Synthesis and Antiproliferative Activity of Novel All-Trans-Retinoic Acid-Podophyllotoxin Conjugate towards Human Gastric Cancer Cells
Abstract
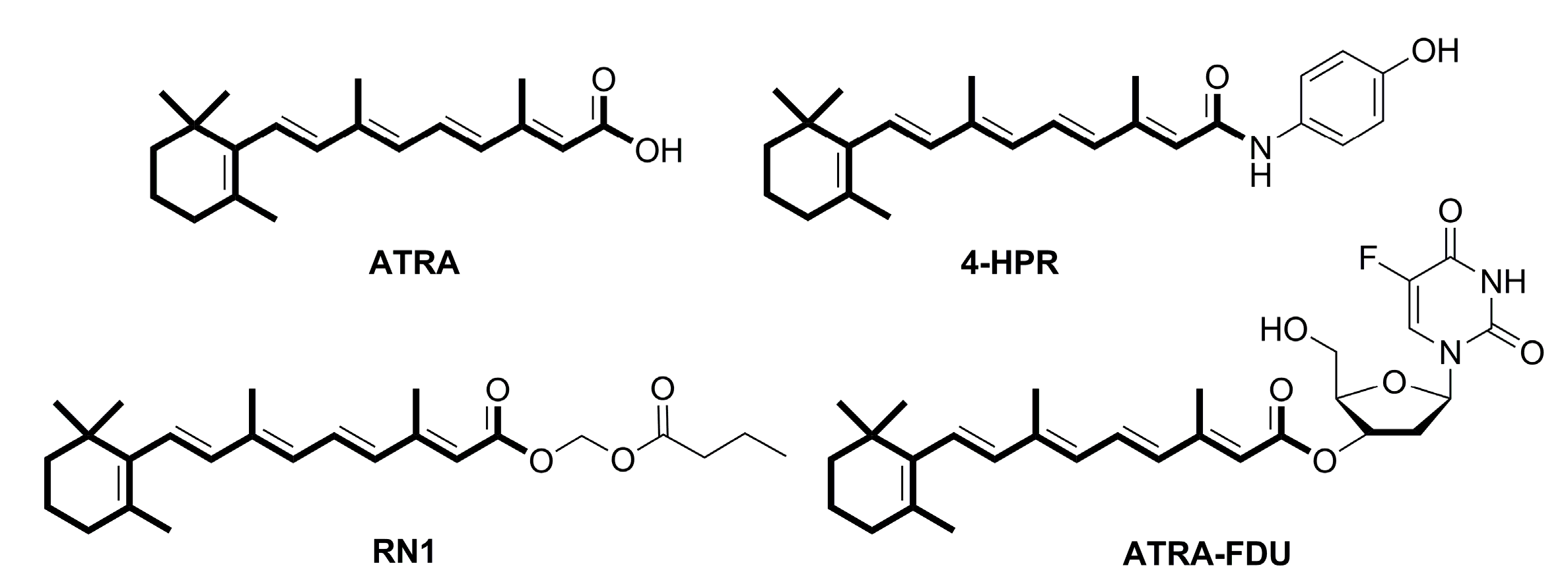
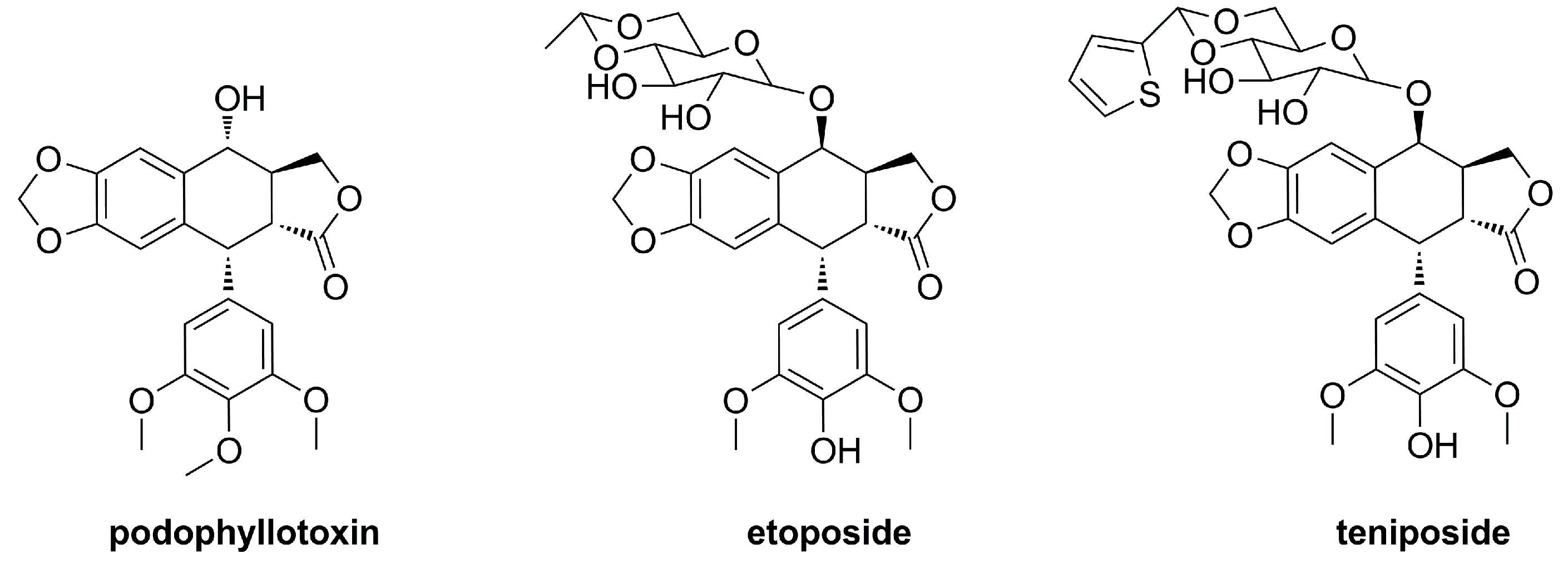
:1. Introduction
2. Results and Discussion
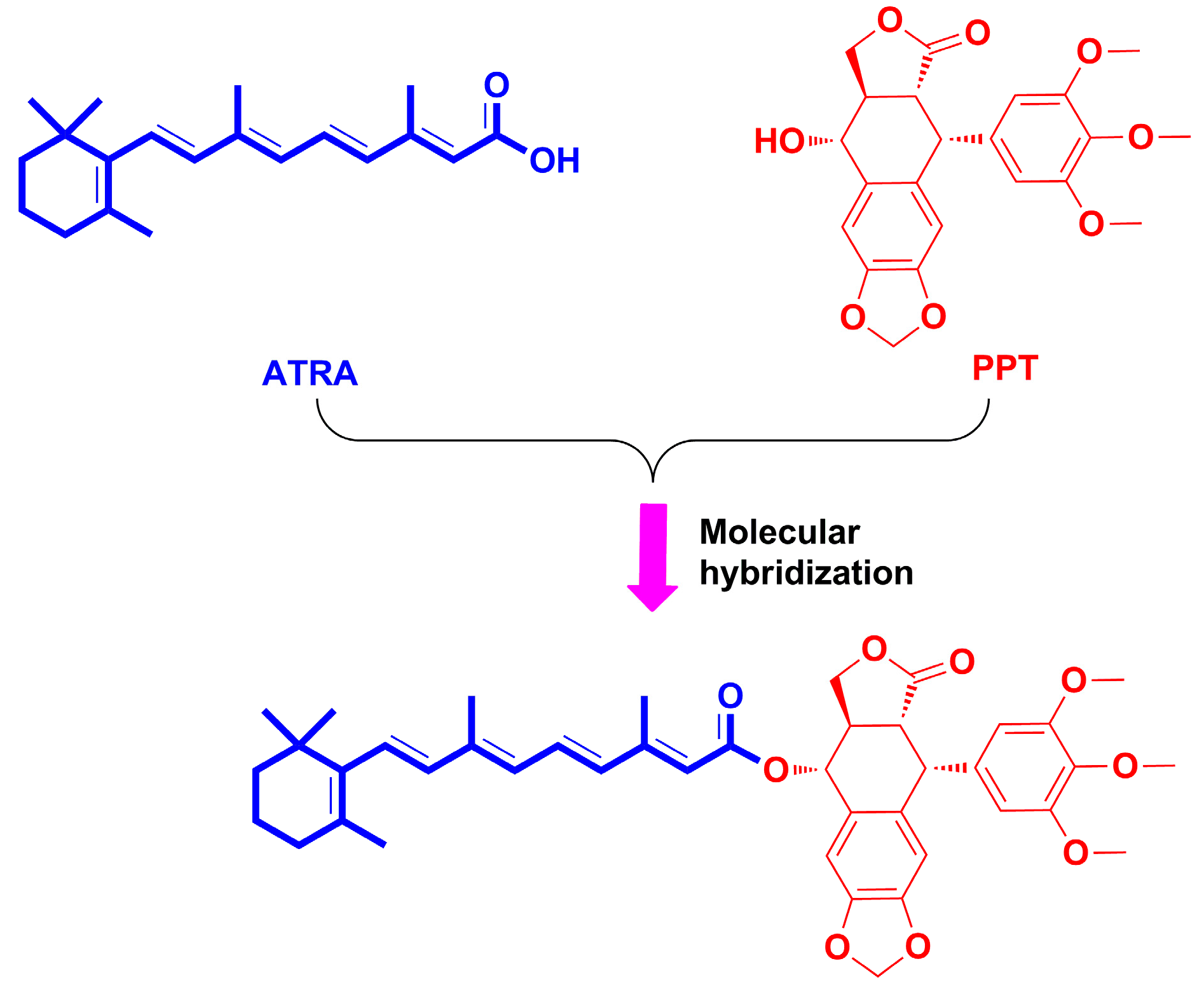
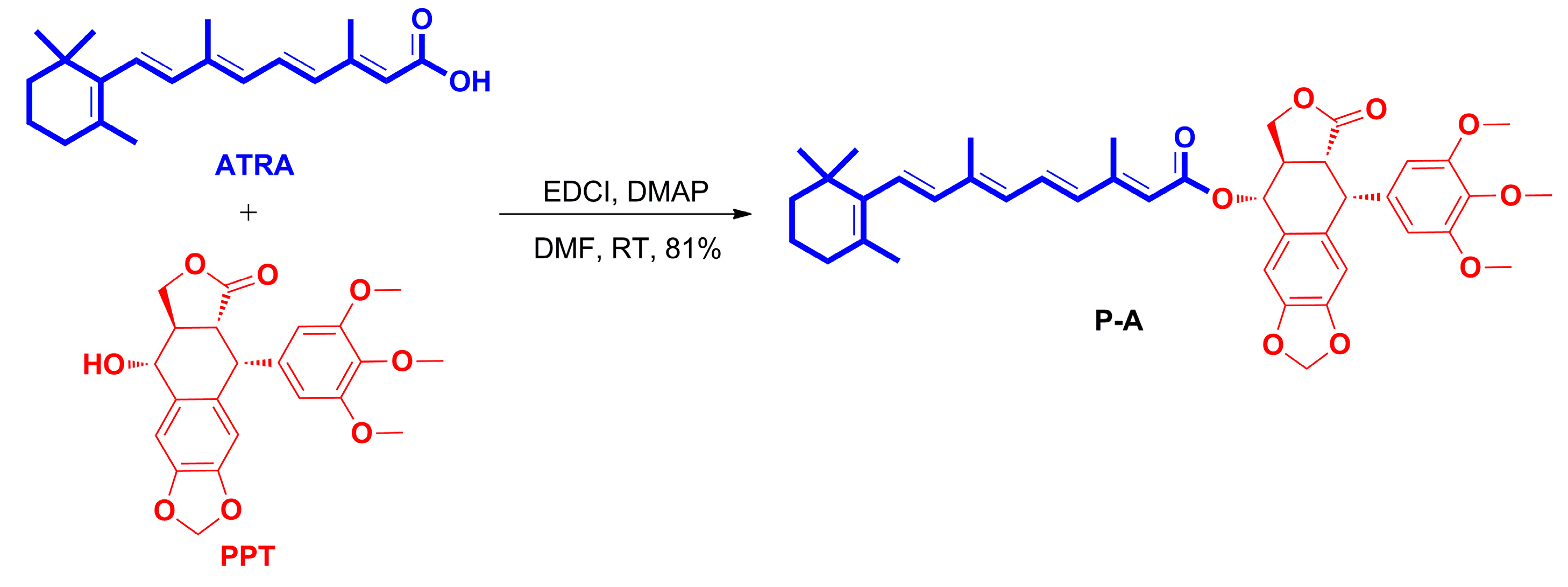
2.1. Chemistry
2.2. Antiproliferative Activity
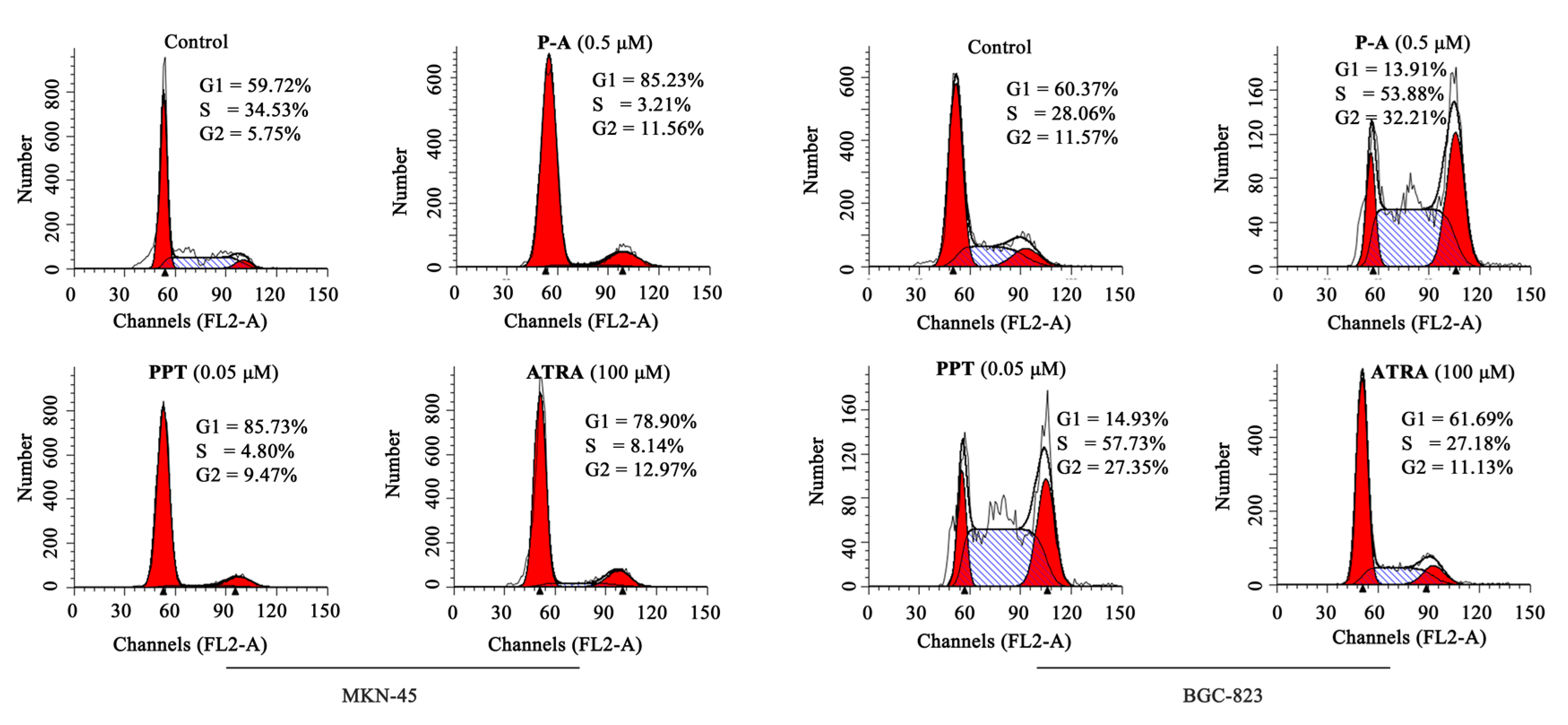
2.3. Cell Cycle Analysis
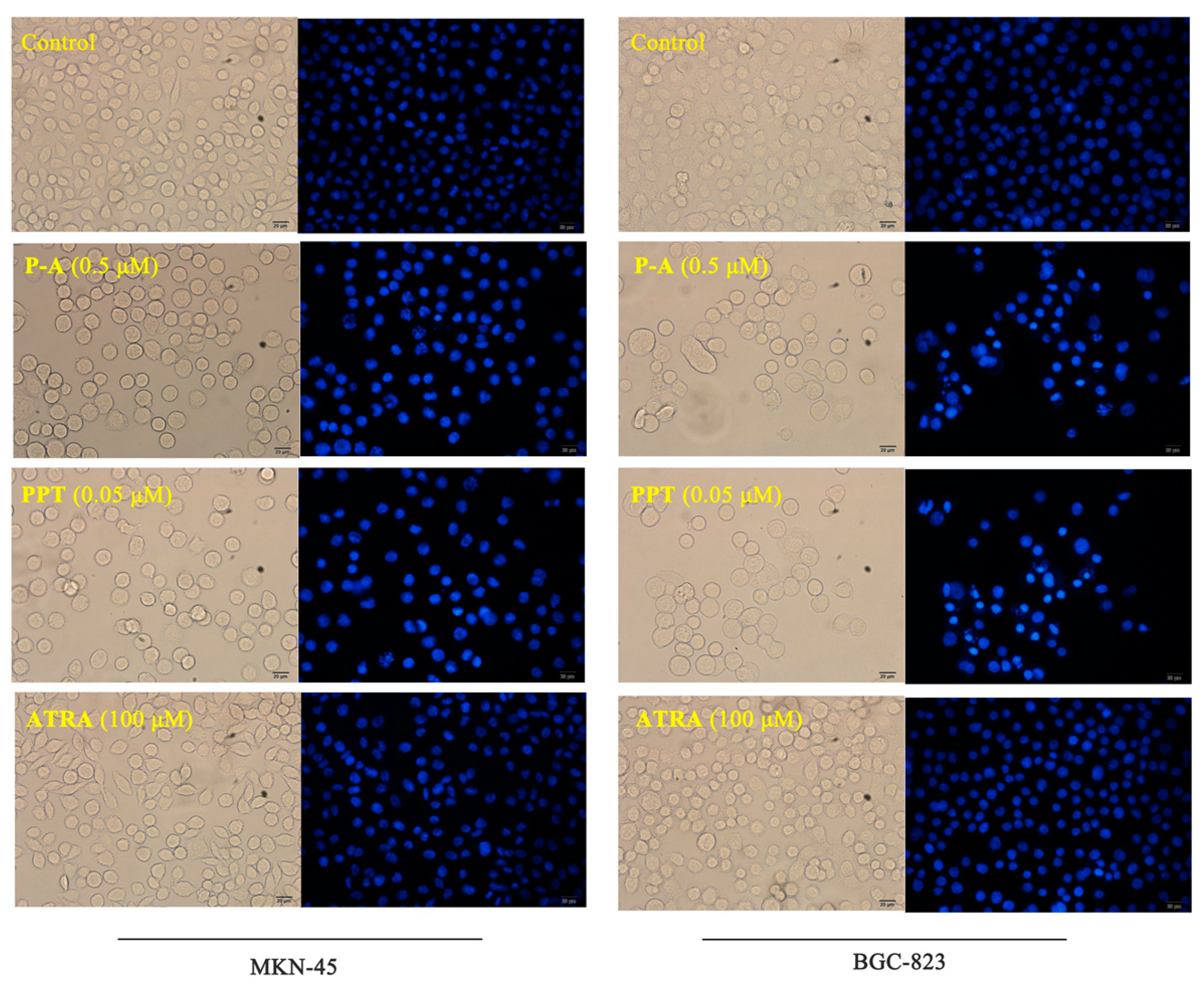
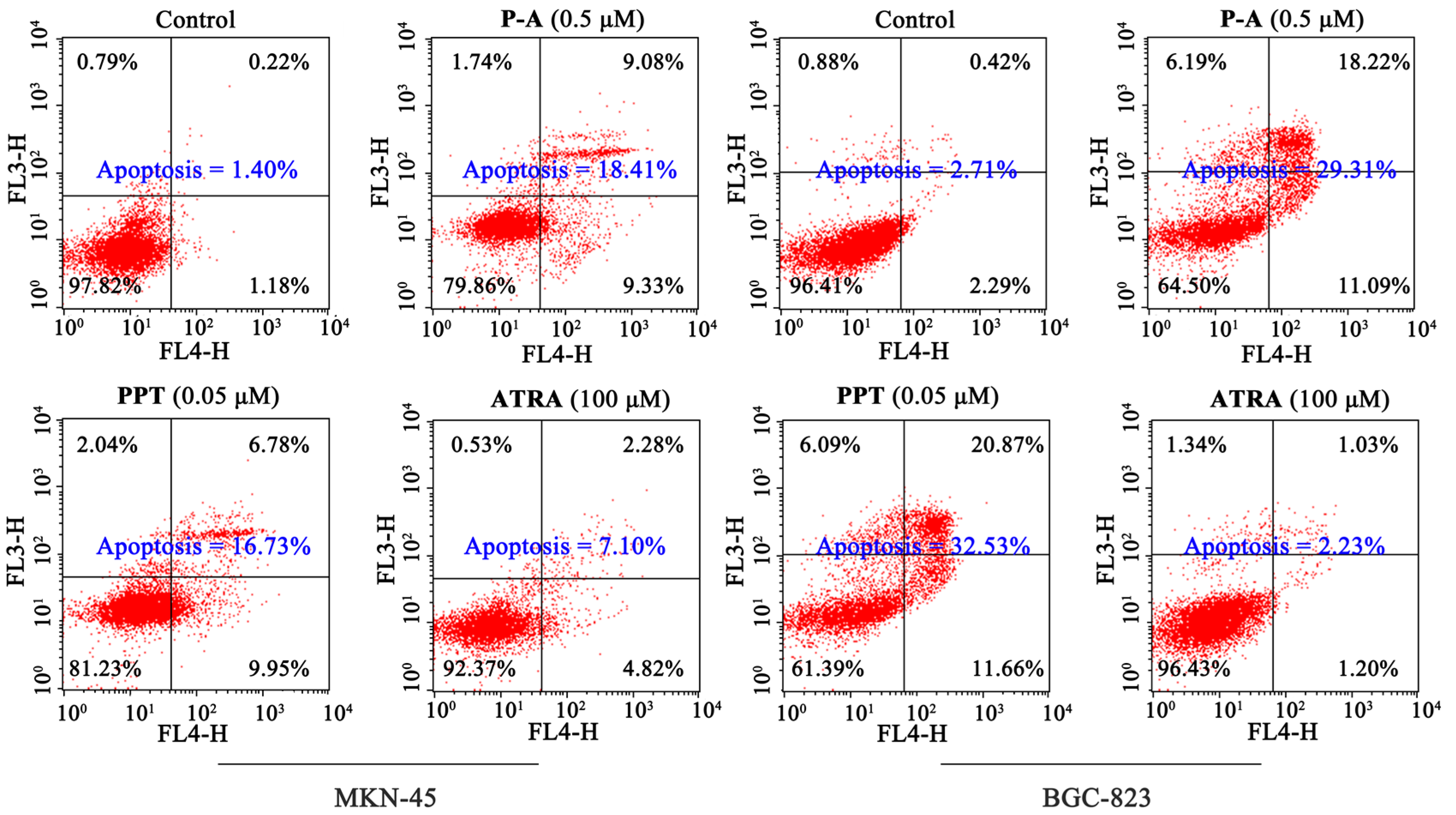
2.4. Cell Apoptosis Analysis
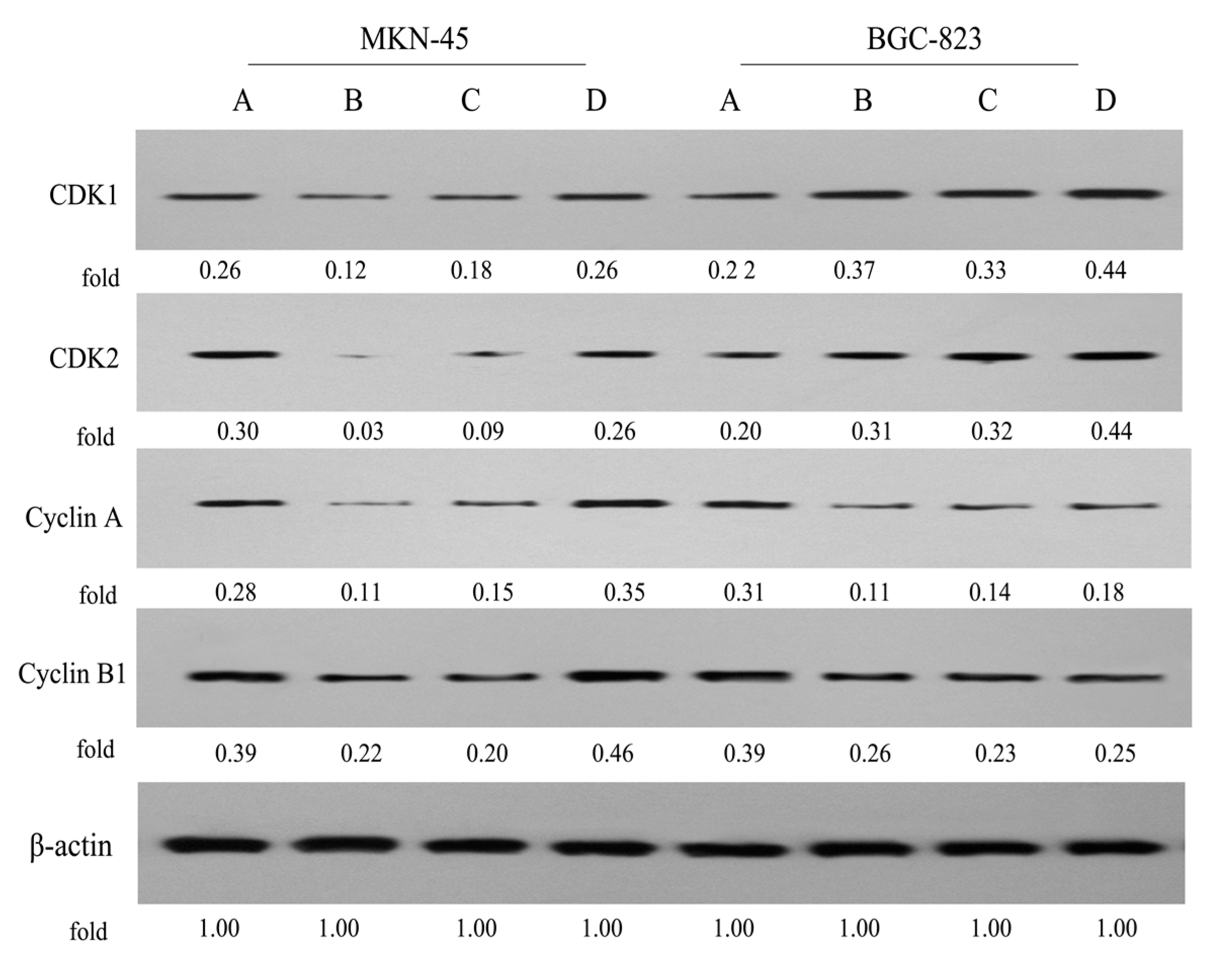
2.5. Expression of Cell Cycle-Related Proteins
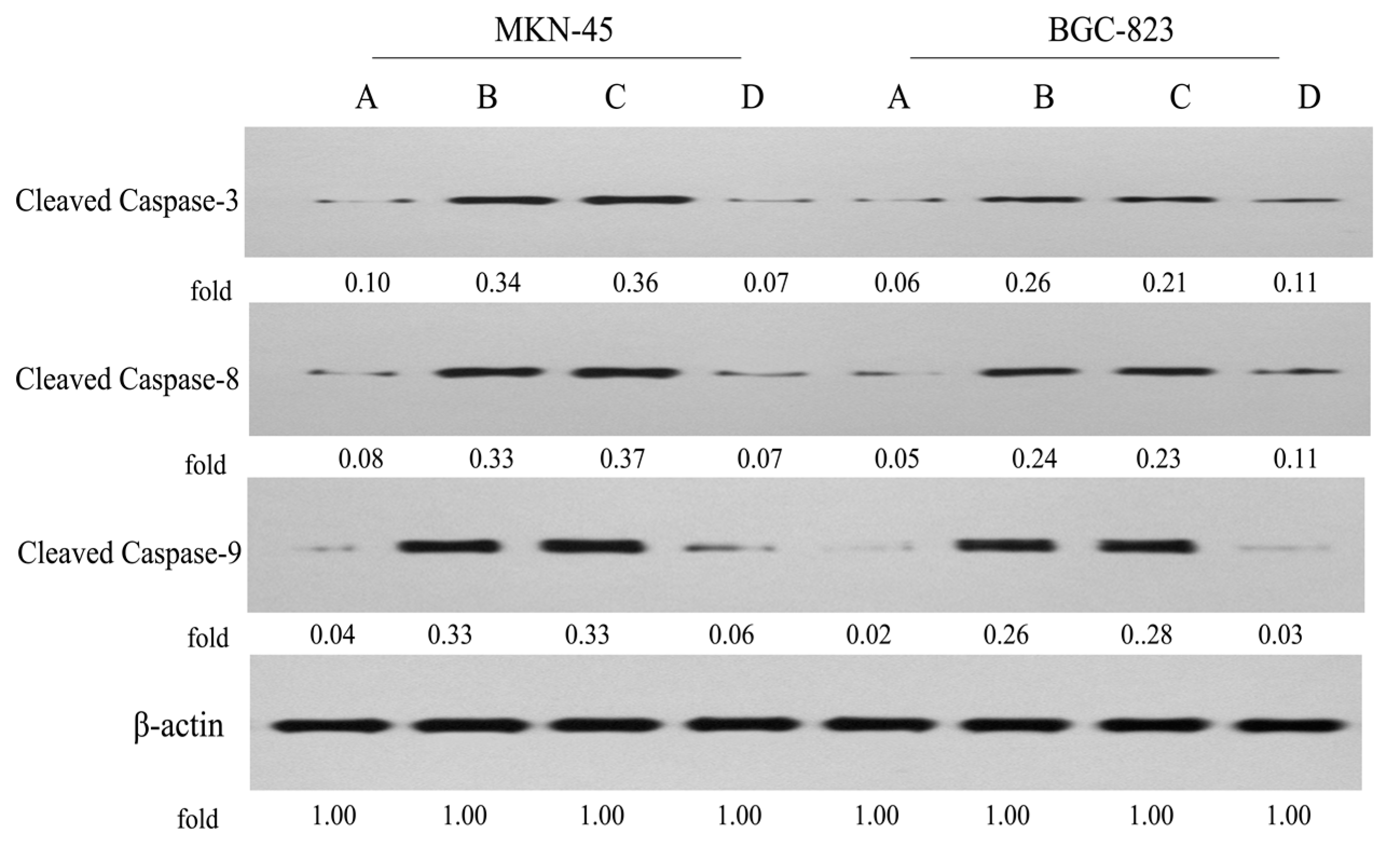
2.6. Expression of Apoptosis-Related Proteins
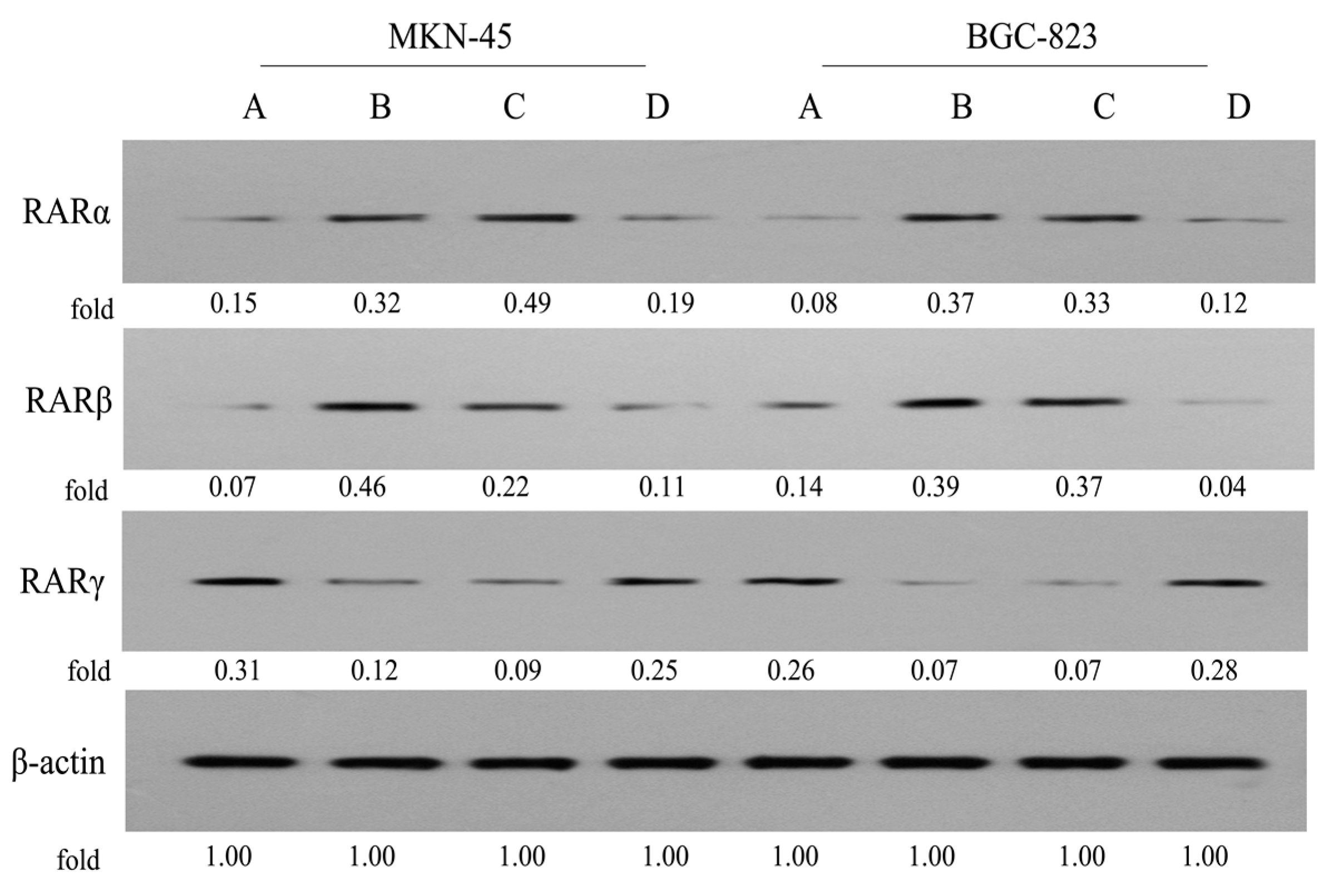
2.7. Expression of RARs
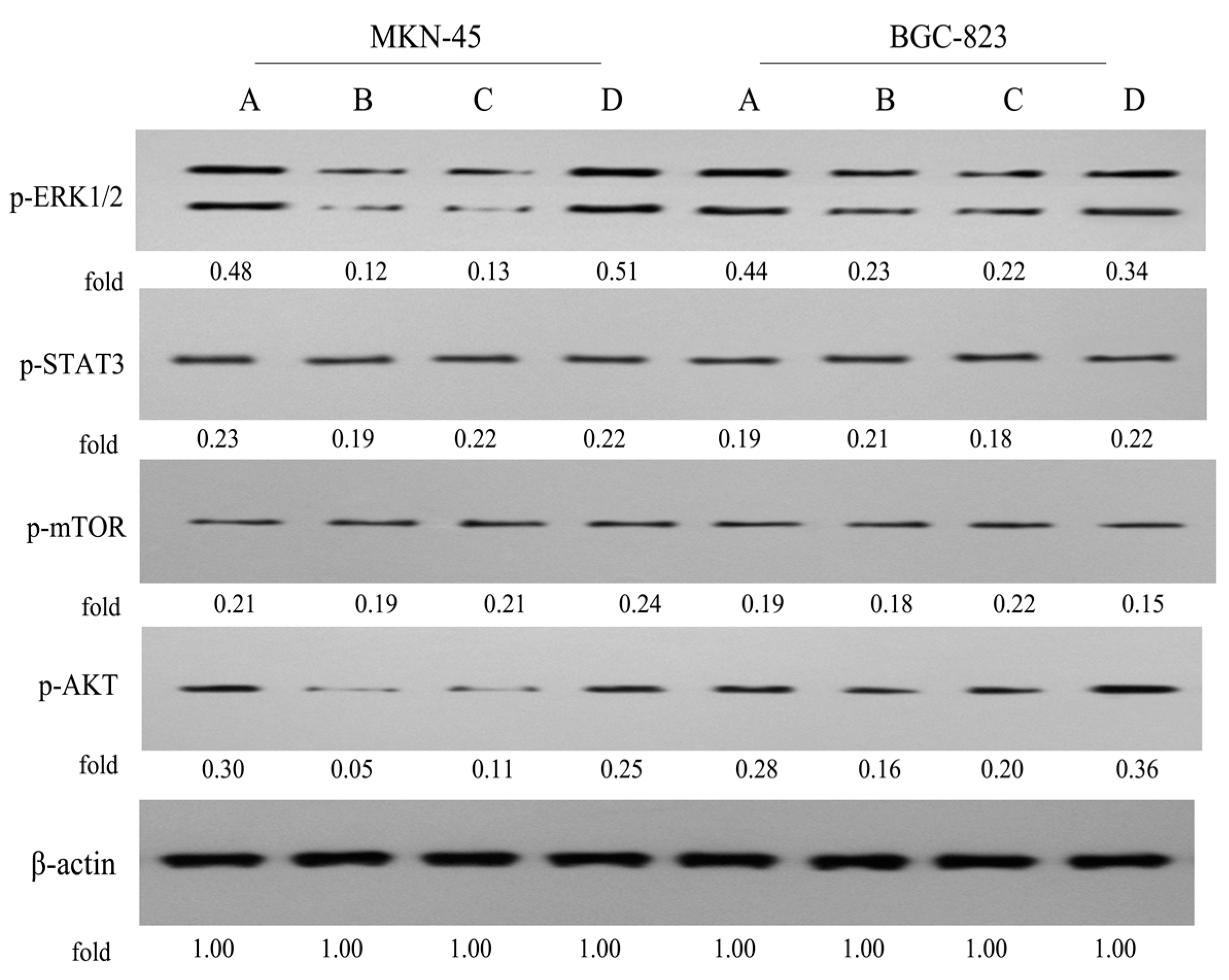
2.8. Effects on the ERK1/2, STAT3, mTOR and AKT Signaling
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Preparation of All-Trans-Retinoic Acid-Podophyllotoxin Conjugate P-A
3.2. Biology
3.2.1. Cytotoxicity Assays
3.2.2. Cell Cycle Analysis
3.2.3. Hoechst 33242 Staining
3.2.4. Cell Apoptosis Analysis
3.2.5. Western Blot Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Roukos, D.H. Current Advances and Changes in Treatment Strategy May Improve Survival and Quality of Life in Patients With Potentially Curable Gastric Cancer. Ann. Surg. Oncol. 1999, 6, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Ohtsu, A.; Shah, M.A.; van Cutsem, E.; Rha, S.Y.; Sawaki, A.; Park, S.R.; Lim, H.Y.; Yamada, Y.; Wu, J.; Langer, B.; et al. Bevacizumab in Combination With Chemotherapy As First-Line Therapy in Advanced Gastric Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. J. Clin. Oncol. 2011, 29, 3968–3976. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Grainger, J.R.; Spencer, S.P.; Belkaid, Y. The Role of Retinoic Acid in Tolerance and Immunity. Immunity 2011, 35, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Maden, M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007, 8, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C. Vitamin A and retinoic acid in T cell–related immunity. Am. J. Clin. Nutr. 2012, 96, 1166S–1172S. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.O.; Yang, H.; Faderl, S.; Estrov, Z.; Giles, F.; Ravandi, F.; Cortes, J.; Wierda, W.G.; Ouzounian, S.; Quezada, A.; et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood 2007, 110, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.K.; Rephaeli, A.; Colosimo, A.L.; Diaz, Z.; Nudelman, A.; Levovich, I.; Jing, Y.; Waxman, S.; Miller, W.H. A Retinoid/Butyric Acid Prodrug Overcomes Retinoic Acid Resistance in Leukemias by Induction of Apoptosis. Mol. Cancer Res. 2003, 1, 903–912. [Google Scholar] [PubMed]
- Nudelman, A.; Rephaeli, A. Novel Mutual Prodrug of Retinoic and Butyric Acids with Enhanced Anticancer Activity. J. Med. Chem. 2000, 43, 2962–2966. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wiebe, L.I.; Miller, G.G.; Knaus, E.E. Synthesis and Biological Evaluation of Butanoate, Retinoate, and Bis(2,2,2-trichloroethyl)phosphate Derivatives of 5-Fluoro-2′-deoxyuridine and 2′,5-Difluoro-2′-deoxyuridine as Potential Dual Action Anticancer Prodrugs. Arch. Pharm. 1999, 332, 286–294. [Google Scholar] [CrossRef]
- Apraiz, A.; Idkowiak-Baldys, J.; Nieto-Rementería, N.; Boyano, M.D.; Hannun, Y.A.; Asumendi, A. Dihydroceramide accumulation and reactive oxygen species are distinct and nonessential events in 4-HPR-mediated leukemia cell death. Biochem. Cell Biol. 2012, 90, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Mariani, L.; Decensi, A.; Formelli, F.; Camerini, T.; Miceli, R.; di Mauro, M.G.; Costa, A.; Marubini, E.; Sporn, M.B.; et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann. Oncol. 2006, 17, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, Z.M.; Su, W.J. Anticancer effect of retinoic acid via AP-1 activity repression is mediated by retinoic acid receptor α and β in gastric cancer cells. Int. J. Biochem. Cell Biol. 2002, 34, 1102–1114. [Google Scholar] [CrossRef]
- Ping, S.; Wang, S.; Zhang, J.; Peng, X. Effect of all-trans-retinoic acid on mRNA binding protein p62 in human gastric cancer cells. Int. J. Biochem. Cell Biol. 2005, 37, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, Q.; Che, Z.M.; Su, W.J. The effect pathway of retinoic acid through regulation of retinoic acid receptor alpha in gastric cancer cells. World J. Gastroenterol. 2001, 7, 662–666. [Google Scholar] [PubMed]
- Zhang, J.P.; Chen, X.Y.; Li, J.S. Effects of all-trans-retinoic on human gastric cancer cells BGC-823. J. Digest. Dis. 2007, 8, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lv, M.; Tian, X. A Review on Hemisynthesis, Biosynthesis, Biological Activities, Mode of Action, and Structure-Activity Relationship of Podophyllotoxins: 2003–2007. Curr. Med. Chem. 2009, 16, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Bohlin, L.; Rosen, B. Podophyllotoxin derivatives: Drug discovery and development. Drug Discov. Today 1996, 1, 343–351. [Google Scholar] [CrossRef]
- Liu, Y.D.; Tian, J.; Qian, K.; Zhao, X.B.; Morris-Natschke, S.L.; Yang, L.; Nan, X.; Tian, X.; Lee, K.H. Recent Progress on C-4-Modified Podophyllotoxin Analogs as Potent Antitumor Agents. Med. Res. Rev. 2015, 35, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Hussaini, S.M.A.; Rahim, A.; Riyaz, S. Podophyllotoxin derivatives: A patent review (2012–2014). Expert Opin. Ther. Pat. 2015, 25, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, L.; Zhang, R.; Tang, J.; Lai, H.; Wang, J.; Wang, G.; Xu, Q.; Chen, T.; Peng, F.; et al. Semi-Synthesis and Biological Evaluation of 1,2,3-Triazole-Based Podophyllotoxin Congeners as Potent Antitumor Agents Inducing Apoptosis in HepG2 Cells. Arch. Pharm. 2012, 345, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Zhao, W.; Zhou, C.; Zhang, Y.X.; Li, H.M.; Tang, Y.L.; Liang, X.H.; Chen, T.; Tang, Y.J. Comparison of carbon-sulfur and carbon-amine bond in therapeutic drug: 4β-S-aromatic heterocyclic podophyllum derivatives display antitumor activity. Sci. Rep. 2015, 5, 14814. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, W.; Li, H.M.; Wan, D.J.; Li, D.S.; Chen, T.; Tang, Y.J. Design and synthesis of the novel DNA topoisomerase II inhibitors: Esterification and amination substituted 4′-demethylepipodophyllotoxin derivates exhibiting anti-tumor activity by activating ATM/ATR signaling pathways. Eur. J. Med. Chem. 2014, 80, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar]
- Fortin, S.; Bérubé, G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Dis. 2013, 8, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Zhang, Z.; Chen, Y.; Lin, Y.; Wang, J. Design, synthesis and evaluation of the multidrug resistance-reversing activity of pyridine acid esters of podophyllotoxin in human leukemia cells. Bioorg. Med. Chem. Lett. 2016, 26, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Zhang, Z.; Chen, Y.; Wang, J. Synthesis and biological evaluation of a novel artesunate–podophyllotoxin conjugate as anticancer agent. Bioorg. Med. Chem. Lett. 2016, 26, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Wang, J.; Chen, Y.; Chen, F.; Lin, Y.; Zhu, X. Potential anti-MDR agents based on the podophyllotoxin scaffold: Synthesis and antiproliferative activity evaluation against chronic myeloid leukemia cells by activating MAPK signaling pathways. RSC Adv. 2016, 6, 2895–2903. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Wang, J.; Chen, Y.; Zhang, Z.; Lin, Y.; Zhu, X. Novel isatin derivatives of podophyllotoxin: Synthesis and cytotoxic evaluation against human leukaemia cancer cells as potent anti-MDR agents. RSC Adv. 2015, 5, 97816–97823. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, F.; Chen, Y.; Lin, Y.; Wang, J. Aromatic heterocyclic esters of podophyllotoxin exert anti-MDR activity in human leukemia K562/ADR cells via ROS/MAPK signaling pathways. Eur. J. Med. Chem. 2016, 123, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Liu, L.; Zheng, C.; Wang, Y.; Chen, Y.; Wei, G. Podophyllotoxin–pterostilbene fused conjugates as potential multifunctional antineoplastic agents against human uveal melanoma cells. RSC Adv. 2017, 7, 10601–10608. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Zheng, C.; Wang, Y.; Nie, X.; Shi, D.; Chen, Y.; Wei, G.; Wang, J. Synthesis and biological evaluation of novel podophyllotoxin-NSAIDs conjugates as multifunctional anti-MDR agents against resistant human hepatocellular carcinoma Bel-7402/5-FU cells. Eur. J. Med. Chem. 2017, 131, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem Res. 2007, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Takeda, T.; Ogawa, N.; Sakamoto, K.; Shimaoka, H.; Fujita, A.; Itoh, T.; Imano, M.; Ishizaka, T.; Satoue, T.; Nishida, S. Overexpression of survivin via activation of ERK1/2, Akt, and NF-κB plays a central role in vincristine resistance in multiple myeloma cells. Leukemia Res. 2015, 39, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, H.; Lin, H.J.; Yang, S.; Lin, J.; Liang, G. Feedback Activation of STAT3 as a Cancer Drug-Resistance Mechanism. Trends Pharmacol. Sci. 2016, 37, 47–61. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the synthsized compound is available from the first author. |
| Compound | IC50 (μM) 1,2 | ||
|---|---|---|---|
| MKN-45 | BGC-823 | L-O2 | |
| P-A | 0.419 ± 0.032 | 0.202 ± 0.055 | 0.293 ± 0.081 |
| PPT | 0.045 ± 0.012 | 0.03 ± 0.005 | 0.037 ± 0.008 |
| ATRA | 88.462 ± 6.931 | 83.076 ± 15.252 | >100 |
| Etoposide | 2.88 ± 0.532 | 1.737 ± 0.294 | 2.006 ± 0.24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, J.; Liu, L.; Zheng, C.; Wang, Y. Synthesis and Antiproliferative Activity of Novel All-Trans-Retinoic Acid-Podophyllotoxin Conjugate towards Human Gastric Cancer Cells. Molecules 2017, 22, 628. https://doi.org/10.3390/molecules22040628
Zhang L, Wang J, Liu L, Zheng C, Wang Y. Synthesis and Antiproliferative Activity of Novel All-Trans-Retinoic Acid-Podophyllotoxin Conjugate towards Human Gastric Cancer Cells. Molecules. 2017; 22(4):628. https://doi.org/10.3390/molecules22040628
Chicago/Turabian StyleZhang, Lei, Jing Wang, Lai Liu, Chengyue Zheng, and Yang Wang. 2017. "Synthesis and Antiproliferative Activity of Novel All-Trans-Retinoic Acid-Podophyllotoxin Conjugate towards Human Gastric Cancer Cells" Molecules 22, no. 4: 628. https://doi.org/10.3390/molecules22040628
APA StyleZhang, L., Wang, J., Liu, L., Zheng, C., & Wang, Y. (2017). Synthesis and Antiproliferative Activity of Novel All-Trans-Retinoic Acid-Podophyllotoxin Conjugate towards Human Gastric Cancer Cells. Molecules, 22(4), 628. https://doi.org/10.3390/molecules22040628





