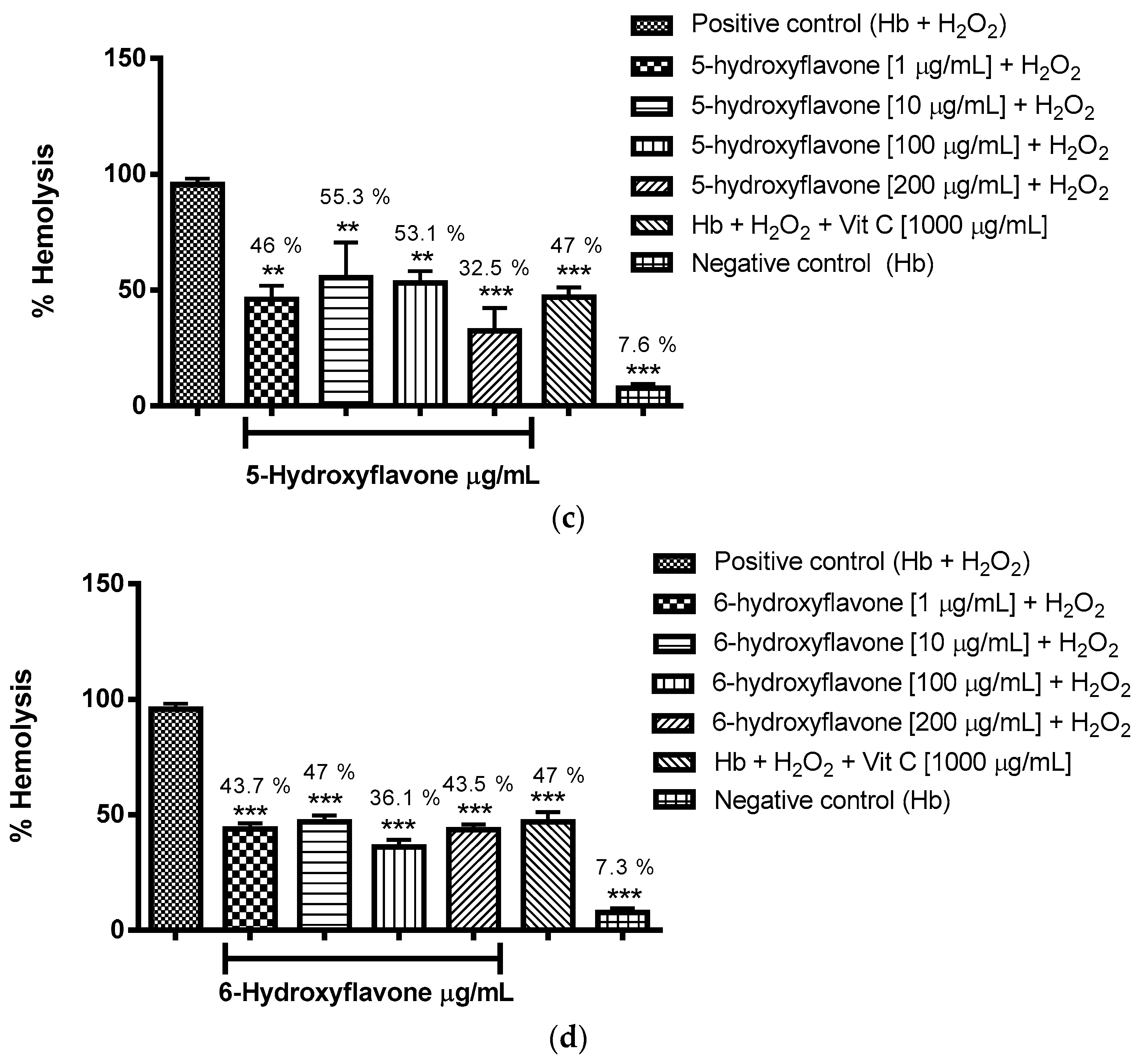
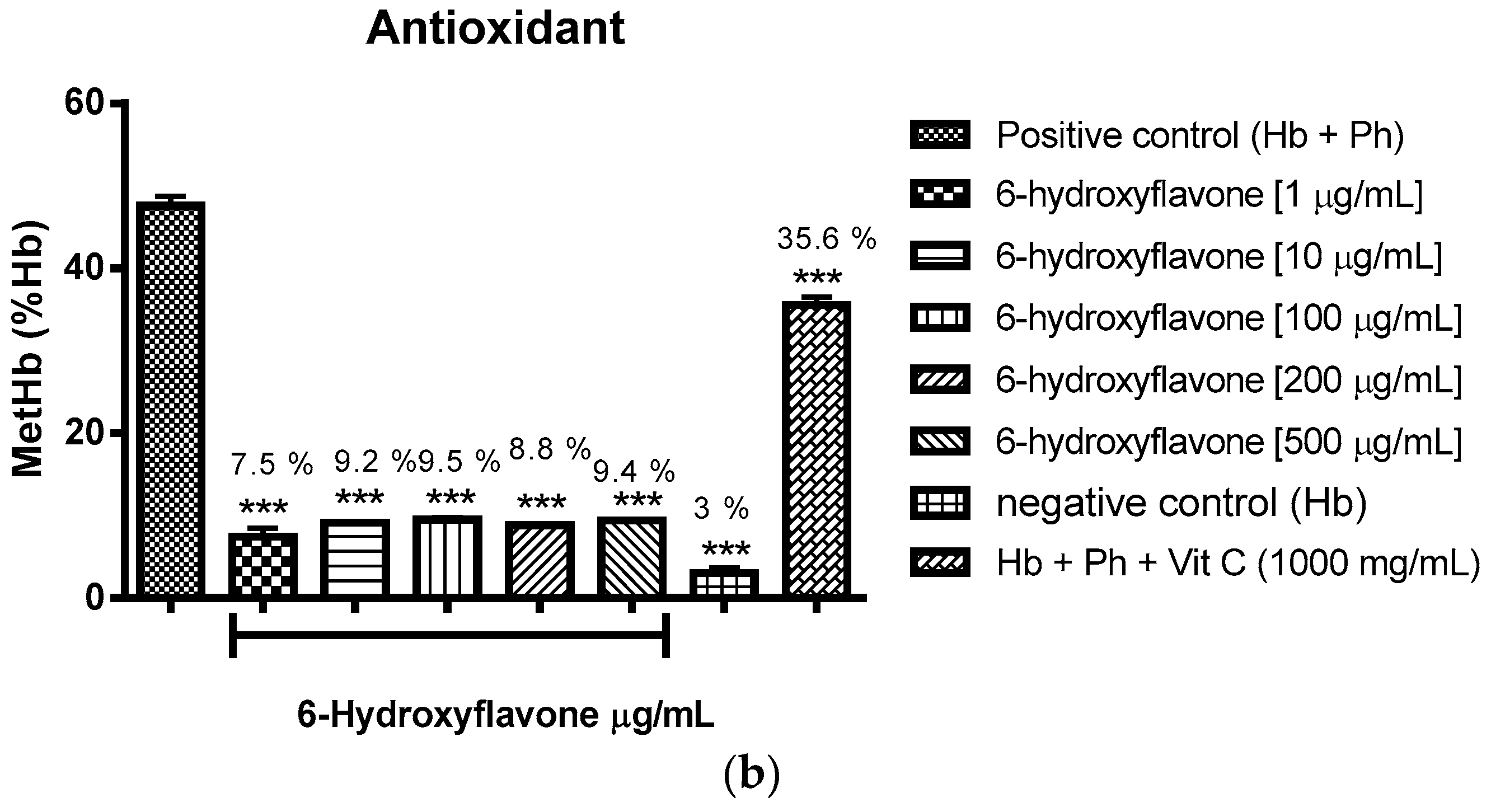
2.3.1. Evaluation of the Antioxidant Potential of these Flavonoids in Human Erythrocytes in the Presence of Reactive Oxygen Species
2.3.2. Assessment of the Oxidant and Antioxidant Potential of Flavonoids in Human Erythrocytes in the Presence of Phenylhydrazine
3. Discussion
4. Materials and Methods
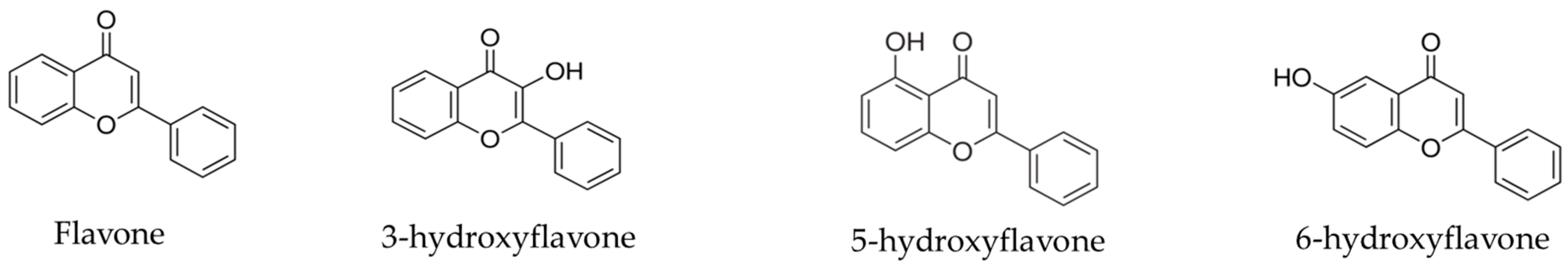
4.1. Flavonoids
4.2. Bacterial Species
4.3. Culture Medium
4.4. Preparation of the Bacterial Inoculum
4.5. Human Erythrocytes
4.6. In Silico Study—PASS Online
4.7. Antibacterial Activity
4.7.1. Determination of Minimum Inhibitory Concentration (MIC)
4.7.2. Determination of Minimum Bactericidal Concentration (MBC)
4.8. Oxidant and Antioxidant Activity Assays
4.8.1. Evaluation of the Antioxidant Potential of Flavonoids in Human Erythrocytes in the Presence of Reactive Oxygen Species
4.8.2. Assessment of Oxidant and Antioxidant Potential for Flavonoids in Human Erythrocytes in the Presence of Phenylhydrazine
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chebil, L.; Anthoni, J.; Humeau, C.; Gerardin, C.; Engasser, J.M.; Ghoul, M. Enzymatic acylation of flavonoids: Effect of the nature of the substrate, origin of lipase, and operating conditions on conversion yield and regioselectivity. J. Agric. Food Chem. 2007, 55, 9496–9502. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Zuanazzi, J.A.S; Montanha, J.A. “Flavonóides”. In Farmacognosia: Da Planta ao Medicamento, 5th ed.; Simões, C.M.O., Schenkel, E.P., Gormann, G., Mello, J.C.P., Mentz, L.A., Petrovick, P.R., Eds.; Editora da UFRGS: Porto Alegre, Brasil, 2004; pp. 577–614. [Google Scholar]
- Peterson, J.; Lagiou, P.; Samoli, E.; Lagiou, A.; Katsouyianni, K.; La Veccia, C.; Dwye, J.; Trichopoulos, D. Flavonoid intake and breast cancer risk: A case-control study in Greece. Br. J. Cancer 2003, 89, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, P.A.; Winn, N.R.; Walle, T. Accumulation and metabolism of the anticancer flavonoid 5,7-dimethoxyflavone compared to its unmethylated analog chrysin in the Atlantic killifish. Chem. Biol. Interact. 2006, 164, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; TA, N.; Kawamori, T.; Wen, X.; Tsuji, P.A.; Walle, U.K. Cancer chemopreventive properties of orally bioavailable flavonoids—Methylated versus unmethylated flavones. Biochem. Pharmacol. 2007, 73, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Choi, J.H.; Yang, H.; Jeong, E.J.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem. Toxicol. 2012, 50, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Cooks, N.C.; Samman, S. Flavonoids: Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Lee, J.C.; Lim, K.T.; Jang, Y.S. Identification of Rhus verniciflua Stokes compounds that exhibit free radical scavenging and anti-apoptotic properties. Biochim. Biophys. Acta Gen. Subj. 2002, 1570, 181–191. [Google Scholar] [CrossRef]
- Venkatachalam, H.; Yogendra, N.; Jayashree, B.S. Synthesis, characterization and antioxidant activities of synthetic chalcones and flavones. APCBEE Proc. 2012, 3, 209–213. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.; Li, D.; Zhang, J.; Qiu, D.; Liu, W.; She, L.; Yang, Z. Tumor inhibition and improved immunity in mice treated with flavone from Cirsium japonicum DC. Int. Immunopharmacol. 2006, 6, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Yang, W.E.; Kuo, W.H.; Chang, H.R.; Chu, S.C.; Hsieh, Y.S. Antimetastatic potentials of flavones on oral cancer cell via an inhibition of matrixdegrading proteases. Arch. Oral Biol. 2008, 53, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Liu, H.F.; Shen, X.; Song, B.A.; Bhadury, P.S.; Zhu, H.L.; Liu, J.X.; Qi, X.B. Synthesis and molecular docking studies of novel 2-chloro-pyridine derivatives containing flavone moieties as potential antitumor agents. Bioorg. Med. Chem. Lett. 2010, 20, 4163–4167. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Hendriks, J.J.; Alblas, J.; Van Der Pol, S.M.; Van Tol, E.A.; Dijkstra, C.D.; De Vries, H.E. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J. Exp. Med. 2004, 200, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jin, S.E.; Min, B.S.; Kim, B.W.; Choi, J.S. Anti-inflammatory activity of Korean thistle Cirsium maackii and its major flavonoid, luteolin 5-O-glucoside. Food Chem. Toxicol. 2012, 50, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.K.; Lee, J.H.; Kim, H.K.; Lee, A.Y.; Lee, S.O.; Kim, Y.S.; Ryu, S.Y.; Kimc, S.Y.; Leed, Y.J.; Ko, B.S. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 2006, 106, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Lee, W.S.; Kim, Y.S.; Curtis-long, M.J.; Lee, B.W.; Ryu, Y.B.; Park, K.H. Isolation of cholinesterase-inhibiting flavonoids from Morus lhou. J. Agric. Food Chem. 2011, 59, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Amoros, M.; Simoes, C.M.; Girre, L.; Sauvager, F.; Cormier, M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992, 55, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, T.; Otsuka, H.; Takeda, Y. Antiviral activity of 5,6,7-trimethoxyflavone and its potentiation of the antiherpes activity of acyclovir. J. Antimicrob. Chemother. 1997, 39, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Kongkuma, N.; Tuchindaa, P.; Pohmakotra, M.; Reutrakula, V.; Piyachaturawatb, P.; Jariyawatb, S.; Suksenb, K.; Yoosookc, C.; Kasisitc, J.; Napaswadc, C. DNA topoisomerase IIα inhibitory and anti-HIV-1 flavones from leaves and twigs of Gardenia carinata. Fitoterapia 2012, 83, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.F.; Tan, R.X.; Yang, L.; Liu, Z.L. Two flavones from Artemisia giraldii and their antimicrobial activity. Planta Med. 1996, 62, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Tarle, D.; Dvorzak, I. Antimicrobial activity of the plant Cirsium oleraceum (L.) Scop. Acta Pharm. Jugosl. 1990, 40, 569–571. [Google Scholar]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlajac, K.; Vuorelaa, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Konan, N.A.; Lincopan, N.; Collantes Díaz, I.E.; Jacysyn, J.F.; Tiba, M.M.T.; Mendes, J.G.P.A.; Bacchid, E.M.; Spira, B. Cytotoxicity of cashew flavonoids towards malignant cell lines. Exp. Toxicol. Pathol. 2012, 64, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.S.; Rodriguez-Amaya, D.B. Flavonols and flavones: Brazilian sources and factors that influence food composition. Alim. Nutr. 2008, 19, 97–108. [Google Scholar]
- Barril, X.; Hubbard, R.E.; Morley, S.D. Virtual screening in structure-based drug discovery. Mini Rev. Med. Chem. 2004, 4, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Ollof, S. Advances in virtual screening. Drug Discov. Today Technol. 2006, 3, 405–411. [Google Scholar]
- Angelo, V.; Max, D.; Markus, A.L. The challenge of predicting drug toxicity in silico. Basic Clin. Pharmacol. Toxicol. 2006, 99, 195–208. [Google Scholar]
- Srinivas, N.; Sandeep, K.S.; Anusha, Y.; Devendra, B.N. In vitro cytotoxic evaluation and detoxification of monocrotaline (Mct) Alkaloid: An In Silico approach. Int. Invent. J. Biochem. Bioinform. 2014, 2, 20–29. [Google Scholar]
- Marchant, C.A. Computational toxicology: A tool for all industries. Wires Comput. Mol. Sci. 2012, 2, 424–434. [Google Scholar] [CrossRef]
- Auld, D.S.; Diller, D.; Ho, K. Targeting signal transduction with large combinatorial collections. Drug Discov. Today 2002, 7, 1206–1213. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci World J. 2013, 2013. [Google Scholar] [CrossRef]
- Maruszak, A.; Safranow, K.; Gustaw, K.; Kijanowska-Haładyna, B.; Jakubowska, K.; Olszewska, M.; Styczyńska, M.; Berdyński, M.; Tysarowski, A.; Chlubek, D.; et al. PIN1 gene variants in Alzheimer's disease. BMC Med. Genet. 2009, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Naccarati, A.; Polakova, V.; Pardini, B.; Vodickova, L.; Hemminki, K.; Kumar, R.; Vodicka, P. Mutations and polymorphisms in TP53 gene—An overview on the role in colorectal cancer. Mutagenesis 2012, 27, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Bjerke, L.; Clarke, P.A.; Workman, P. Drugging PI3K in cancer: Refining targets and therapeutic strategies. Curr. Opin. Pharmacol. 2015, 23, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Yamagishi, H.; Ono, Y.; Ueda, Y. Versatile inhibitory effects of the flavonoid-derived PI3K/Akt inhibitor, LY294002, on ATP-binding cassette transporters that characterize stem cells. Clin. Transl. Med. 2012, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Chen, W.K.; Wang, C.J.; Lin, W.L.; Tseng, T.H. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and β4 integrin function in MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2008, 226, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J.; Jeong, Y.J.; Park, J.W.; Kwon, T.K. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem. Biophys. Res. Commun. 2004, 325, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lu, Y.; Zang, X.; Wu, T.; Qi, X.J.; Pan, S.; Xu, X. 3D-QSAR and docking studies of flavonoids as potent Escherichia coli inhibitors. Sci. Rep. 2016, 6, 23634. [Google Scholar] [CrossRef] [PubMed]
- Shakhatreh, M.A.K.; Al-Smadi, M.; Khabour, O.F.; Shuaibu, F.A.; Hussein, E.I.; Alzoubi, K.H. Study of the antibacterial and antifungal activities of synthetic benzyl bromides, ketones, and corresponding chalcone derivatives. Drug Des. Dev. Ther. 2016, 10, 3653–3660. [Google Scholar] [CrossRef] [PubMed]
- Lovewell, R.R.; Hayes, S.M.; O’Toole, G.A.; Berwin, B. Pseudomonas aeruginosa flagellar motility activates the phagocyte PI3K/Akt pathway to induce phagocytic engulfment. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, L698–L707. [Google Scholar] [CrossRef] [PubMed]
- Sandhar, H.K.; Kumar, B.; Prasher, S.; Tiwari, P.; Salhan, M.; Sharma, P. A Review of phytochemistry and pharmacology of flavonoids. Int. Pharm. Sci. 2011, 1, 25–41. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Q.; Wen, Y.F.; Bhadauria, M.; Nirala, S.K.; Sharma, A.; Shrivastava, S.; Shukla, S.; Agrawal, O.P.; Mathur, R. Protective effects of propolis on inorganic mercury induced oxidative stress in mice. Indian J. Exp. Biol. 2009, 47, 264–269. [Google Scholar] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2006, 26, 343–356. [Google Scholar] [CrossRef]
- Packer, J.F.; Luz, M.M.S. Evaluation and research method for natural products inhibitory activity. Rev. Bras. Farmacogn. 2007, 17, 102–107. [Google Scholar] [CrossRef]
- Srour, M.A.; Bilto, Y.Y.; Juma, M. Evaluation of different methods used to measure malonyldial-dehyde in human erythrocytes. Clin. Hemorheol. Microcirc. 2000, 23, 23–30. [Google Scholar] [PubMed]
- Jager, A.K.; Saaby, L. Flavonoids and the CNS. Molecules. 2011, 16, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Snijman, P.W.; Swanevelder, S.; Elizabeth Joubert, E.; Green, I.R.; Gelderblom, W.C.A. The antimutagenic activity of the major flavonoids of rooibos (Aspalathus linearis): Some dose-response effects on mutagen activationflavonoid interactions. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 631, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Boersma, M.G.; Vervoort, J.; Szymusiak, H.; Lemanska, K.; Tyrakowska, B.; Cenas, N.; Segura-Aguilar, J.; Rietjens, I. Regioselectivity and reversibility of the glutathione conjugation of quercetin quinone methide. Chem. Res. Toxicol. 2000, 13, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, R.; Squires, E. Evalution of new antimicrobials in vitro and in experimental animal infections. In Antibiotics in Laboratory Medicine, 3rd ed.; Lorian, V.M.D., Ed.; Willians & Wilkins: Baltimore, MD, USA, 1991; pp. 739–788. ISBN 0683051687. [Google Scholar]
- Hadacek, F.; Greger, H. Testing of antifungal natural products: Methodologies, comparatibility of results and assay choice. Phytochem. Anal. 2000, 11, 137–147. [Google Scholar] [CrossRef]
- Gerhardt, P.; Murray, R.G.E.; Wood, W.A.; Krieg, N.R. Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, USA, 1994; p. 791. ISBN 1555810489. [Google Scholar]
- Bilton, Y.Y.; Suboh, S.; Aburjai, T.; Abdalla, S. Structure-activity relationships regarding the antioxidant effects of the flavonoids on human erythrocytes. Nat. Sci. 2012, 4, 740–747. [Google Scholar]
- Rangel, M.; Malpezzi, E.L.A.; Susini, S.M.M.; Freitas, J.C. Hemolytic activity in extracts of the diatom Nitzschia. Toxicon 1997, 35, 305–309. [Google Scholar] [CrossRef]
- Arbós, K.A.; Claro, L.M.; Borges, L.; Santos, C.A.; Weffort-Santos, A.M. Human erythrocytes as a system for evaluating the antioxidant capacity of vegetable extracts. Nutr. Res. 2008, 28, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Camargo, T.M.; Alves, M.I.F.; Oliveira, S.J.; Oshima-Franco, Y. Comparative study between two methemoglobin (MHb) dosing techniques. Rev. Bras. Anal. Clin. 2007, 39, 95–98. [Google Scholar]
Sample Availability: Samples of the compounds flavone, 3-hydroxyflavone, 5-hydroxyflavone and 6-hydroxyflavone are available from the authors. |
| PA | PI | Activity |
|---|---|---|
| 0.952 | 0.001 | Inhibitor of 4-nitrophenol 2-monooxygenase |
| 0.952 | 0.003 | HIF1A expression inhibitor |
| 0.947 | 0.004 | Agonist of membrane integrity |
| 0.943 | 0.002 | Inhibitor 27-hydroxycholesterol 7α-monooxygenas |
| 0.938 | 0.002 | Kinase inhibitor |
| 0.933 | 0.002 | Inhibitor of colestanetriol 26-monooxygenase |
| 0.929 | 0.003 | Anaphylatoxin receptor antagonist |
| 0.914 | 0.003 | Inhibitor of membrane permeability |
| 0.913 | 0.004 | Chlordecone reductase inhibitor |
| 0.913 | 0.009 | CYP2C12 substrate |
| 0.903 | 0.002 | CYP2B5 substrate |
| 0.895 | 0.002 | Aryl-alcohol dehydrogenase (NADP+) inhibitor |
| 0.893 | 0.002 | CYP1A2 inducer |
| 0.874 | 0.014 | Aspulvinone dimethylallyltransferase inhibitor |
| 0.872 | 0.002 | Inhibitor of P-benzoquinone reductase (NADPH) |
| 0.857 | 0.004 | Inhibitor of 2-dehydropantoate 2-reductase |
| 0.857 | 0.006 | Aldehyde oxidase inhibitor |
| 0.854 | 0.003 | CYP1A inducer |
| 0.850 | 0.012 | Methylenetetrahydrofolate reductase inhibitor (NADPH) |
| 0.849 | 0.004 | Vasoprotector |
| 0.848 | 0.002 | CYP2A4 substrate |
| 0.829 | 0.002 | Quercetin 2.3-dioxygenase inhibitor |
| 0.829 | 0.002 | Inhibitor of Leukotriene-B4 20-monooxygenase |
| 0.820 | 0.005 | Inhibitor of complement factor D |
| 0.819 | 0.005 | Alkane 1-monooxygenase inhibitor |
| 0.814 | 0.003 | CYP2A11 substrate |
| 0.813 | 0.018 | CYP2J substrate |
| 0.813 | 0.022 | Testosterone 17beta-dehydrogenase (NADP+) inhibitor |
| 0.809 | 0.002 | CYP1A1 inducer |
| 0.807 | 0.003 | MAP kinase stimulant |
| 0.806 | 0.006 | Inhibitor of oxidoreductase |
| 0.804 | 0.005 | Peroxidase inhibitor |
| 0.803 | 0.003 | Total ecdysone 20-monooxygenase inhibitor |
| 0.801 | 0.004 | Inhibitor of Pin1 |
| 0.800 | 0.018 | mucous membrane protector |
| 0.795 | 0.004 | Antimutagenic |
| 0.787 | 0.006 | Inhibitor of nitrate reductase (cytochrome) |
| PA | PI | Activity |
|---|---|---|
| 0.962 | 0.003 | Agonist of membrane integrity |
| 0.957 | 0.003 | HIF1A expression inhibitor |
| 0.954 | 0.002 | Chlordecone reductase inhibitor |
| 0.947 | 0.001 | Aryl-alcohol dehydrogenase (NADP+) inhibitor |
| 0.947 | 0.002 | Kinase inhibitor |
| 0.939 | 0.003 | Inhibitor of membrane permeability |
| 0.938 | 0.001 | Inhibitor of p-benzoquinone reductase (NADPH) |
| 0.929 | 0.001 | Quercetin 2.3-dioxygenase inhibitor |
| 0.922 | 0.002 | Inhibitor of 2-dehydropantoate 2-reductase |
| 0.917 | 0.002 | Peroxidase inhibitor |
| 0.896 | 0.009 | Aspulvinone dimethylallyltransferase inhibitor |
| 0.896 | 0.013 | CYP2C12 substrate |
| 0.894 | 0.002 | MAP kinase stimulant |
| 0.893 | 0.002 | CYP1A inducer |
| 0.892 | 0.002 | Colestanetriol inhibitor 26-monooxygenase |
| 0.889 | 0.002 | 2-Enoate reductase inhibitor |
| 0.888 | 0.002 | Inhibitor of 4-nitrophenol 2-monooxygenase |
| 0.888 | 0.007 | Inhibitor ubiquinol, cytochrome-c reductase |
| 0.880 | 0.005 | CYP1A substrate |
| 0.877 | 0.003 | Inhibitor of alcohol dehydrogenase (NADP+) |
| 0.876 | 0.002 | Inhibitor of NADPH-ferrihemoprotein reductase |
| 0.870 | 0.003 | Antimutagenic |
| 0.870 | 0.003 | Inhibitor 27-hydroxycholesterol 7α-monooxygenase |
| 0.861 | 0.002 | CYP1A1 inducer |
| 0.859 | 0.007 | Enhances expression of TP53 |
| 0.856 | 0.011 | Methylenetetrahydrofolate reductase inhibitor (NADPH) |
| 0.843 | 0.004 | CYP1A1 substrate |
| 0.843 | 0.008 | Anaphylatoxin receptor antagonist |
| 0.837 | 0.001 | Inhibitor of glycerol dehydrogenase (NADP+) |
| 0.827 | 0.003 | Enhances expression of HMOX1 |
| 0.825 | 0.005 | CYP1A2 substrate |
| 0.819 | 0.004 | UGT1A9 substrate |
| 0.816 | 0.002 | Inhibitor of 2-dehydropantolactone reductase (A-specific) |
| 0.804 | 0.009 | Inhibitor dehydro-L-gulonate decarboxylase |
| 0.802 | 0.003 | Inhibitor of β-carotene 15.15'-monooxygenase |
| 0.800 | 0.005 | Inhibitor of nitrate reductase (cytochrome) |
| 0.800 | 0.008 | Agonist of apoptosis |
| 0.799 | 0.006 | Alkane 1-monooxygenase inhibitor |
| 0.798 | 0.021 | The substrate CYP2J |
| PA | PI | Activity |
|---|---|---|
| 0.963 | 0.003 | Agonist of membrane integrity |
| 0.956 | 0.002 | Chlordecone reductase inhibitor |
| 0.953 | 0.003 | HIF1A expression inhibitor |
| 0.942 | 0.005 | Substrate of CYP2C12 |
| 0.937 | 0.003 | Inhibitor of membrane permeability |
| 0.935 | 0.002 | Kinase inhibitor |
| 0.934 | 0.003 | Anaphylatoxin receptor antagonist |
| 0.930 | 0.001 | Aryl alcohol dehydrogenase inhibitor (NADP+) |
| 0.923 | 0.004 | Aldehyde oxidase inhibitor |
| 0.921 | 0.002 | Inhibitor of P-benzoquinone reductase (NADPH) |
| 0.920 | 0.003 | Inhibitor 2-dehydropantoate 2-reductase |
| 0.909 | 0.002 | Inhibitor of 4-nitrophenol 2-monooxygenase |
| 0.904 | 0.002 | Colestanetriol 26-monooxygenase inhibitor |
| 0.903 | 0.001 | Quercetin 2.3-dioxygenase inhibitor |
| 0.901 | 0.003 | Vasoprotector |
| 0.900 | 0.008 | Aspulvinone dimethylallyltransferase inhibitor |
| 0.897 | 0.002 | CYP1A inducer |
| 0.897 | 0.002 | Histidine kinase inhibitor |
| 0.895 | 0.003 | Inhibitor 27-hydroxycholesterol 7α-monooxygenase |
| 0.888 | 0.003 | Peroxidase inhibitor |
| 0.887 | 0.008 | Inhibitor of ubiquinol-cytochrome-c reductase |
| 0.882 | 0.002 | Antimutagenic |
| 0.880 | 0.002 | Inhibitor of NADPH-ferrihemoprotein reductase |
| 0.878 | 0.005 | CYP1A substrate |
| 0.871 | 0.007 | Enhances expression of TP53 |
| 0.868 | 0.002 | CYP1A1 inducer |
| 0.864 | 0.004 | UGT1A6 substrate |
| 0.854 | 0.003 | Inhibitor of alcohol dehydrogenase (NADP+) |
| 0.849 | 0.002 | 2-Enoate reductase inhibitor |
| 0.847 | 0.004 | Alkane 1-monooxygenase inhibitor |
| 0.842 | 0.003 | Enhances expression of HMOX1 |
| 0.841 | 0.002 | Inhibitor of β-carotene 15.15′-monooxygenase |
| 0.840 | 0.004 | UGT1A9 substrate |
| 0.838 | 0.001 | Inhibitor of glycerol dehydrogenase (NADP+) |
| 0.838 | 0.012 | Anti-seborrheic |
| 0.837 | 0.005 | CYP1A1 substrate |
| 0.835 | 0.003 | CYP2B5 substrate |
| 0.830 | 0.003 | CYP2A4 substrate |
| 0.827 | 0.003 | Inhibitor of Pin1 |
| 0.818 | 0.002 | SULT1A3 substrate |
| 0.814 | 0.008 | Inhibitor dihydrocholic L-gulonate decarboxylase |
| 0.812 | 0.002 | Inhibitor of Leukotriene-B4 20-monooxygenase |
| 0.807 | 0.008 | Agonist of apoptosis |
| 0.806 | 0.003 | Inhibitor of histamine release |
| 0.804 | 0.003 | Inhibitor of nitrite reductase [NAD(P)H] |
| 0.802 | 0.002 | Inhibitor 2-dehydropantolactone reductase (A-specific) |
| 0.795 | 0.003 | MAP kinase stimulant |
| PA | PI | Activity |
|---|---|---|
| 0.957 | 0.003 | Agonist of membrane integrity |
| 0.949 | 0.002 | Chlordecone reductase inhibitor |
| 0.948 | 0.004 | HIF1A expression inhibitor |
| 0.945 | 0.004 | CYP2C12 substrate |
| 0.935 | 0.003 | Inhibitor of membrane permeability |
| 0.932 | 0.001 | Aryl alcohol dehydrogenase inhibitor (NADP+) |
| 0.932 | 0.001 | Inhibitor of 4-nitrophenol 2-monooxygenase |
| 0.929 | 0.003 | Inhibitor aldehyde oxidase |
| 0.924 | 0.002 | Inhibitor of p-benzoquinone reductase (NADPH) |
| 0.915 | 0.002 | Inhibitor colestanetriol 26-monooxygenase |
| 0.914 | 0.003 | Kinase inhibitor |
| 0.907 | 0.002 | Inhibitor 27-Hydroxycolesterol 7α-monooxygenase |
| 0.901 | 0.003 | Inhibitor 2-2-dehydropantoate reductase |
| 0.895 | 0.004 | Anaphylatoxin receptor antagonist |
| 0.894 | 0.002 | CYP1A inducer |
| 0.892 | 0.003 | Peroxidase inhibitor |
| 0.888 | 0.002 | Antimutagenic |
| 0.887 | 0.011 | Aspulvinone dimethylallyltransferase inhibitor |
| 0.884 | 0.002 | MAP kinase stimulant |
| 0.880 | 0.001 | Quercetin 2.3-dioxygenase inhibitor inhibitor |
| 0.875 | 0.006 | Anti-seborreic |
| 0.868 | 0.002 | CYP1A1 inducer |
| 0.852 | 0.007 | Enhances expression of TP53 |
| 0.851 | 0.005 | CYP1A substrate |
| 0.850 | 0.003 | Inhibitor of NADPH-ferrihemoprotein reductase |
| 0.850 | 0.003 | CYP2B5 substrate |
| 0.844 | 0.003 | Enhances expression of HMOX1 |
| 0.843 | 0.003 | CYP2A4 substrate |
| 0.843 | 0.004 | UGT1A9 substrate |
| 0.840 | 0.003 | Inhibitor of Pin1 |
| 0.840 | 0.004 | Vasoprotector |
| 0.841 | 0.005 | Expression of JAK2 inhibitor |
| 0.833 | 0.003 | Inhibitor of alcohol dehydrogenase (NADP+) |
| 0.829 | 0.002 | Inhibitor of leukotriene-B4 20-monooxygenase |
| 0.828 | 0.005 | Alkane 1-monooxygenase inhibitor |
| 0.822 | 0.005 | Inhibitor of oxidoreductase |
| 0.820 | 0.016 | Methylenetetrahydrofolate reductase inhibitor (NADPH) |
| 0.817 | 0.005 | Substrate of CYP1A1 |
| 0.815 | 0.003 | Inhibitor of β-carotene 15.15′-monooxygenase |
| 0.815 | 0.004 | Histidine kinase inhibitor |
| 0.809 | 0.002 | 2-Enoate reductase inhibitor |
| 0.809 | 0.013 | 5 Hydroxytryptamine release stimulant |
| 0.808 | 0.003 | Inhibitor of nitrite reductase [NAD (P) H] |
| 0.806 | 0.004 | UGT1A6 substrate |
| 0.805 | 0.017 | Mucous membrane protector |
| 0.802 | 0.016 | Gluconate 2-dehydrogenase inhibitor |
| 0.801 | 0.002 | Inhibitor of Glycerol dehydrogenase (NADP+) |
| 0.797 | 0.002 | CYP1A2 inducer |
| 0.797 | 0.003 | Inhibitor of histamine release |
| Test Group | Flavone | 3-Hydroxyflavone | 5-Hydroxyflavone | 6-Hydroxyflavone | Chloramphenicol | DMSO | |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| B. subtilis CCT 0516 | >200 | 200 | >200 | >200 | 100 | + | |
| S. aureus ATCC 25619 | 100 | 100 | 200 | 200 | 100 | + | |
| S. aureus ATCC 25925 | 200 | 200 | >200 | >200 | 100 | + | |
| Test Group | Flavone | 3-Hydroxyflavone | 5-Hydroxyflavone | 6-Hydroxyflavone | Chloramphenicol | DMSO | |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| P. aeruginosa ATCC 8027 | 200 | 200 | >200 | >200 | 100 | + | |
| P. aeruginosa ATCC 23243 | 50 | 200 | 200 | 200 | 100 | + | |
| E. coli ATCC 2536 | 100 | 100 | 200 | >200 | 100 | + | |
| E. coli 101 | 100 | 200 | 200 | 200 | 100 | + | |
| E. coli 103 | 25 | 200 | >200 | >200 | 100 | + | |
| E. coli 104 | 200 | 100 | 200 | >200 | 100 | + | |
| E. coli 105 | 25 | 200 | 200 | >200 | 100 | + | |
| E. coli 108 | 200 | 100 | 25 | 200 | 100 | + | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, C.D.A.; Gonçalves, G.F.; Oliveira Filho, A.A.d.; Lira, A.B.; Cassiano, T.T.M.; Lima, N.T.R.d.; Barbosa-Filho, J.M.; Diniz, M.D.F.F.M.; Pessôa, H.L.F. In Silico Study and Bioprospection of the Antibacterial and Antioxidant Effects of Flavone and Its Hydroxylated Derivatives. Molecules 2017, 22, 869. https://doi.org/10.3390/molecules22060869
Montenegro CDA, Gonçalves GF, Oliveira Filho AAd, Lira AB, Cassiano TTM, Lima NTRd, Barbosa-Filho JM, Diniz MDFFM, Pessôa HLF. In Silico Study and Bioprospection of the Antibacterial and Antioxidant Effects of Flavone and Its Hydroxylated Derivatives. Molecules. 2017; 22(6):869. https://doi.org/10.3390/molecules22060869
Chicago/Turabian StyleMontenegro, Camila De Albuquerque, Gregório Fernandes Gonçalves, Abrahão Alves de Oliveira Filho, Andressa Brito Lira, Thays Thyara Mendes Cassiano, Natanael Teles Ramos de Lima, José Maria Barbosa-Filho, Margareth De Fátima Formiga Melo Diniz, and Hilzeth Luna Freire Pessôa. 2017. "In Silico Study and Bioprospection of the Antibacterial and Antioxidant Effects of Flavone and Its Hydroxylated Derivatives" Molecules 22, no. 6: 869. https://doi.org/10.3390/molecules22060869
APA StyleMontenegro, C. D. A., Gonçalves, G. F., Oliveira Filho, A. A. d., Lira, A. B., Cassiano, T. T. M., Lima, N. T. R. d., Barbosa-Filho, J. M., Diniz, M. D. F. F. M., & Pessôa, H. L. F. (2017). In Silico Study and Bioprospection of the Antibacterial and Antioxidant Effects of Flavone and Its Hydroxylated Derivatives. Molecules, 22(6), 869. https://doi.org/10.3390/molecules22060869