Camellia sinensis L. Extract and Its Potential Beneficial Effects in Antioxidant, Anti-Inflammatory, Anti-Hepatotoxic, and Anti-Tyrosinase Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity Assay
2.1.1. Determination of Lipid Peroxidation Inhibition Using the Ferric Thiocyanate (FTC) Method
2.1.2. Determination of Free Radical Scavenging Activity Using the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.1.3. Chelation of Ferrous Metal Ions
2.2. Determination of In Vitro Anti-Inflammatory Activity by the Inhibition of 5-Lipoxygenase (5-LOX)
2.3. Determination of the Anti-Hepatotoxic Effects of the FTE on CCl4-Induced Liver Injury in Rats
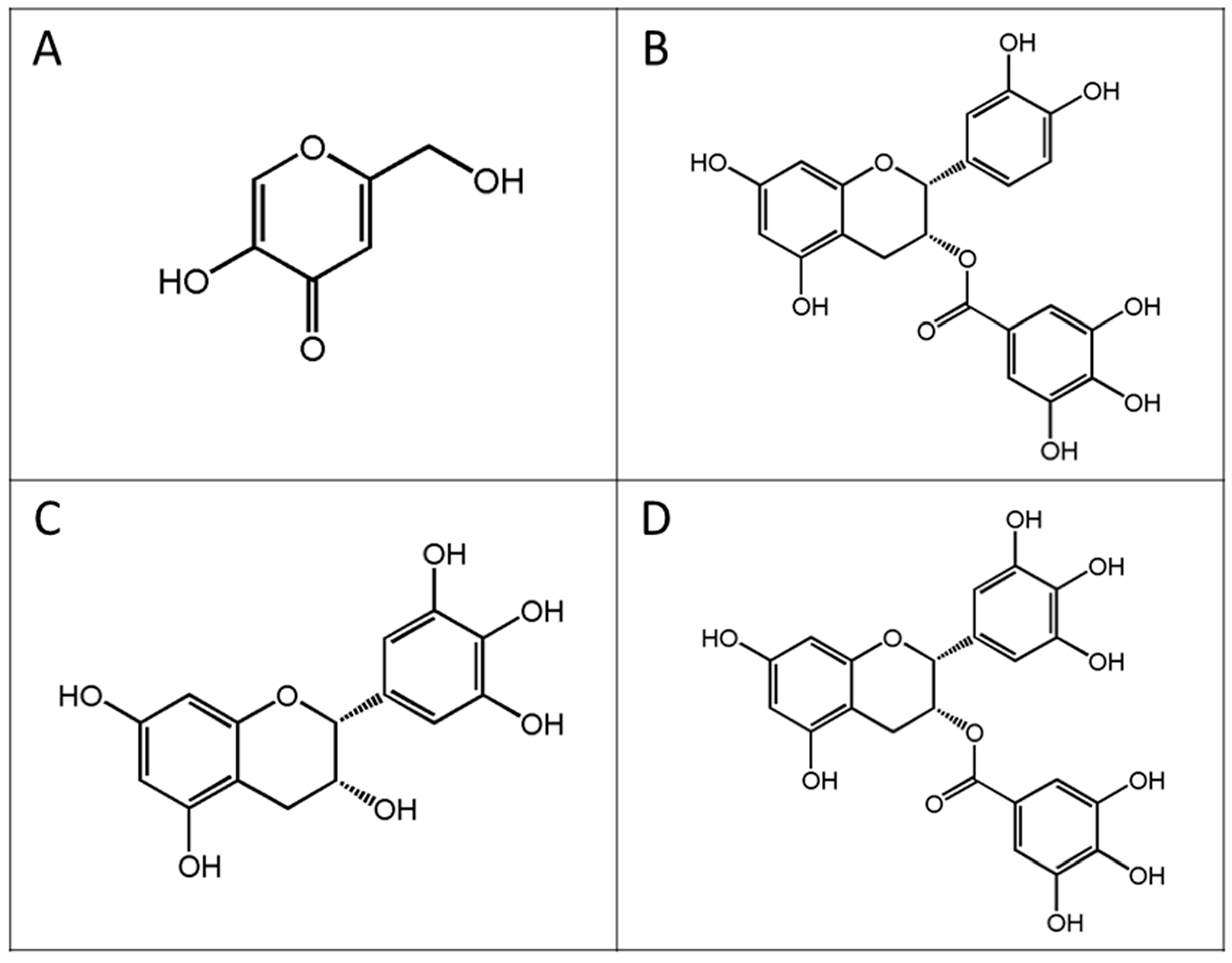
2.4. Determination of Mushroom Tyrosinase Inhibition In Vitro
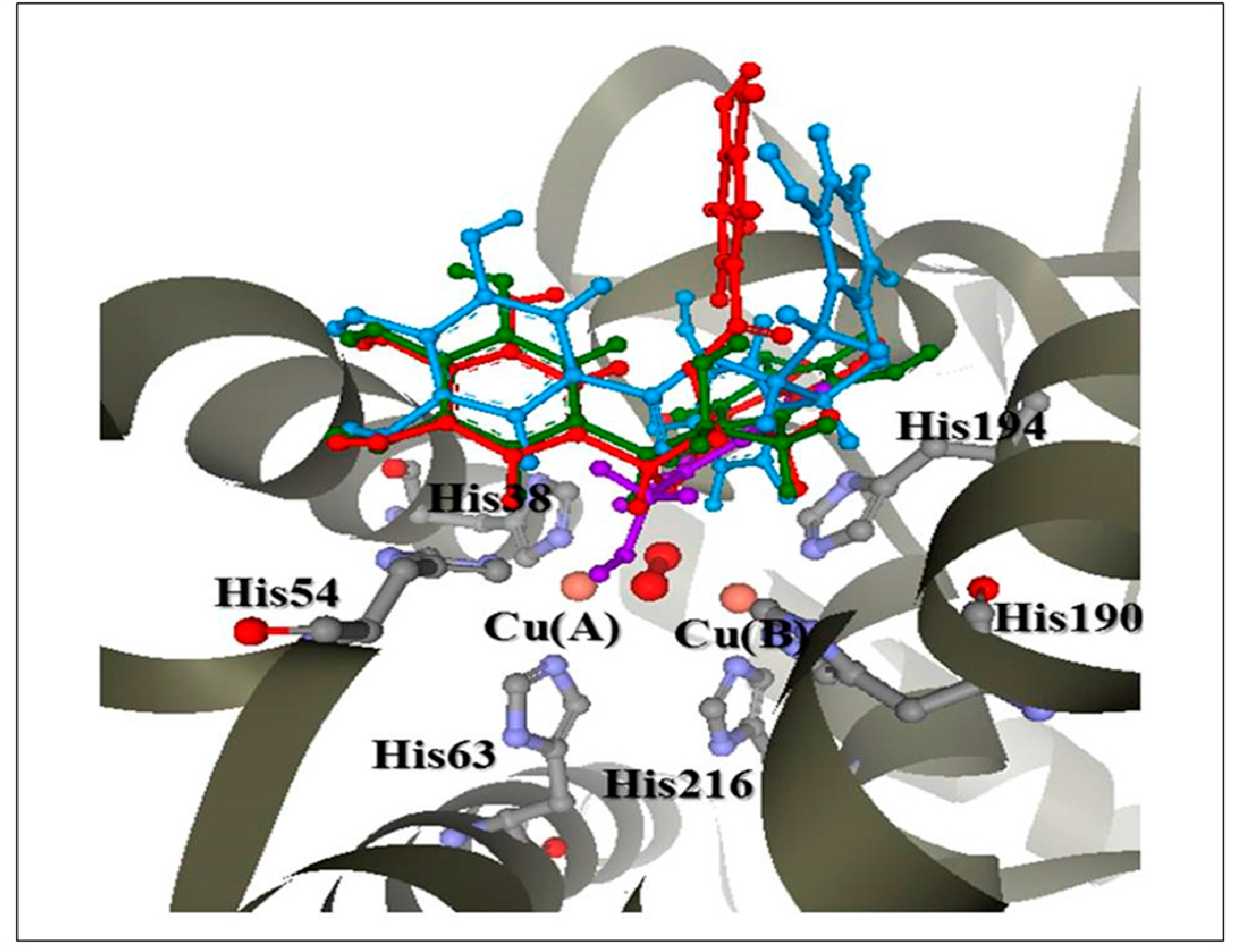
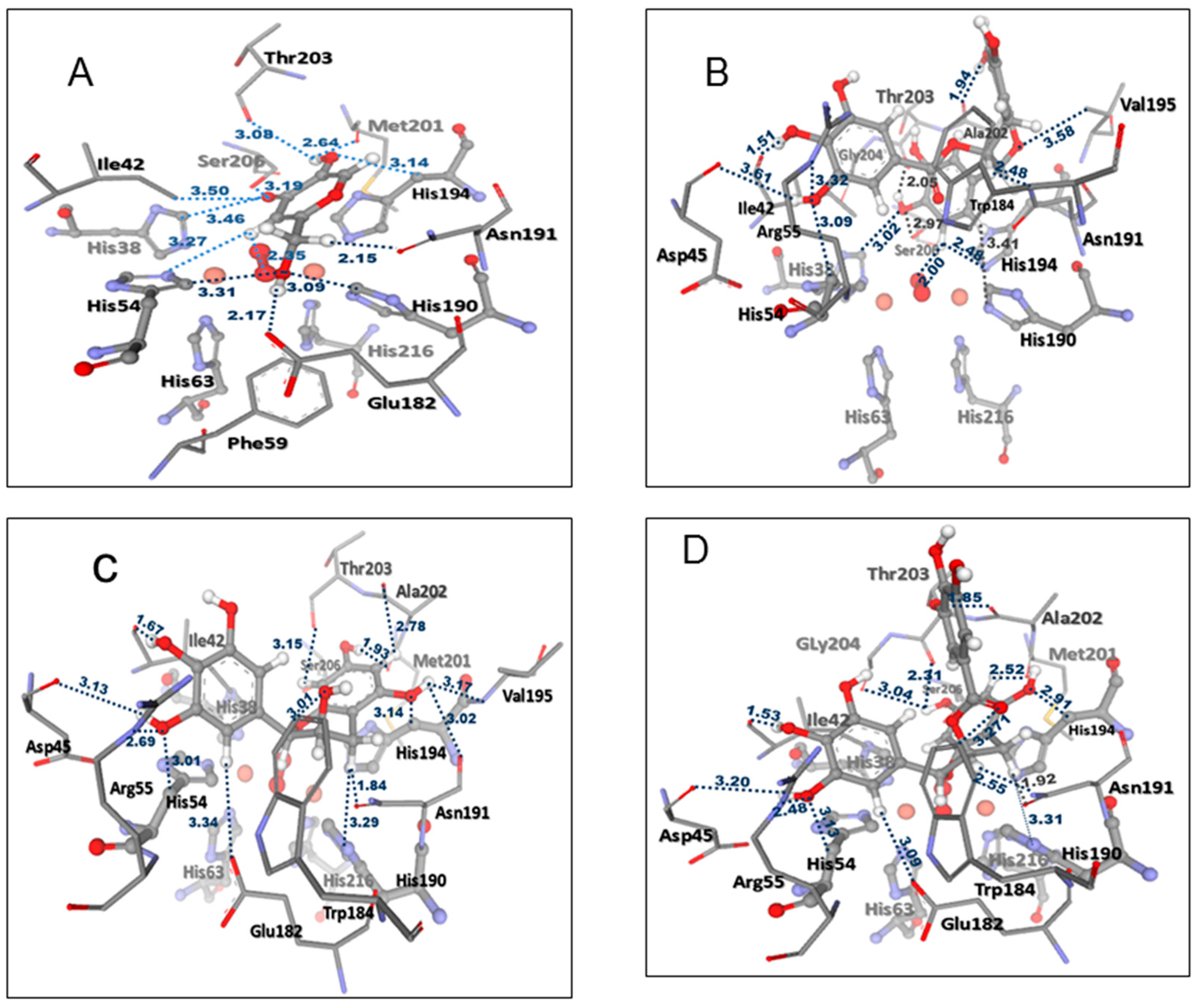
2.5. Molecular Modelling
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Animals
3.4. Preparation and Standardization of Plant Extract
3.5. Antioxidant Activity Assay
3.5.1. Determination of Lipid Peroxidation Inhibition Using the FTC Method
3.5.2. Determination of Radical Scavenging Activity Using the DPPH Test
3.5.3. Chelation of Ferrous Metal Ions
3.6. Determination of In Vitro Anti-Inflammatory Activity by the Inhibition of 5-LOX
3.7. Determination of the Anti-Hepatotoxic Effects of the FTE on CCl4-Induced Liver Injury in Rats
3.7.1. ALT Assay
3.7.2. Thiobarbituric Acid (TBA) Assay
3.8. Determination of Mushroom Tyrosinase Inhibition In Vitro
3.9. Molecular Modelling
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poli, G. Liver damage due to free radicals. Br. Med. Bull. 1993, 49, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Modriansky, M. Oxidative burst of Kupffer cells: Target for liver injury treatment. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2002, 146, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S.; Sharma, A.; Chakraborti, K.K. Natural products and plants as liver protecting drugs. Fitoterapia 1986, 57, 307–352. [Google Scholar]
- Henning, S.M.; Niu, Y.; Liu, Y.; Lee, N.H.; Hara, Y.; Thames, G.D.; Minutti, R.R.; Carpenter, C.L.; Wang, H.; Heber, D. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J. Nutr. Biochem. 2005, 16, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Camargo, L.E.; Pedroso, L.S.; Vendrame, S.C.; Mainardes, R.M.; Khalil, N.M. Antioxidant and antifungal activities of Camellia sinensis (L.) Kuntze leaves obtained by different forms of production. Braz. Biol. 2016, 76, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Górecka, D. Antioxidative potential, nutritional value and sensory profiles of confectionery fortified with green and yellow tea leaves (Camellia sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Obanda, M.; Owuor, P.O.; Taylor, S.J. Flavanol composition and caffeine content of green leaf as quality potential indicators of Kenyan black teas. J. Sci. Food Agric. 1997, 74, 209–215. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Agarwal, R.; Zaim, M.T.; Mukhtar, H. Protection against N-nitrosodiethylamine and benzo[a]pyrene-induced forestomach and lung tumorigenesis in A/J mice by green tea. Carcinogenesis 1993, 14, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Hara, Y.; Sano, M.; Tomita, I. Antioxidative effects of black tea theaflavins and thearubigin on lipid peroxidation of rat liver homogenates induced by tert-butyl hydroperoxide. Biol. Pharm. Bull. 1994, 17, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Hung, M.W.; Fung, M.L. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Choi, S.Y.; Park, E.Y. Anti-melanogenic effects of black, green, and white tea extracts on immortalized melanocytes. J. Vet. Sci. 2015, 16, 135–143. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Noda, Y.; Sugiyama, K. Green tea suppresses lipopolysaccharide-induced liver injury in d-galactosamine-sensitized rats. J. Nutr. 2001, 131, 1560–1567. [Google Scholar] [PubMed]
- Ostrowska, J.; Łuczaj, W.; Kasacka, I.; Rózański, A.; Skrzydlewska, E. Green tea protects against ethanol-induced lipid peroxidation in rat organs. Alcohol 2004, 32, 25–32. [Google Scholar] [CrossRef] [PubMed]
- El-Beshbishy, H.A. Hepatoprotective effect of green tea (Camelliasinensis) extract against tamoxifen-induced liver injury in rats. J. Biochem. Mol. Biol. 2005, 38, 563–570. [Google Scholar] [PubMed]
- Issabeagloo, E.; Taghizadieh, M. Hepatomodulatory action of Camellia sinensis aqueous extract against isoniazid-rifampicin combination induced oxidative stress in rat. Adv. Biomed. Res. 2012, 3, 18–27. [Google Scholar]
- Lodhi, P.; Tandan, N.; Singh, N.; Kumar, D.; Kumar, M. Camellia sinensis (L.) Kuntze extract ameliorates chronic ethanol-induced hepatotoxicity in albino rats. Evid.-Based Complement. Altern. Med. 2014, 2014, 787153. [Google Scholar] [CrossRef] [PubMed]
- Nerya, O.; Ben-Arie, R.; Luzzatto, T.; Musa, R.; Khatib, S.; Vaya, J. Prevention of Agaricus bisporus postharvest browning with tyrosinase inhibitors. Postharvest Biol. Technol. 2006, 39, 272–277. [Google Scholar] [CrossRef]
- Hearing, V.J.; Jimenez, M. Analysis of mammalian pigmentation at the molecular level. Pigment Cell Res. 1989, 2, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocytes. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar] [PubMed]
- Shimogaki, H.; Tanaka, Y.; Tamai, H.; Masuda, M. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int. J. Cosmet. Sci. 2000, 22, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Kumagai, T.; Yamamoto, A.; Yoshitsu, H.; Sugiyama, M. Crystallographic evidence that the dinuclear coppercenter of tyrosinase is flexible during catalysis. J. Biol. Chem. 2006, 281, 8981–8990. [Google Scholar] [CrossRef] [PubMed]
- Nithitanakool, S.; Pithayanukul, P.; Bavovada, R.; Saparpakorn, P. Molecular docking studies and anti-tyrosinase activity of Thai mango seed kernel extract. Molecules 2009, 14, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Pokorný, J. Addition of antioxidants for food stabilization to control oxidative rancidity. Czech J. Food Sci. 1986, 4, 299–307. [Google Scholar]
- Farhoosh, R.; Golmovahhed, G.A.; Khodaparast, M.H.H. Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.). Food Chem. 2007, 100, 231–236. [Google Scholar] [CrossRef]
- Young, R.N. Inhibitors of 5-lipoxygenase: A therapeutic potential yet to be fully realized? Eur. J. Med. Chem. 1999, 34, 671–685. [Google Scholar] [CrossRef]
- Frum, Y.; Viljoen, A.M. In vitro 5-lipoxygenase and anti-oxidant activities of South African medicinal plants commonly used topically for skin diseases. Skin Pharmacol. Physiol. 2006, 19, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Nerya, O.; Musa, R.; Tamir, S.; Peter, T.; Vaya, J. Enhanced substituted resorcinol hydrophobicity augments tyrosinase inhibition potency. J. Med. Chem. 2007, 50, 2676–2681. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Wang, H.; Helliwell, K.; You, X. Isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem. 2000, 68, 115–121. [Google Scholar]
- Kikuzaki, H.; Nakatani, N. Antioxidant effects of some ginger constituents. J. Food Sci. 1993, 58, 1407–1410. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tailor, C.S.; Goyal, A. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. leaves. Am. J. Ethnomed. 2014, 1, 244–249. [Google Scholar]
- Gülçin, I.; Elmastaş, M.; Aboul-Enein, H.Y. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. 2007, 21, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Baylac, S.; Racine, P. Inhibition of 5-lipoxygenase by essential oils and other natural fragrant extracts. Int. J. Aromather. 2003, 13, 138–142. [Google Scholar] [CrossRef]
- Mishra, S.; Sahoo, S.; Rout, K.K.; Nayak, S.K.; Mishra, S.K.; Panda, P.K. Hepatoprotective effect of Barringtonia acutangula Linn. leaves on carbon tetrachloride-induced acute liver damage in rats. Indian J. Nat. Prod. Resour. 2011, 2, 515–519. [Google Scholar]
- Pandit, S.; Sur, T.K.; Jana, U.; Debnath, P.K.; Sen, S.; Bhattacharyya, D. Prevention of carbon tetrachloride-induced hepatotoxicity in rats by Adhatoda vasica leaves. Indian J. Pharmacol. 2004, 36, 312–320. [Google Scholar]
- Mahli, A.; Koch, A.; Czech, B.; Peterburs, P.; Lechner, A.; Haunschild, J.; Müller, M.; Hellerbrand, C. Hepatoprotective effect of oral application of a silymarin extract in carbon tetrachloride-induced hepatotoxicity in rats. Clin. Phytosciol. 2015. [Google Scholar] [CrossRef]
- Bezenjani, S.N.; Pouraboli, I.; Afshar, R.M.; Mohammadi, G. Hepatoprotective effect of Otostegia persica Boiss. shoot extract on carbon tetrachloride-induced acute liver damage in rats. Iran. J. Pharm. Res. 2012, 11, 1235–1241. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Iida, K.; Hase, K.; Shimomura, K.; Sudo, S.; Kadota, S.; Namba, T. Potent inhibitors of tyrosinase activity and melanin biosynthesis from Rheum officinale. Planta Med. 1995, 61, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
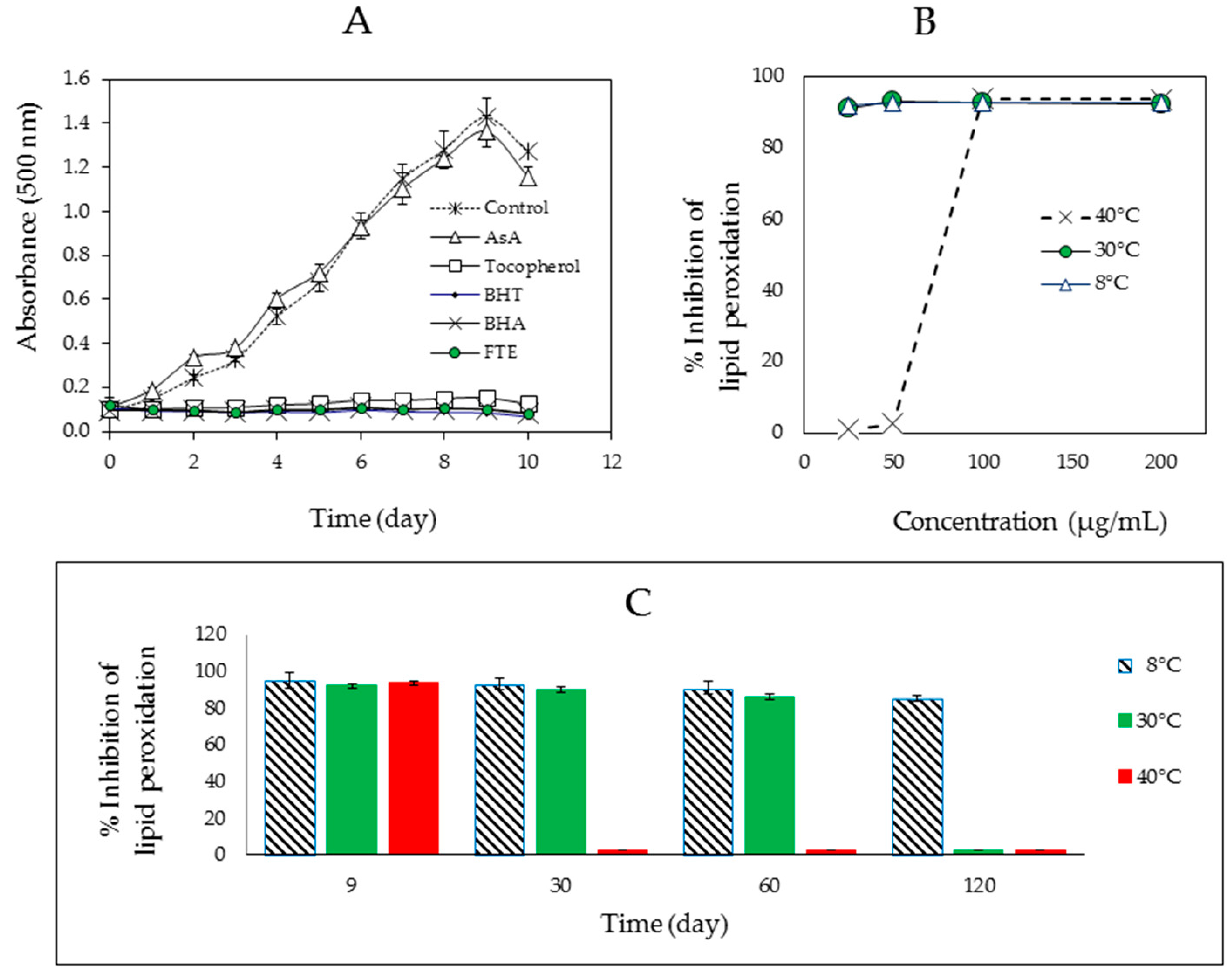
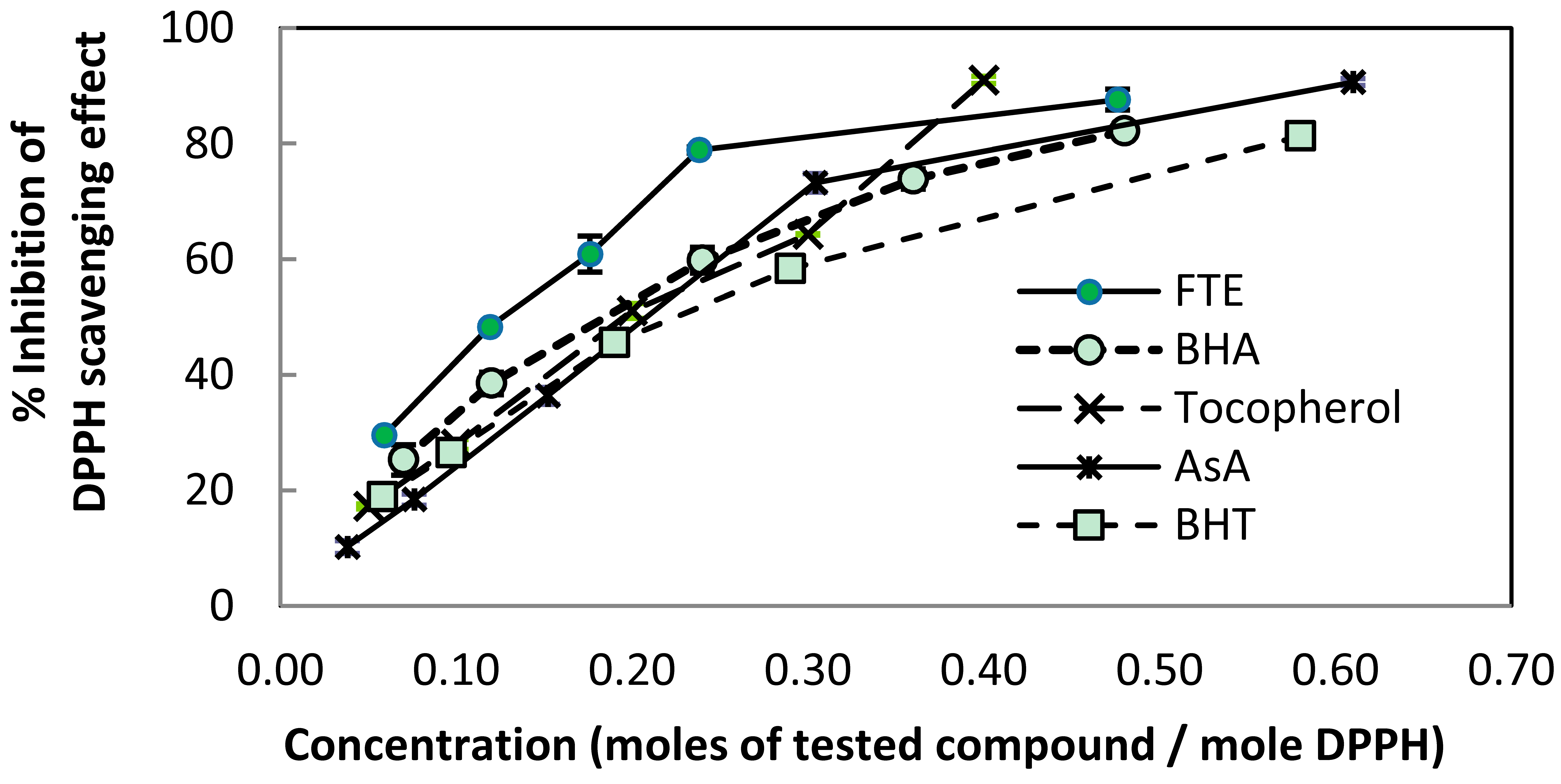

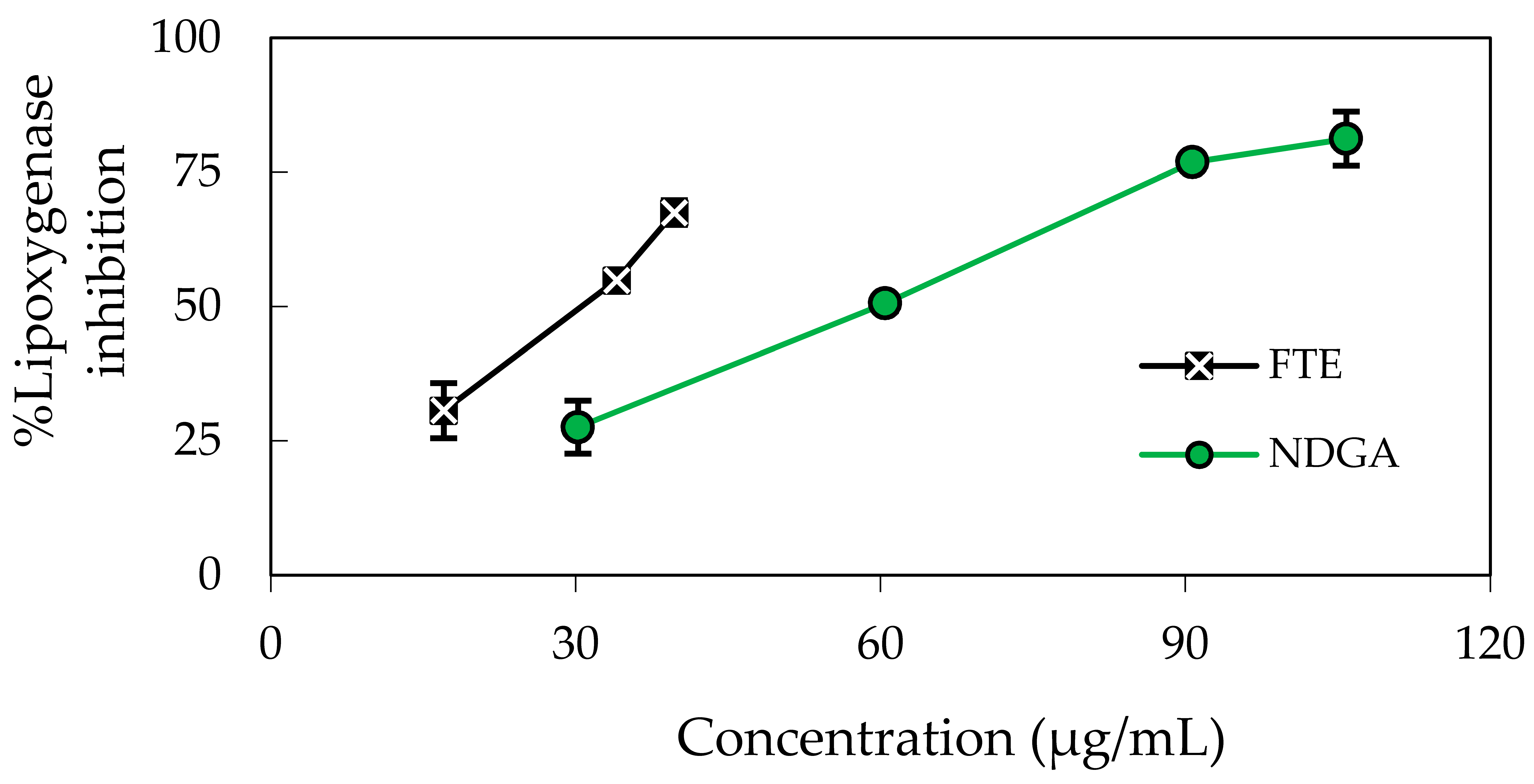
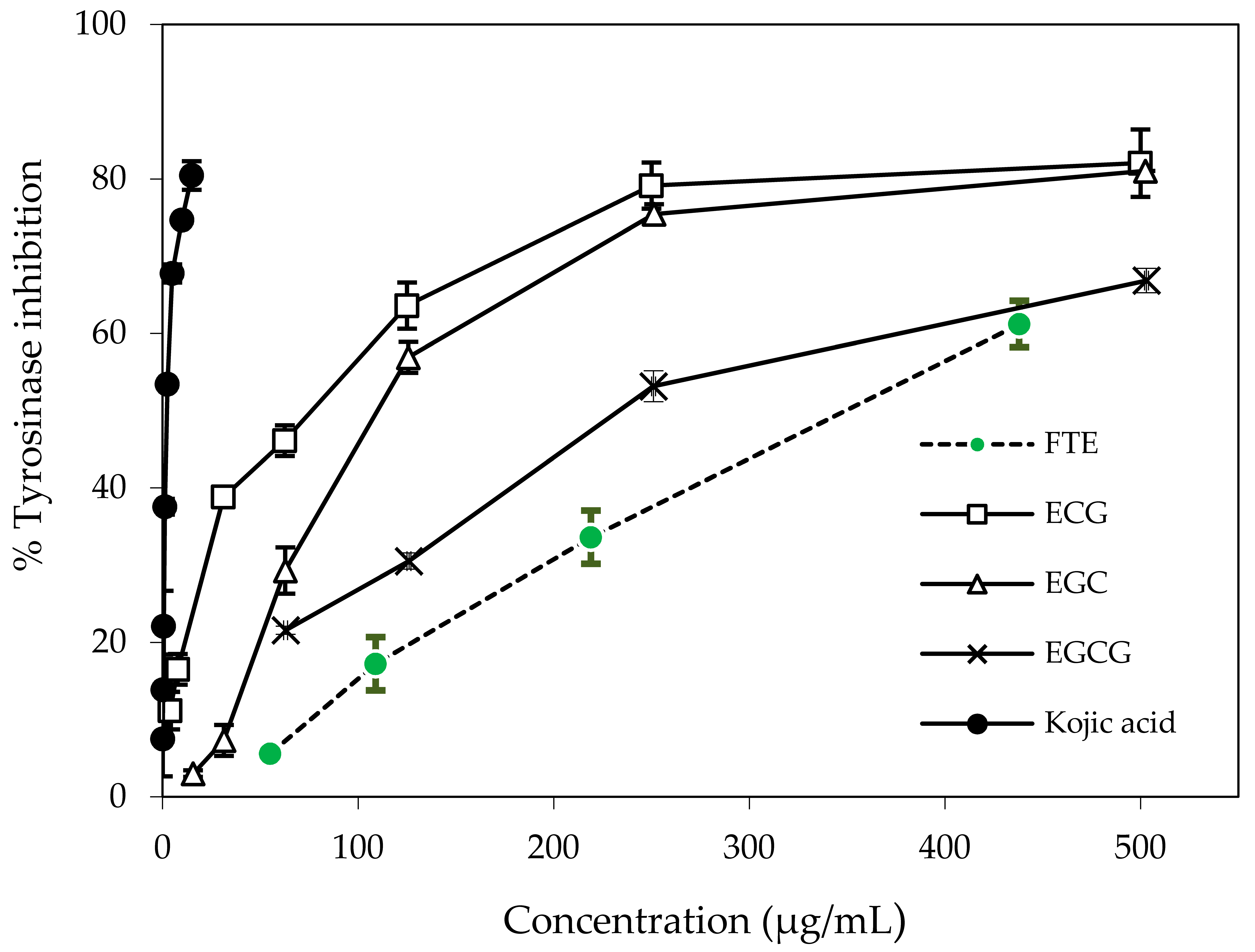
| Tested Samples | SC50 (Moles of Tested Compound/Mole DPPH) |
|---|---|
| FTE | 0.128 ± 0.004 |
| Reference standards: | |
| BHA | 0.184 ± 0.011 |
| BHT | 0.224 ± 0.005 |
| Tocopherol | 0.196 ± 0.005 |
| AsA | 0.209 ± 0.006 |
| FTE and Its Isolated Compounds | Contents a | Iron(II)-Chelating b (IC50, mg/mL) | Anti-Lipoxygenase b (IC50, µg/mL ) | Anti-Tyrosinase a (IC50, µg/mL ) | |
|---|---|---|---|---|---|
| % w/w | mg/g Dry Weight | ||||
| FTE c, total phenolic content | 24.52 | 245.20 ± 4.92 | 2.08 ± 0.10 | 30.60 ± 0.40 | 349.00 d ± 9.00 |
| Constituents: | |||||
| Epicatechin (EC) | 1.60 | 16.05± 0.25 | - | - | Nd |
| Epicatechin-3-gallate (ECG) | 1.17 | 11.70 ± 0.20 | - | - | 76.50 d,e ± 1.50 |
| Epigallocatechin (EGC) | 8.97 | 89.70 ± 2.00 | - | - | 110.00 d,e ± 0.00 |
| Epigallocatechin-3-gallate (EGCG) | 7.21 | 72.10 ± 2.30 | - | - | 234.00 d,e ± 6.00 |
| Unidentified phenolic content | 5.57 | - | - | - | - |
| Reference Standards: | |||||
| Ethylenediaminetetraacetic acid (EDTA) | - | 0.01 ± 0.00 | - | - | |
| Nordihydroguaiaretic acid (NDGA) | - | - | 59.26 ± 0.92 | - | |
| Kojic acid (KA) | - | - | - | 2.28 ± 0.03 | |
| Groups | ALT (U/L) | MDA (nmol/g Liver) |
|---|---|---|
| Normal | 25.49 ± 2.41 | 0.02 ± 0.00 |
| CCl4 | 301.04 a ± 13.88 | 0.14 a ± 0.01 |
| FTE (500 mg/kg) + CCl4 | 292.34 ± 9.38 (3.16%) b | 0.12 ± 0.01 (24.44%) b |
| FTE (1000 mg/kg) + CCl4 | 206.42 c ± 18.42 (34.34%) b | 0.09 c ± 0.02 (48.96%) b |
| FTE (2000 mg/kg) + CCl4 | 111.64 c ± 2.91 (68.74%) b | 0.04 c ± 0.00 (90.27%) b |
| FTE (2000 mg/kg) | 28.64 c ± 2.39 | 0.03 c ± 0.00 |
| Silymarin (100 mg/kg) + CCl4 | 79.38 c ± 28.22 (80.43%) b | 0.05 c ± 0.01 (78.78%) b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thitimuta, S.; Pithayanukul, P.; Nithitanakool, S.; Bavovada, R.; Leanpolchareanchai, J.; Saparpakorn, P. Camellia sinensis L. Extract and Its Potential Beneficial Effects in Antioxidant, Anti-Inflammatory, Anti-Hepatotoxic, and Anti-Tyrosinase Activities. Molecules 2017, 22, 401. https://doi.org/10.3390/molecules22030401
Thitimuta S, Pithayanukul P, Nithitanakool S, Bavovada R, Leanpolchareanchai J, Saparpakorn P. Camellia sinensis L. Extract and Its Potential Beneficial Effects in Antioxidant, Anti-Inflammatory, Anti-Hepatotoxic, and Anti-Tyrosinase Activities. Molecules. 2017; 22(3):401. https://doi.org/10.3390/molecules22030401
Chicago/Turabian StyleThitimuta, Surached, Pimolpan Pithayanukul, Saruth Nithitanakool, Rapepol Bavovada, Jiraporn Leanpolchareanchai, and Patchreenart Saparpakorn. 2017. "Camellia sinensis L. Extract and Its Potential Beneficial Effects in Antioxidant, Anti-Inflammatory, Anti-Hepatotoxic, and Anti-Tyrosinase Activities" Molecules 22, no. 3: 401. https://doi.org/10.3390/molecules22030401
APA StyleThitimuta, S., Pithayanukul, P., Nithitanakool, S., Bavovada, R., Leanpolchareanchai, J., & Saparpakorn, P. (2017). Camellia sinensis L. Extract and Its Potential Beneficial Effects in Antioxidant, Anti-Inflammatory, Anti-Hepatotoxic, and Anti-Tyrosinase Activities. Molecules, 22(3), 401. https://doi.org/10.3390/molecules22030401





