Silane Modified Diopside for Improved Interfacial Adhesion and Bioactivity of Composite Scaffolds
Abstract
:1. Introduction
2. Results and Discussion
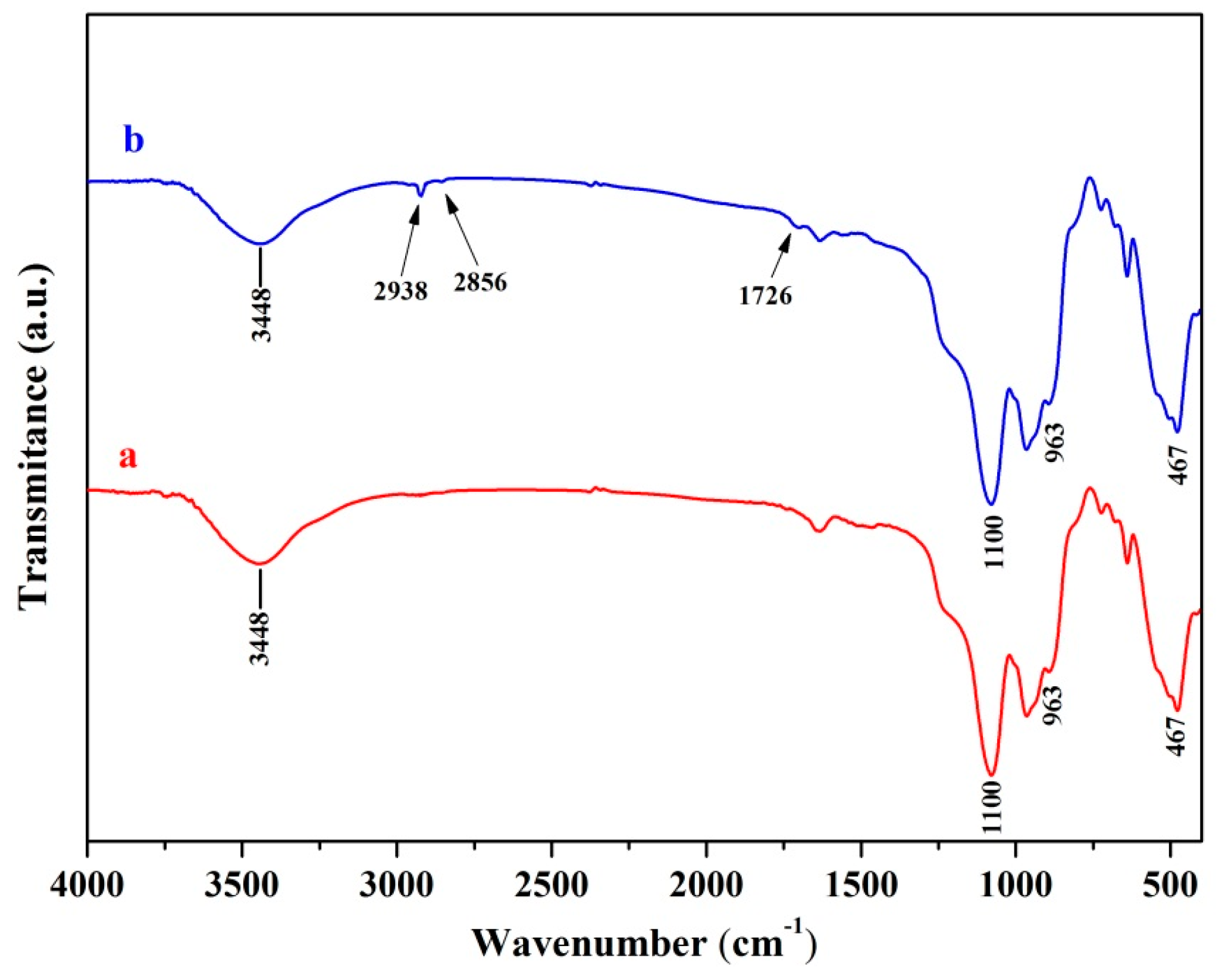
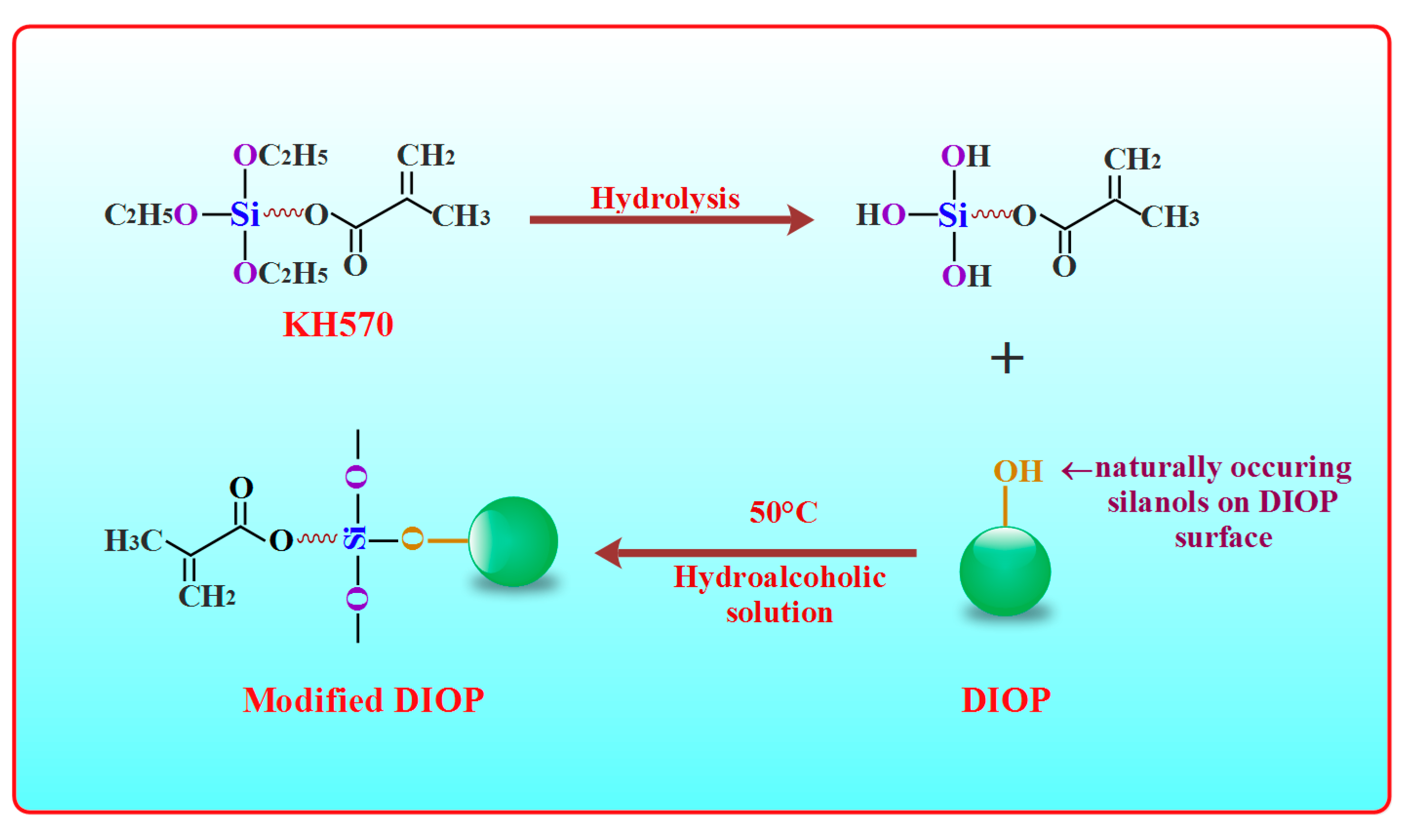
2.1. Surface Modification of DIOP
2.2. Scaffold Fabrication
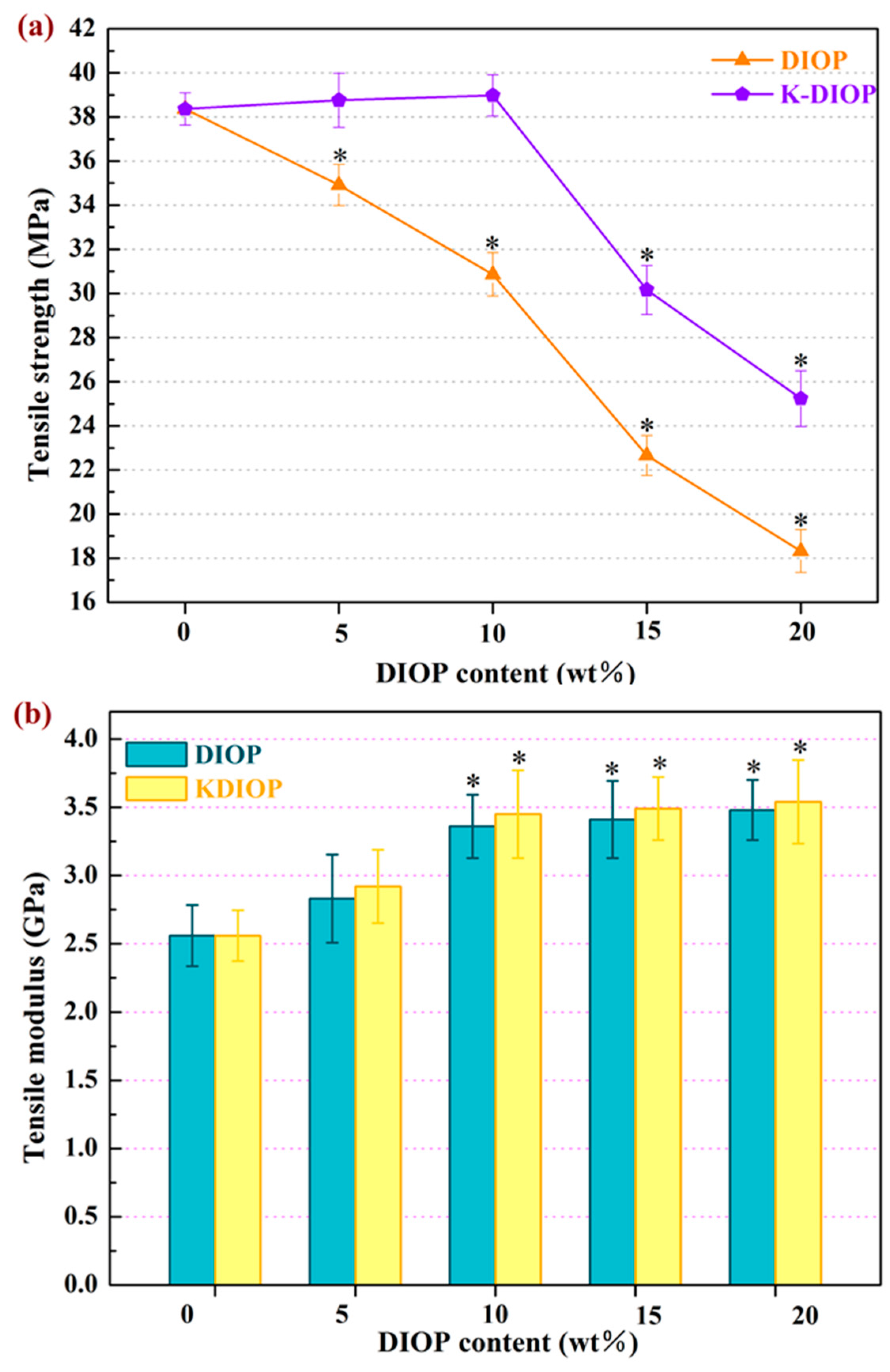
2.3. Microstructure and Mechanical Properties
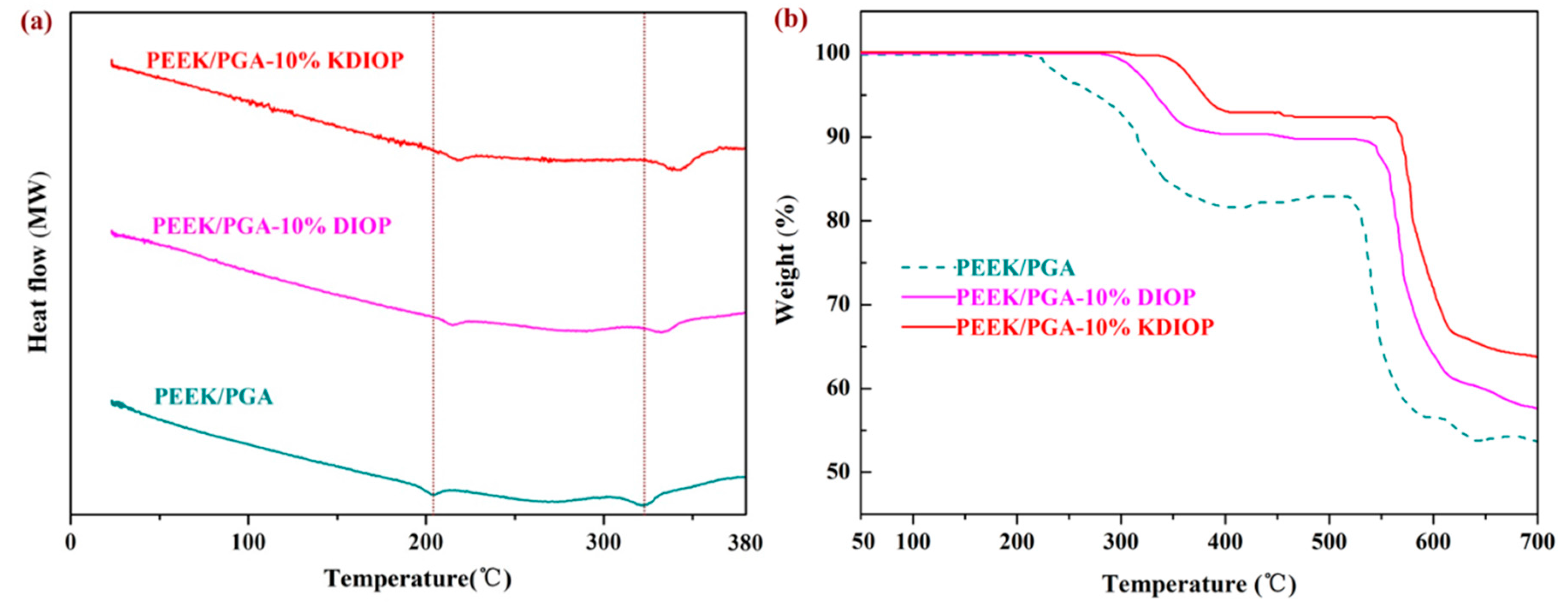
2.4. Thermal Properties
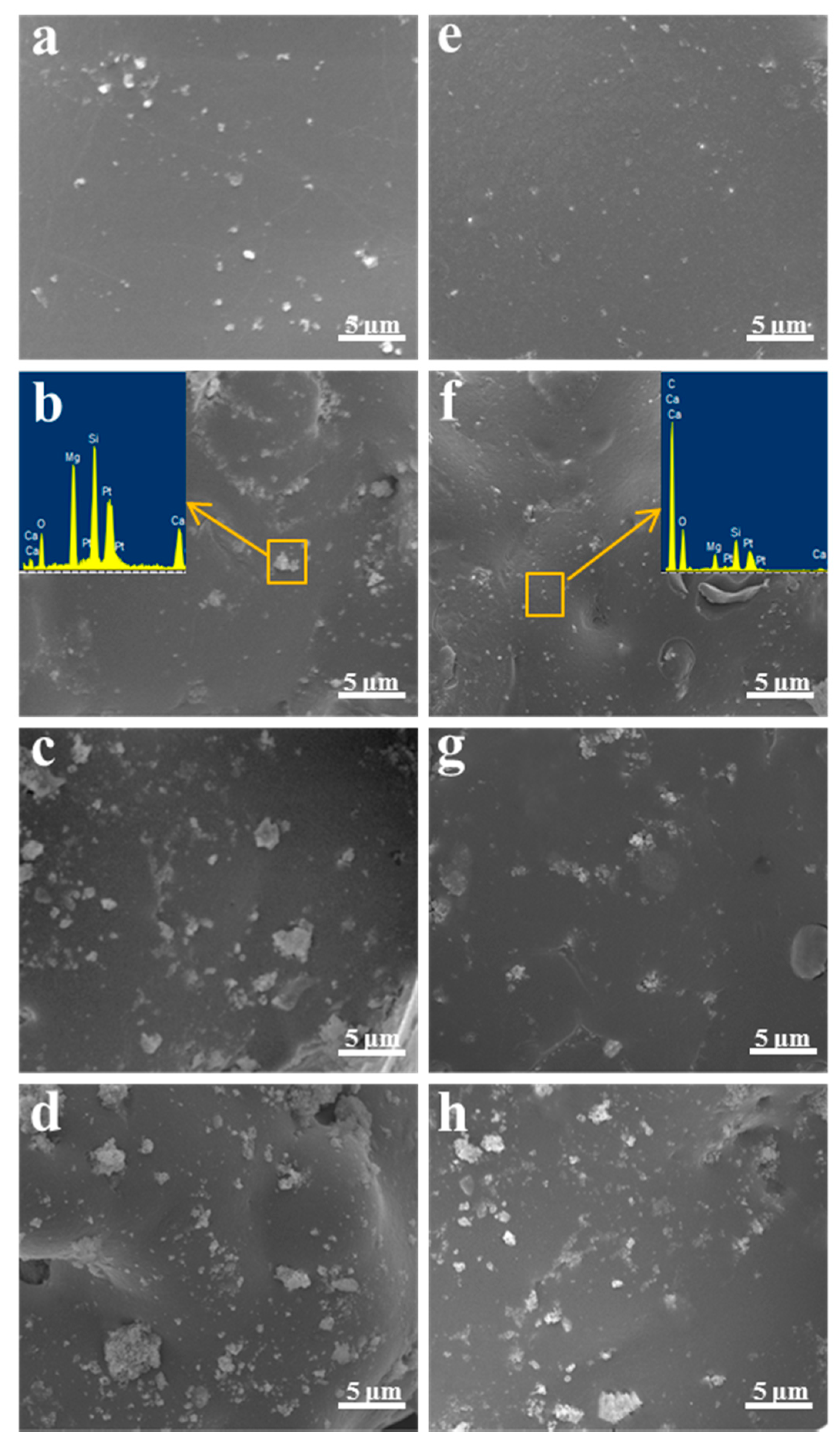
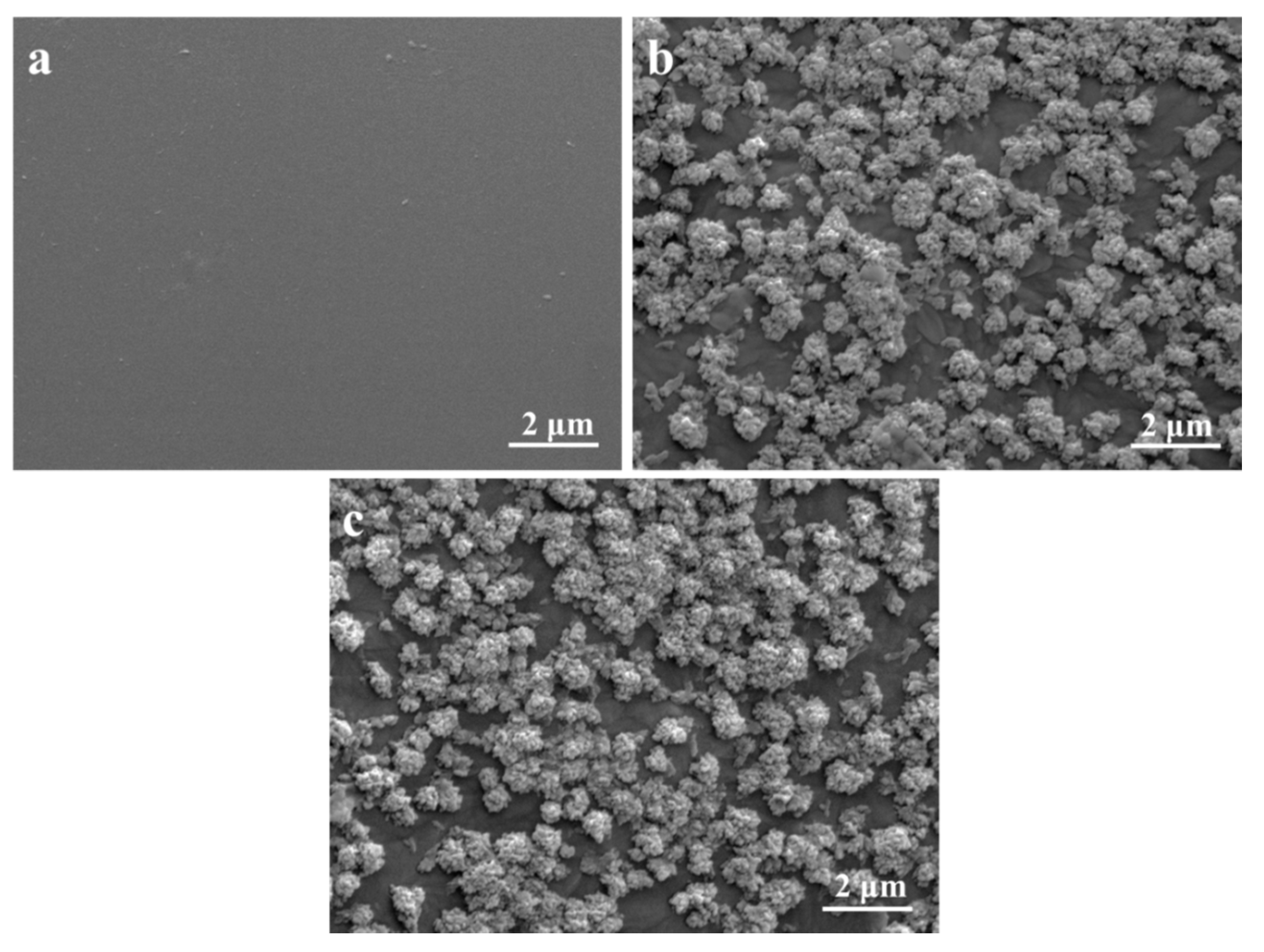
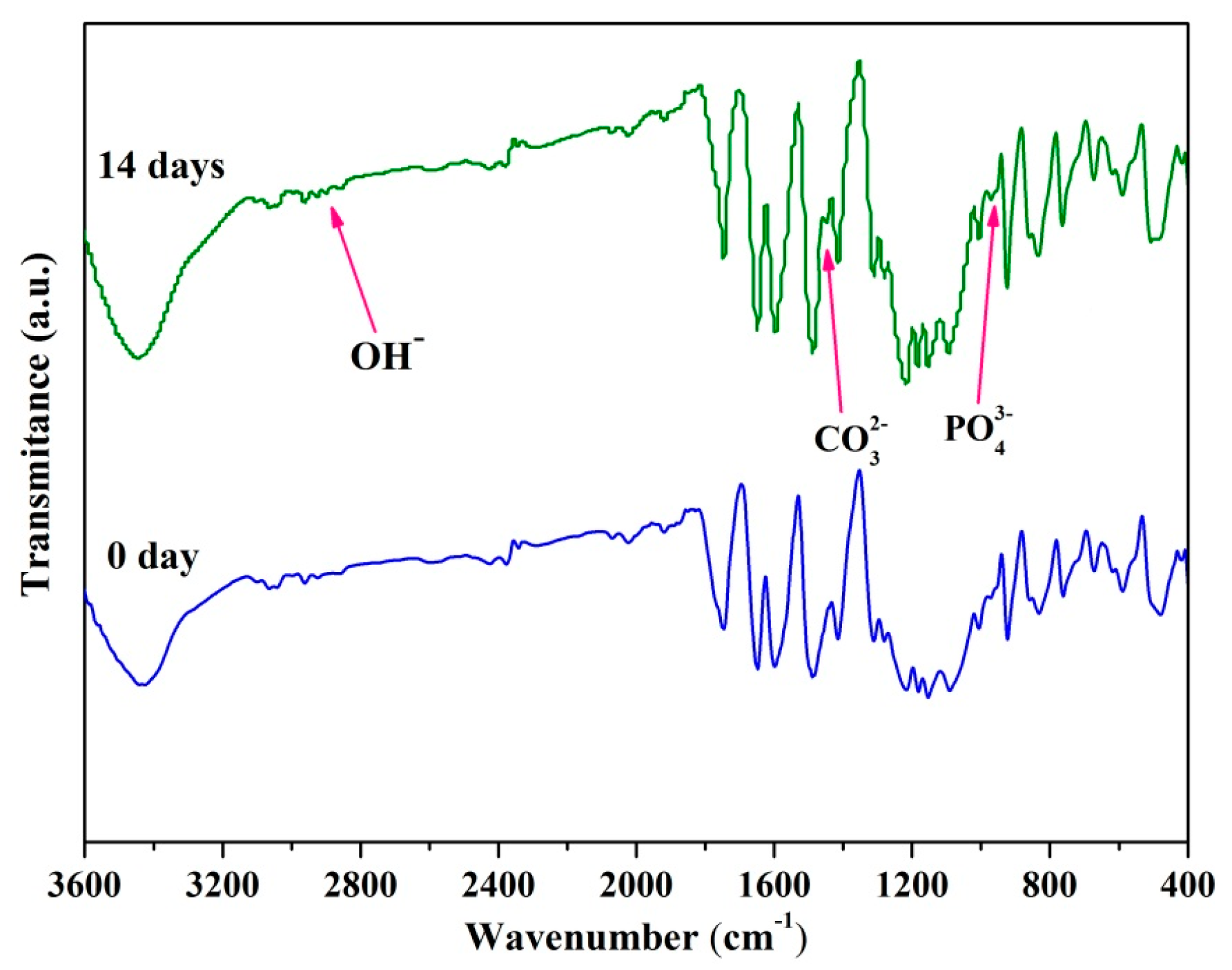
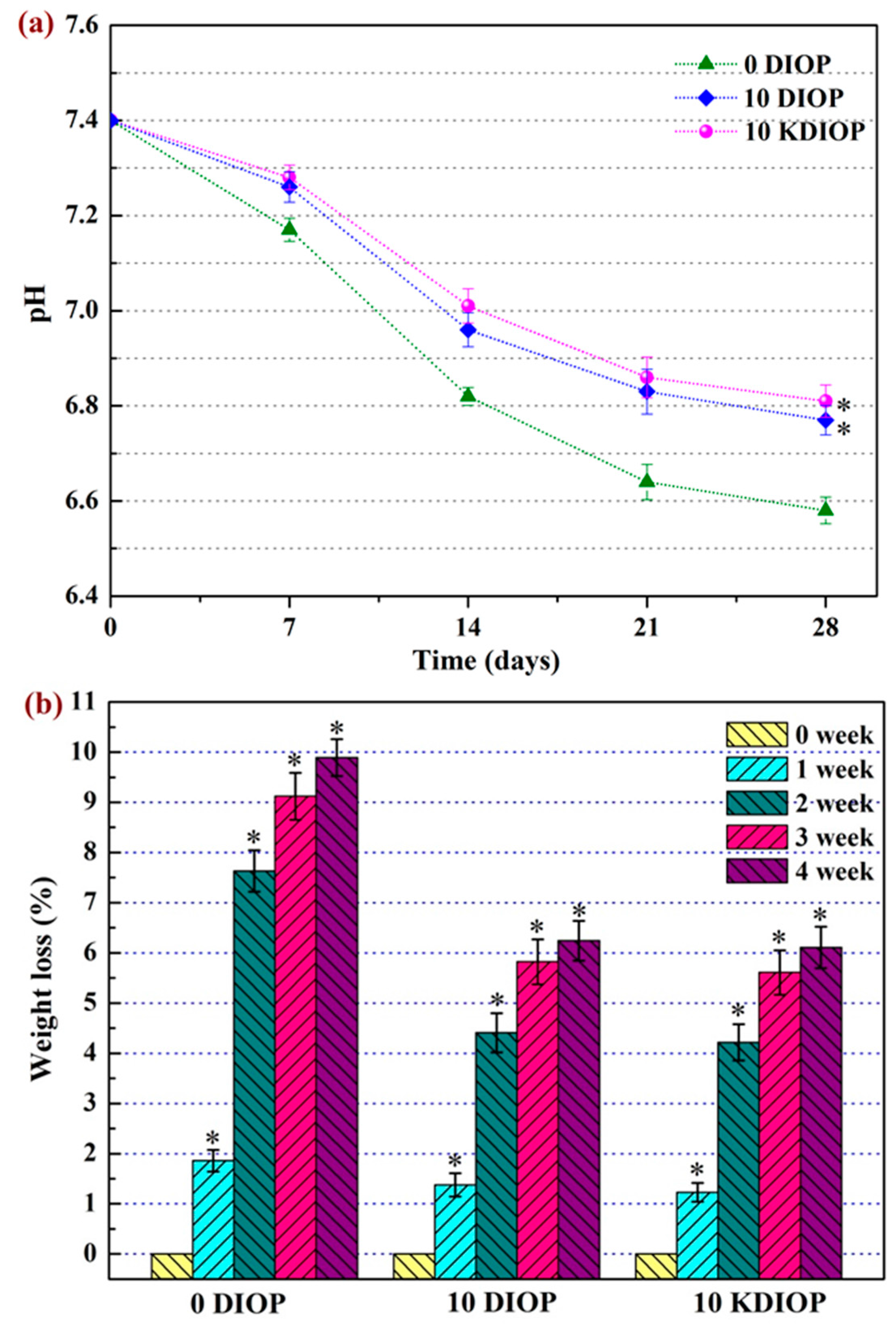
2.5. In Vitro Bioactivity and Degradability
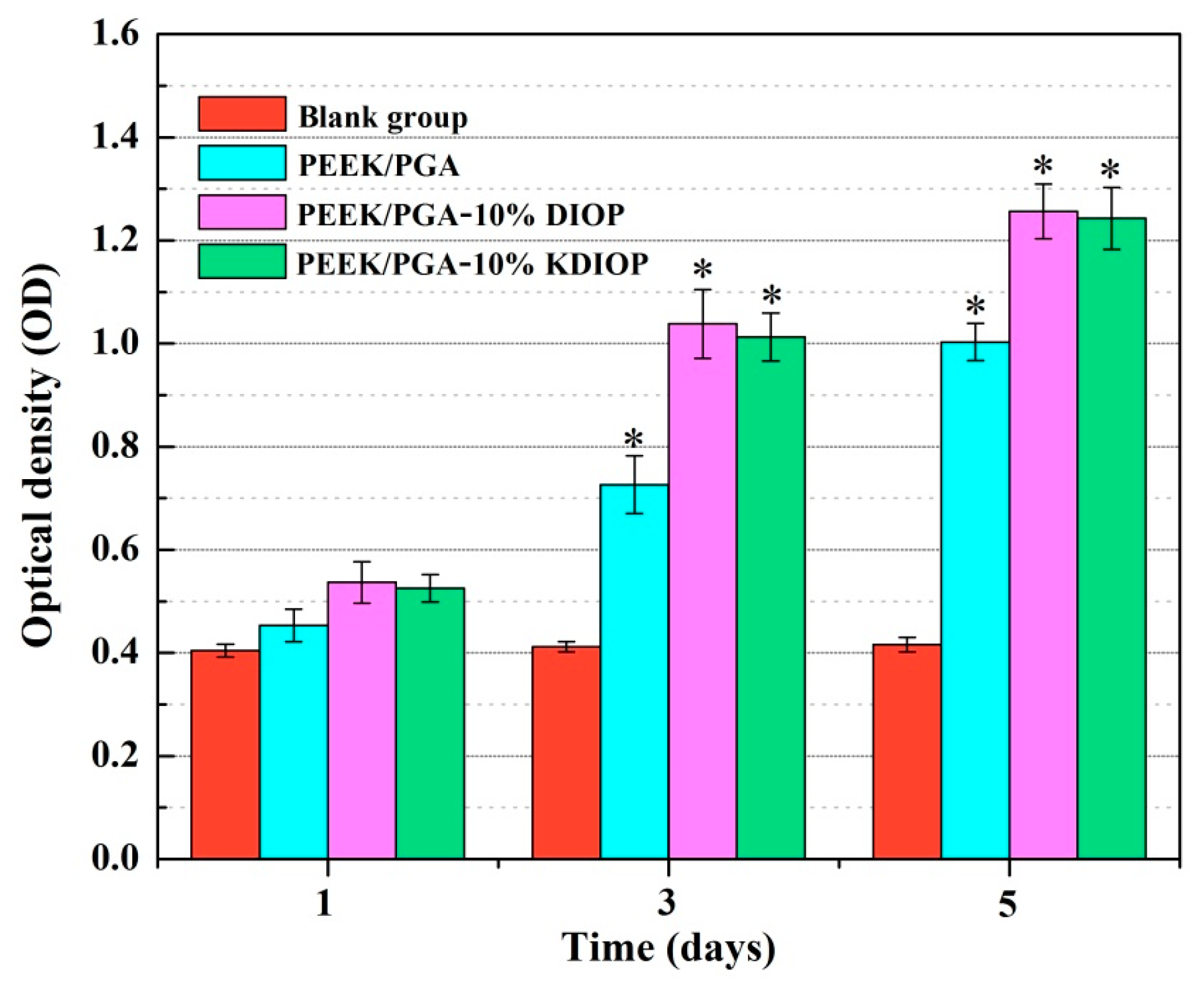
2.6. Biocompatibility Studies
3. Materials and Methods
3.1. Materials
3.2. Surface Modification of DIOP
3.3. Scaffolds Preparation
3.4. Characterization
3.5. Biomineralization and Degradation
3.6. Cell Culture
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vaezi, M.; Black, C.; Gibbs, D.M.R.; Oreffo, R.O.C.; Yang, S. Characterization of new PEEK/HA composites with 3D HA network fabricated by extrusion freeforming. Molecules 2016, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gan, K.; Liu, H.; Song, X.; Liu, X.; Niu, D.; Chen, T.; Liu, C. Preparation and mechanical properties of polyethereketone biocomposite for prosthodontics. J. Biomater. Tissue Eng. 2017, 7, 45–52. [Google Scholar] [CrossRef]
- Liu, C.; Chan, K.; Shen, J.; Liao, C.; Yeung, K.; Tjong, S. Polyetheretherketone hybrid composites with bioactive nanohydroxyapatite and multiwalled carbon nanotube fillers. Polymers 2016, 8, 425. [Google Scholar]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. A novel bioactive PEEK/HA composite with controlled 3D interconnected HA network. Int. J. Bioprint. 2015, 1, 66–76. [Google Scholar] [CrossRef]
- Dragojevic, S.; Ryu, J.S.; Raucher, D. Polymer-based prodrugs: Improving tumor targeting and the solubility of small molecule drugs in cancer therapy. Molecules 2015, 20, 21750. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Dębek, C.; Olędzka, E.; Kozłowski, R. Polymeric systems of antimicrobial peptides--strategies and potential applications. Molecules 2013, 18, 14122. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, R.M.; Najarian, S.; Shakhesi, S.; Khanlari, S.; Shaabani, K.; Sharifi, S. Investigating the effect of pga on physical and mechanical properties of electrospun pcl/pga blend nanofibers. J. Appl. Polym. Sci. 2012, 124, 123–131. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, X.; Xu, A.; Wang, L.; Luo, Z.; Zheng, Y.; Deng, F.; Wei, J.; Tang, Z.; Wei, S. Effect of surface roughness on osteogenesis in vitro and osseointegration in vivo of carbon fiber-reinforced polyetheretherketone-nanohydroxyapatite composite. Int. J. Nanomed. 2015, 10, 1425. [Google Scholar]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Liu, T.; Gao, C.; Feng, P.; Xiao, T.; Yu, K.; Peng, S. Mechanical and structural characterization of diopside scaffolds reinforced with graphene. J. Alloy Compd. 2016, 655, 86–92. [Google Scholar]
- Fiocco, L.; Elsayed, H.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive wollastonite-diopside foams from preceramic polymers and reactive oxide fillers. Materials 2015, 8, 2480–2494. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Zreiqat, H. Porous diopside (CaMgSi(2)O(6)) scaffold: A promising bioactive material for bone tissue engineering. Acta Biomater. 2010, 6, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Liu, X.; Wei, L.; Wang, G.; Meng, F. Proliferation and gene expression of osteoblasts cultured in dmem containing the ionic products of dicalcium silicate coating. Biomed. Pharmacother. 2009, 63, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, M.; Shamanian, M.; Fathi, M.; Samadikuchaksaraei, A.; Mehdipour, A. Synthesis, characterization, in vitro bioactivity and biocompatibility evaluation of hydroxyapatite/bredigite (Ca7MgSi4O16) composite nanoparticles. JOM 2016, 68, 1061–1070. [Google Scholar] [CrossRef]
- Hosseini, Y.; Emadi, R.; Kharaziha, M.; Doostmohammadi, A. Reinforcement of electrospun poly(ε-caprolactone) scaffold using diopside nanopowder to promote biological and physical properties. J. Appl. Polym. Sci. 2016, 134, 6. [Google Scholar] [CrossRef]
- Kumar, J.P.; Lakshmi, L.; Jyothsna, V.; Balaji, D.R.P.; Saravanan, S.; Moorthi, A.; Selvamurugan, N. Synthesis and characterization of diopside particles and their suitability along with chitosan matrix for bone tissue engineering in vitro and in vivo. J. Biomed. Nanotechnol. 2014, 10, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ji, J.; Tang, S.; Qian, J.; Yan, Y.; Yu, B.; Su, J.; Wei, J. Biocompatibility, degradability, bioactivity and osteogenesis of mesoporous/macroporous scaffolds of mesoporous diopside/poly(l-lactide) composite. J. R. Soc. Interface 2015, 12, 20150507. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Bunk, J.K.G.; Drechsler, A.; Rauch, S.; Uhlmann, P.; Stamm, M.; Rennekamp, R. The distribution of hydrophobized inorganic nanoparticles in thermoresponsive polymer nanocomposite films investigated by scanning probe and electron microscopy. Eur. Polym. J. 2013, 49, 1994. [Google Scholar] [CrossRef]
- Kongsinlark, A.; Rempel, G.L.; Prasassarakich, P. Synthesis of monodispersed polyisoprene–silica nanoparticles via differential microemulsion polymerization and mechanical properties of polyisoprene nanocomposite. Chem. Eng. J. Chem. Eng. J. 2012, 193, 215–226. [Google Scholar] [CrossRef]
- Hristov, V.; Krumova, M.; Michler, G. The influence of excess coupling agent on the microdeformation processes and mechanical properties of poly(propylene)/wood-flour composites. Macromol. Mater. Eng. 2010, 291, 677–683. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Cao, D.R.; Wang, D.S.; Chen, J.J. Study on the interface modification of bagasse fibre and the mechanical properties of its composite with PVC. Compos. Part A Appl. Sci. Manuf. 2007, 38, 20–25. [Google Scholar] [CrossRef]
- Abdelwahab, N.A.; Helaly, F.M. Chemically modified sugarcane bagasse as an improving agent for the properties of styrene-butadiene rubber. J. Appl. Polym. Sci. 2010, 113, 3470–3476. [Google Scholar]
- Kim, S.; Wang, X.; Ando, S.; Wang, X. Hybrid ternary composites of hyperbranched and linear polyimides with SiO2: A research for low dielectric constant and optimized properties. RSC Adv. 2014, 4, 42737–42746. [Google Scholar] [CrossRef]
- Han, Q.; Pei, Y.; Zhong, Y.; Zhang, K.; Tang, L.; Wu, L. Fabrication and properties of chemically bonded polysilsesquioxane-polyacrylate/silica hybrid latex films with high silicon content. Polym. Compos. 2015, 36, 389–396. [Google Scholar] [CrossRef]
- Pu, Z.; Tang, H.; Huang, X.; Yang, J.; Zhan, Y.; Zhao, R.; Liu, X. Effect of surface functionalization of SiO2 particles on the interfacial and mechanical properties of pen composite films. Colloids Surf. A 2012, 415, 125–133. [Google Scholar] [CrossRef]
- Feng, P.; Niu, M.; Gao, C.; Peng, S.; Shuai, C. A novel two-step sintering for nano-hydroxyapatite scaffolds for bone tissue engineering. Sci. Rep. 2013, 4, 5599. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.T.; Lin, C.C.; Chen, Y.W.; Yeh, C.H.; Fang, H.Y.; Shie, M.Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 56, 165. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Curto, M.; Crescenzio, F.D. A method to design biomimetic scaffolds for bone tissue engineering based on voronoi lattices. Virtual Phys. Prototyp. 2016, 1–14. [Google Scholar] [CrossRef]
- Deplaine, H.; Acosta-Santamaría, V.A.; Vidaurre, A.; Gómez Ribelles, J.L.; Doblaré, M.; Ochoa, I.; Gallego Ferrer, G. Evolution of the properties of a poly(l-lactic acid) scaffold with double porosity during in vitro degradation in a phosphate-buffered saline solution. J. Appl. Polym. Sci. 2014, 131, 1366–1373. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Tissue engineering: Toward strong and tough glass and ceramic scaffolds for bone repair. Adv. Funct. Mater. 2013, 23, 5461–5476. [Google Scholar] [CrossRef]
- Chauhan, S.R.; Thakur, S. Effects of particle size, particle loading and sliding distance on the friction and wear properties of cenosphere particulate filled vinylester composites. Mater. Des. 2013, 51, 398–408. [Google Scholar] [CrossRef]
- Bahari, S.A.; Krause, A. Utilizing malaysian bamboo for use in thermoplastic composites. J. Clean. Prod. 2015, 110, 16–24. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, P.; Pandey, K.N.; Verma, V.; Kumar, V. Poly(ether ether)ketone/poly(ether) imide nanocomposites. Asian J. Res. Chem. 2012, 5, 703–706. [Google Scholar]
- Wang, Y.C.; Lin, M.C.; Wang, D.M.; Hsieh, H.J. Fabrication of a novel porous pga-chitosan hybrid matrix for tissue engineering. Biomaterials 2003, 24, 1047–1057. [Google Scholar] [CrossRef]
- Handel, M.; Hammer, T.R.; Nooeaid, P.; Boccaccini, A.R.; Hoefer, D. 45s5-bioglass(®)-based 3D-scaffolds seeded with human adipose tissue-derived stem cells induce in vivo vascularization in the cam angiogenesis assay. Tissue Eng. Part A 2013, 19, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Nishimura, D.; Doi, H.; Nomura, N.; Hanawa, T. Cathodic alkaline treatment of zirconium to give the ability to form calcium phosphate. Acta Biomater. 2010, 6, 4161–4166. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Shoham, O.; Orr, N.; Muhsen, K. An inverse and independent association between helicobacter pylori infection and the incidence of shigellosis and other diarrheal diseases. Clin. Infect. Dis. 2012, 54, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Li, Q.; Lin, W.; Zhang, X.; Lin, F.; Luo, Z.; Bai, X.; Lei, M. Mechanical properties and in vivo study of modified-hydroxyapatite/polyetheretherketone biocomposites. Mat. Sci. Eng. C 2017, 73, 429–439. [Google Scholar]
- Wong, H.M.; Chu, P.K.; Leung, F.K.; Cheung, K.M.; Luk, K.D.; Yeung, K.W. Engineered polycaprolactone–magnesium hybrid biodegradable porous scaffold for bone tissue engineering. Prog. Nat. Sci. 2014, 24, 561–567. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Bian, H.; Zhou, Y.; Gao, C.; Shuai, C. System development, formability quality and microstructure evolution of selective laser-melted magnesium. Virtual Phys. Prototyp. 2016, 11, 173–181. [Google Scholar] [CrossRef]
- Gao, C.; Liu, T.; Shuai, C.; Peng, S. Enhancement mechanisms of graphene in nano-58s bioactive glass scaffold: Mechanical and biological performance. Sci. Rep. 2014, 4, 4712. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Bolelli, G.; Cannillo, V.; Cattini, A.; Sola, A. In situ raman spectroscopy investigation of bioactive glass reactivity: Simulated body fluid solution vs tris-buffered solution. Mater. Charact. 2011, 62, 1021–1028. [Google Scholar] [CrossRef]
- Sun, H.; He, S.; Wu, P.; Gao, C.; Feng, P.; Xiao, T.; Deng, Y.; Shuai, C. A novel mgo-cao-sio2 system for fabricating bone scaffolds with improved overall performance. Materials 2016, 9, 287. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Preventing bacterial adhesion on scaffolds for bone tissue engineering. Int. J. Bioprint. 2016, 2, 20–23. [Google Scholar] [CrossRef]
Sample Availability: Samples of the PEEK/PGA–KDIOP are available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, C.; Shuai, C.; Feng, P.; Yang, Y.; Xu, Y.; Qin, T.; Yang, S.; Gao, C.; Peng, S. Silane Modified Diopside for Improved Interfacial Adhesion and Bioactivity of Composite Scaffolds. Molecules 2017, 22, 511. https://doi.org/10.3390/molecules22040511
Shuai C, Shuai C, Feng P, Yang Y, Xu Y, Qin T, Yang S, Gao C, Peng S. Silane Modified Diopside for Improved Interfacial Adhesion and Bioactivity of Composite Scaffolds. Molecules. 2017; 22(4):511. https://doi.org/10.3390/molecules22040511
Chicago/Turabian StyleShuai, Cijun, Chenying Shuai, Pei Feng, Youwen Yang, Yong Xu, Tian Qin, Sheng Yang, Chengde Gao, and Shuping Peng. 2017. "Silane Modified Diopside for Improved Interfacial Adhesion and Bioactivity of Composite Scaffolds" Molecules 22, no. 4: 511. https://doi.org/10.3390/molecules22040511
APA StyleShuai, C., Shuai, C., Feng, P., Yang, Y., Xu, Y., Qin, T., Yang, S., Gao, C., & Peng, S. (2017). Silane Modified Diopside for Improved Interfacial Adhesion and Bioactivity of Composite Scaffolds. Molecules, 22(4), 511. https://doi.org/10.3390/molecules22040511







