Ultraviolet B (UVB) Photosensitivities of Tea Catechins and the Relevant Chemical Conversions
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Photostabilities of Catechins under UVB Radiation
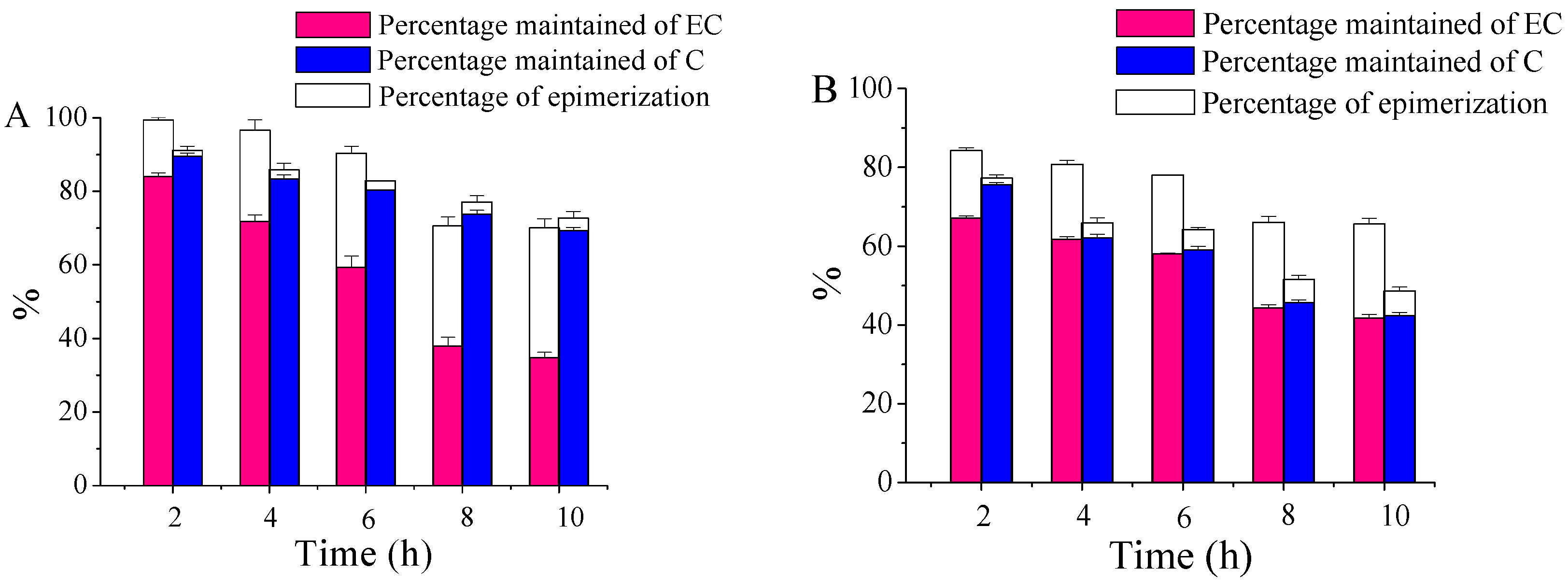
2.2. The Chemical Transformations of EC and C with UV B Radiation Time
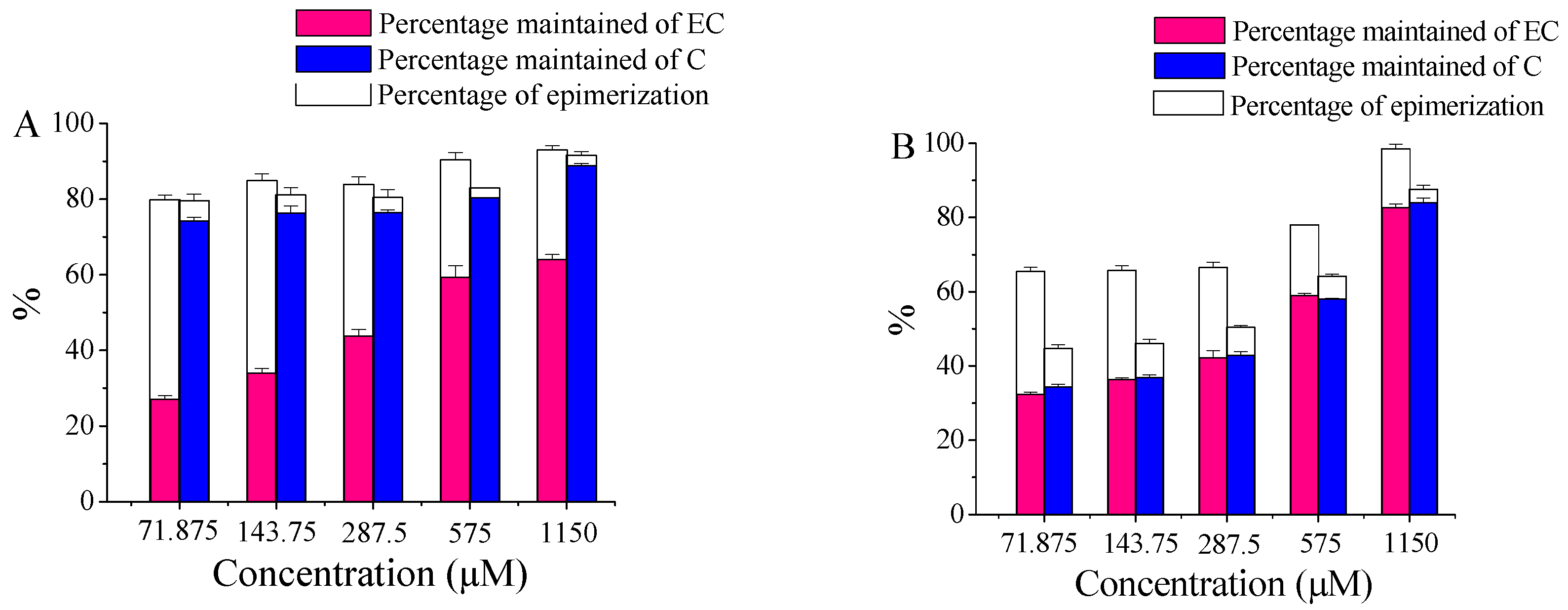
2.3. The Effect of Substrate Concentrations of EC and C
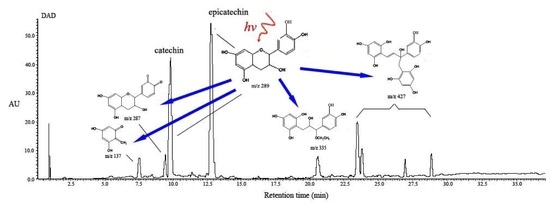
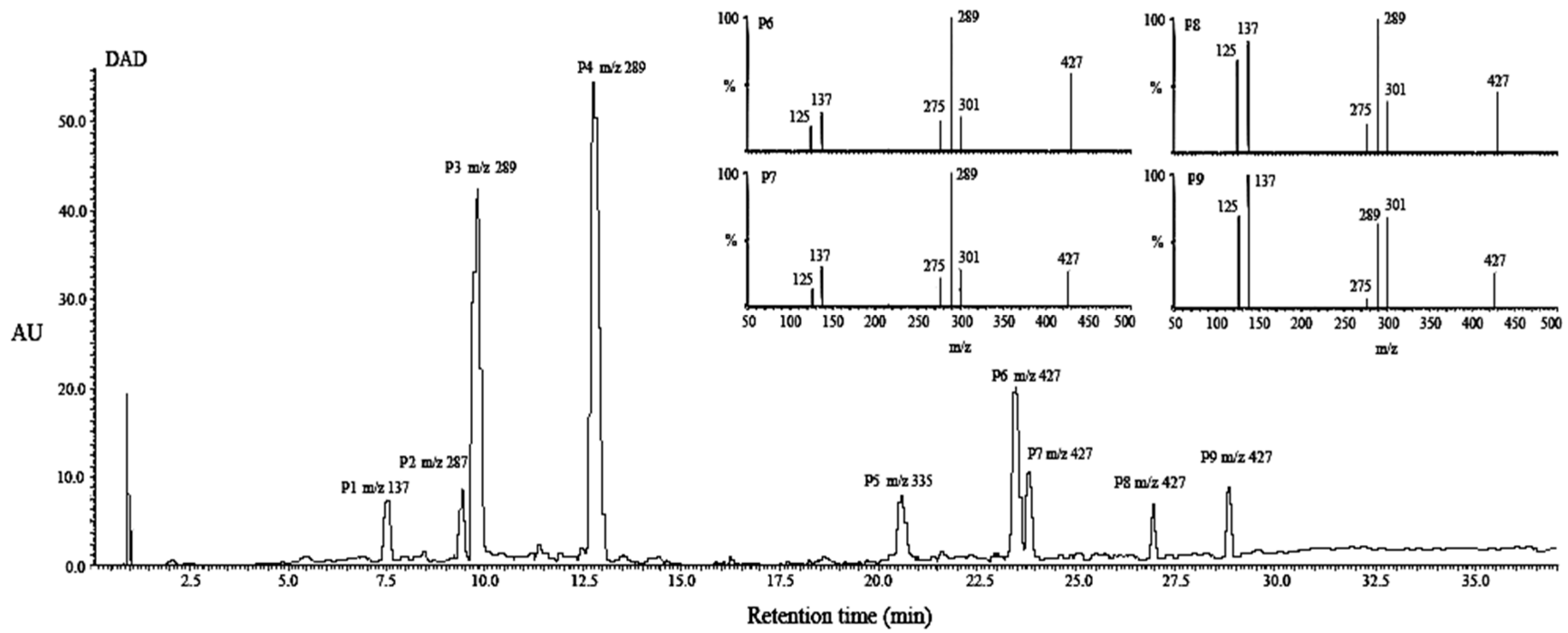
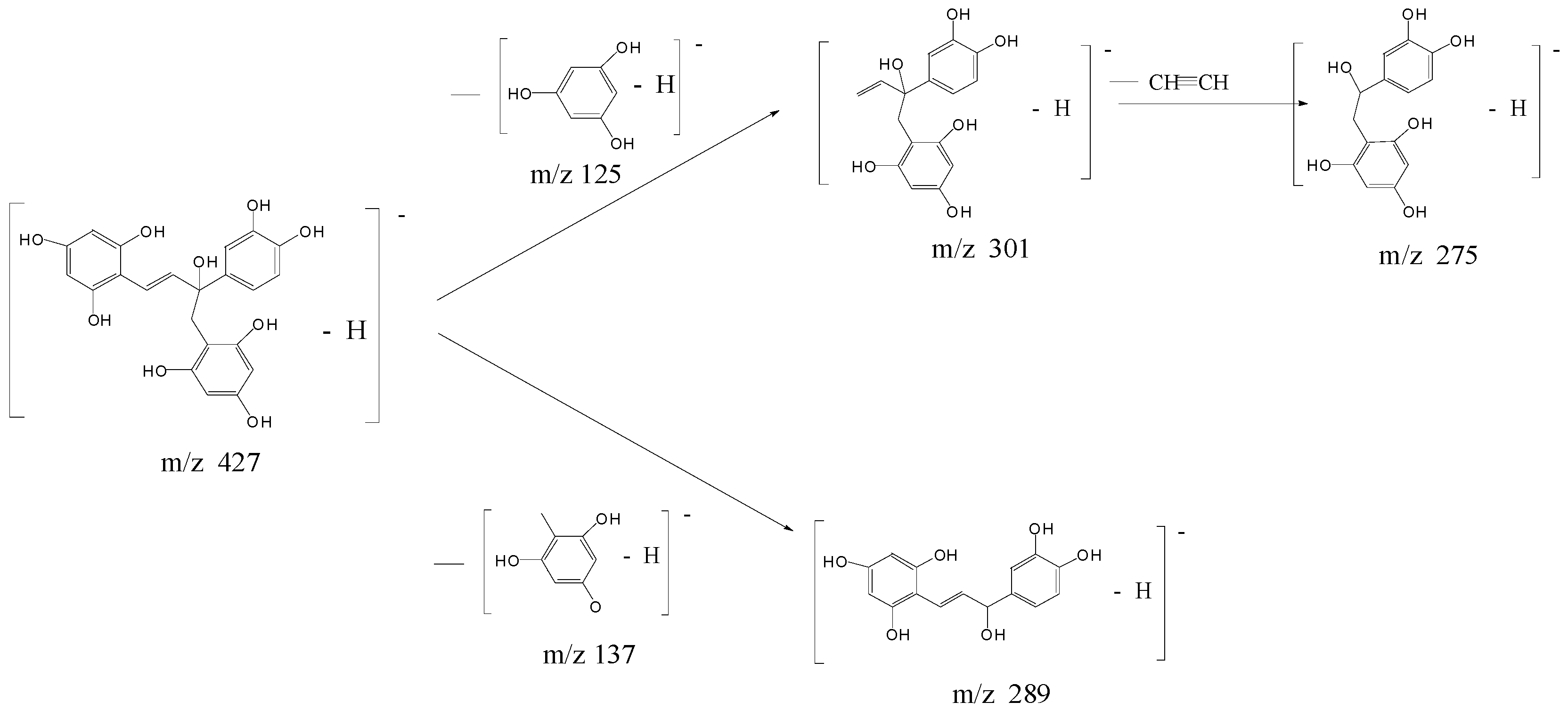
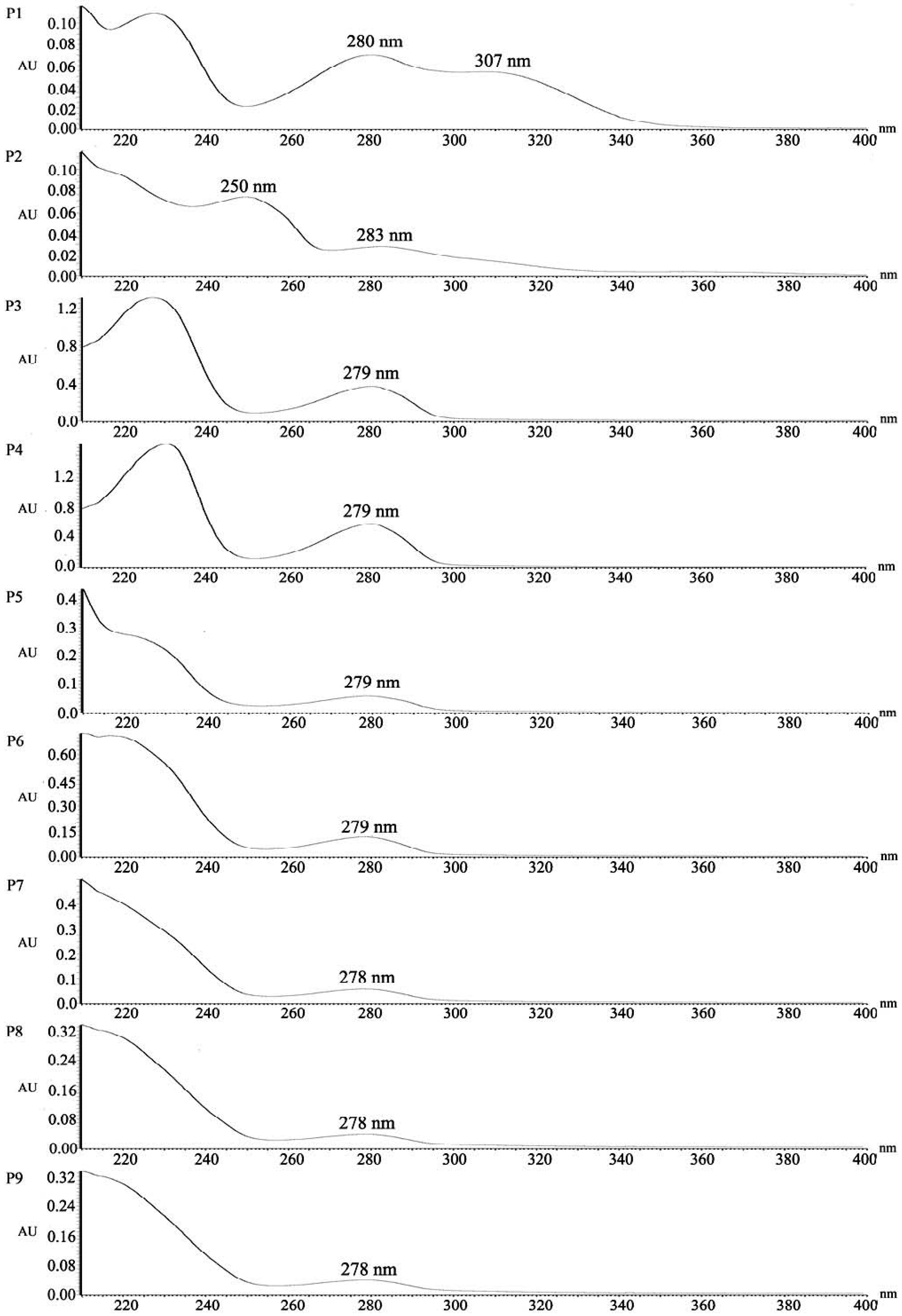
2.4. Structural Characterization of Photoproducts
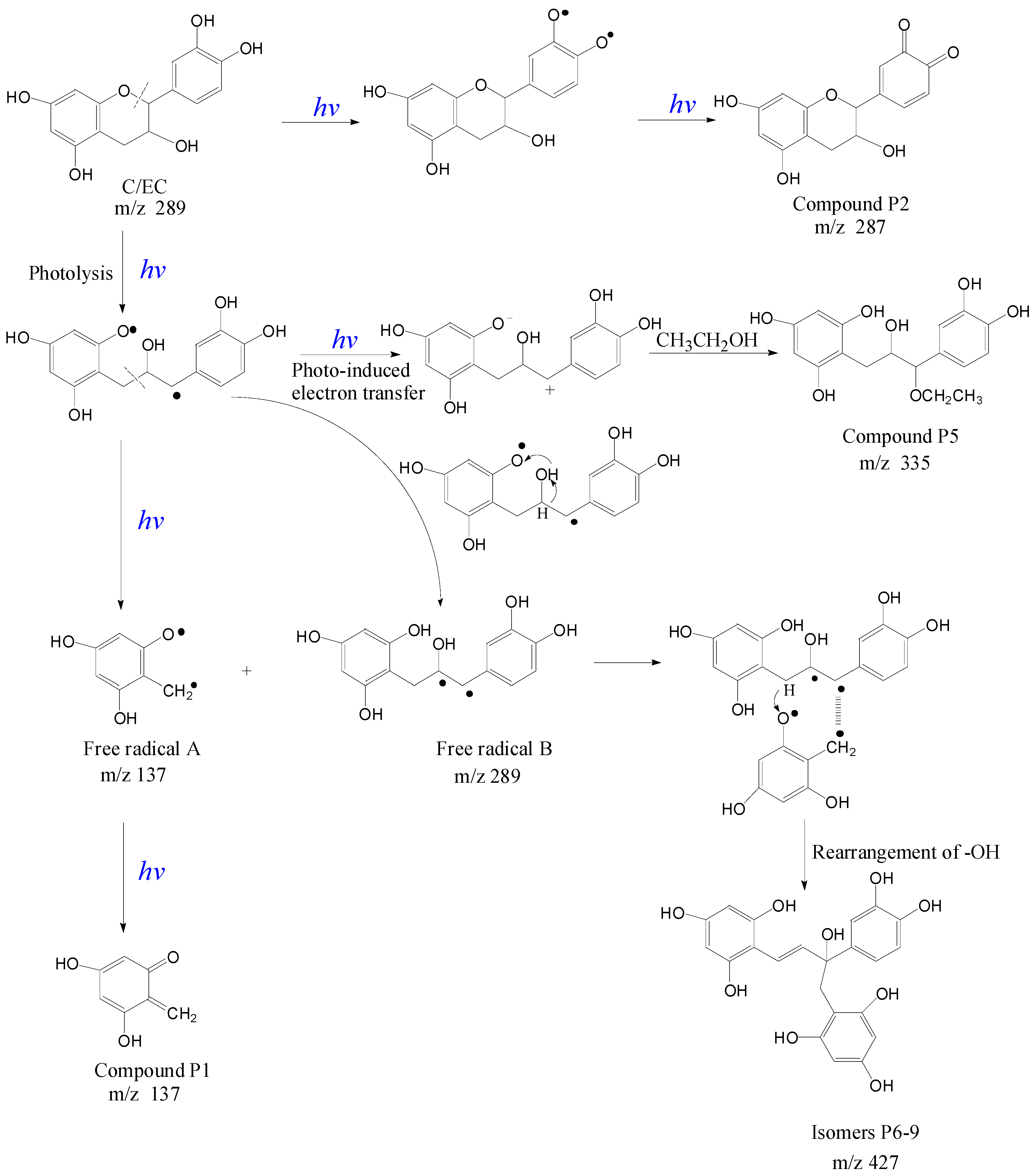
2.5. Proposed Reaction Mechanisms
2.6. Antioxidant Activities
3. Materials and Methods
3.1. Chemicals
3.2. Photosensitivities of Eight Catechins to UVB
3.3. Effect of UVB Radiation Time on Conversions of EC and C
3.4. Effect of Substrate Concentration on Conversions of EC and C
3.5. HPLC Analysis
3.6. Identification of the Photoproducts of EC and C
3.6.1. UPLC-DAD-MS/MS Analysis
3.6.2. 1H Nuclear Magnetic Resonance (NMR) Measurement
3.7. Antioxidant Activity Measurement
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, L.; Zhang, H.Y. Cancer preventive mechanisms of the green tea polyphenol (−)-epigallocatechin-3-gallate. Molecules 2007, 12, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Monobe, M.; Ema, K.; Matsunaga, A.; Maeda-Yamamoto, M.; Horie, H. Effects of flavonol-rich green tea cultivar (Camellia sinensis L.) on plasma oxidized LDL levels in hypercholesterolemic mice. Biosci. Biotech. Bioch. 2016, 80, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.E.; Chan, J.L.; Kern, D.G.; Kohler, S.; Rehmus, W.E.; Kimball, A.B. Double-blinded, placebo-controllecl trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol. Surg. 2005, 31, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Wu, W.B.; Fang, J.Y.; Chiang, H.S.; Chen, S.K.; Chen, B.H.; Chen, Y.T.; Hung, C.F. (−)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules 2007, 12, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.E.; Choi-Kwon, S.; Park, K.A.; Lee, H.J.; Park, M.S.; Lee, J.H.; Kwon, S.B.; Park, K.C. Dietary supplementation of (+)-catechin protects against UVB-induced skin damage by modulating antioxidant enzyme activities. Photodermatol. Photoimmunol. Photomed. 2003, 19, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.W.; Jo, C.; Lee, J.W.; Jo, S.K.; Kim, K.S. Application of radiation technology to develop green tea leaf as a natural resource for the cosmetic industry. Radiat. Phys. Chem. 2004, 71, 485–487. [Google Scholar] [CrossRef]
- Frauen, M.; Rode, T.; Rapp, C.; Steinhart, H. Determination of green-tea catechins in cosmetic formulations and in in-vitro skin extracts by high-performance liquid chromatography coupled with electrospray ionization mass spectrometry. Chromatographia 2002, 55, 43–48. [Google Scholar] [CrossRef]
- Gianeti, M.D.; Mercurio, D.G.; Maia Campos, P.M. The use of green tea extract in cosmetic formulations: Not only an antioxidant active ingredient. Dermatol. Ther. 2013, 26, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Marchetti, N.; Bianchi, A. Comparative evaluation of different co-antioxidants on the photochemical-and functional-stability of epigallocatechin-3-gallate in topical creams exposed to simulated sunlight. Molecules 2013, 18, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Seto, Y.; Ochi, M.; Inoue, R.; Ito, H.; Hatano, T.; Yamada, S. In vitro photochemical and phototoxicological characterization of major constituents in St. John’s Wort (Hypericum perforatum) extracts. Phytochemistry 2011, 72, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Chen, H.; Zhang, L.B.; Ye, J.H.; Lu, J.L.; Liang, Y.R. Effects of temperature, illumination, and sodium ascorbate on browning of green tea infusion. Food Sci. Biotechnol. 2009, 18, 932–938. [Google Scholar]
- Ananingsih, V.K.; Sharma, A.; Zhou, W.B. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Fan, F.Y.; Shi, M.; Nie, Y.; Zhao, Y.; Ye, J.H.; Liang, Y.R. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016, 196, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Leung, L.K.; Huang, Y.; Chen, Z.Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar]
- Seto, R.; Nakamura, H.; Nanjo, F.; Hara, Y. Preparation of epimers of tea catechins by heat treatment. Biosci. Biotech. Bioch. 1997, 61, 1434–1439. [Google Scholar] [CrossRef]
- Lavker, R.M.; Gerberick, G.F.; Veres, D.; Irwin, C.J.; Kaidbey, K.H. Cumulative effects from repeated exposures to suberythemal doses of UV B and UV A in human skin. J. Am. Acad. Dermatol. 1995, 32, 53–62. [Google Scholar] [CrossRef]
- Dobashi, Y.; Hirano, T.; Hirano, M.; Ohkatsu, Y. Antioxidant and photo-antioxidant abilities of catechins. J. Photochem. Photobiol. A 2008, 197, 141–148. [Google Scholar] [CrossRef]
- Fourie, T.G.; Ferreira, D.; Roux, D.G. Flavonoid synthesis based on photolysis of flavan-3-ols, 3-hydroxyflavanones, and 2-benzylbenzofuranones. J. Chem. Soc. Perkin Trans. 1 1997, 125–133. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, M.; Ye, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Photo-induced chemical reaction of trans-resveratrol. Food Chem. 2015, 171, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Huvaere, K.; Sinnaeve, B.; Van Bocxlaer, J.; Skibsted, L.H. Flavonoid deactivation of excited state flavins: Reaction monitoring by mass spectrometry. J. Agric. Food Chem. 2012, 60, 9261–9272. [Google Scholar] [CrossRef] [PubMed]
- Huvaere, K.; Skibsted, L.H. Flavonoids protecting food and beverages against light. J. Sci. Food Agric. 2015, 95, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Shirato, M.; Ikai, H.; Kanno, T.; Sasaki, K.; Kohno, M.; Niwano, Y. Photo-irradiation of proanthocyanidin as a new disinfection technique via reactive oxygen species formation. PLoS ONE 2013, 8, e60053. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Marchetti, N.; Scalia, S. Photodegradation of (−)-epigallocatechin-3-gallate in topical cream formulations and its photostabilization. J. Pharm. Biomed. Anal. 2011, 56, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Cren-Olivé, C.; Lebrun, S.; Hapiot, P.; Pinson, J.; Rolando, C. Selective protection of catechin gives access to the intrinsic reactivity of the two phenol rings during H-abstraction and photo-oxidation. Tetrahedron Lett. 2000, 41, 5847–5851. [Google Scholar] [CrossRef]
- Wang, H.F.; Helliwell, K. Epimerisation of catechins in green tea infusions. Food Chem. 2000, 70, 337–344. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Color and chemical stability of tea polyphenol (−)-epigallocatechin-3-gallate in solution and solid states. Food Res. Int. 2013, 53, 909–921. [Google Scholar] [CrossRef]
- Forest, K.; Wan, P.; Preston, C.M. Catechin and hydroxybenzhydrols as models for the environmental photochemistry of tannins and lignins. Photochem. Photobiol. Sci. 2004, 3, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sano, M.; Yoshida, R.; Degawa, M.; Miyase, T.; Maeda-Yamamoto, M. Epimerization of tea catechins and O-methylated derivatives of (−)-epigallocatechin-3-O-gallate: Relationship between epimerization and chemical structure. J. Agric. Food Chem. 2003, 51, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Munro, M.H.G.; Powell, H.K.J.; Porter, L.J.; Foo, L.Y. The protonation reactions of catechin, epicatechin and related-compounds. Aust. J. Chem. 1984, 37, 885–892. [Google Scholar] [CrossRef]
- Sousa, W.R.; da Rocha, C.; Cardoso, C.L.; Silva, D.H.S.; Zanoni, M.V.B. Determination of the relative contribution of phenolic antioxidants in orange juice by voltammetric methods. J. Food Compos. Anal. 2004, 17, 619–633. [Google Scholar] [CrossRef]
- Lukeman, M. Photochemical generation and characterization of quinone methides. In Quinone Methides; Rokita, S.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wilke, T.; Schneider, M.; Kleinermanns, K. 1,4-Hydroquinone is a hydrogen reservoir for fuel cells and recyclable via photocatalytic water splitting. Open J. Phys. Chem. 2013, 3, 97–102. [Google Scholar] [CrossRef]
- Hailey, C.; Burks, J.W. Contact dermatitis from currently popular tanning agents. JAMA 1960, 174, 2072–2073. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Y.; Sun, J.; Wei, X.Y.; Shi, J.; Cheng, G.P.; Zhao, M.M.; Wang, J.S.; Yang, B.; Jiang, Y.M. Identification of (−)-epicatechin as the direct substrate for polyphenol oxidase from longan fruit pericarp. LWT-Food Sci. Technol. 2008, 41, 1742–1747. [Google Scholar] [CrossRef]
- Wilhelm-Mouton, A.; Bonnet, S.L.; Ding, Y.Q.; Li, X.C.; Ferreira, D.; van der Westhuizen, J.H. Photochemistry synthesis. Part 2: Enantiomerically pure polyhydroxy-1,1,3-triarylpropan-2-ols. J. Photochem. Photobiol. A 2012, 227, 18–24. [Google Scholar] [CrossRef]
- Faria, J.L.; Steenken, S. Photoionization (λ = 248 or 308 nm) of triphenylmethyl radical in aqueous-solution-formation of triphenylmethyl carbocation. J. Am. Chem. Soc. 1990, 112, 1277–1279. [Google Scholar] [CrossRef]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.L.; Liang, Y.R.; Dong, J.J.; Lu, J.L.; Xu, H.R.; Wang, H. Decaffeination of fresh green tea leaf (Camellia sinensis) by hot water treatment. Food Chem. 2007, 101, 1451–1456. [Google Scholar] [CrossRef]
- Jo, C.; Son, J.H.; Lee, H.J.; Byun, M.W. Irradiation application for color removal and purification of green tea leaves extract. Radiat. Phys. Chem. 2003, 66, 179–184. [Google Scholar] [CrossRef]
- Sample Availability: Samples of UV B irradiated products of EC and C are available from the authors.
| Catechins 2 | Molecular Structures | After UV Radiation 3 | Compositions | Water 4 | Ethanol 4 |
|---|---|---|---|---|---|
| EC |  |  | EC | 341.0 ± 6.3 (59.3 ± 3.1%) | 333.7 ± 1.1 (58.0 ± 0.2%) |
| Epimer | 178.5 ± 3.6 (31.1 ± 1.2%) | 115.0 ± 0.7 (20.0 ± 0.1%) | |||
| C |  |  | C | 461.5 ± 4.5 (80.3 ± 0.0%) | 339.2 ± 3.4 (59.0 ± 0.6%) |
| Epimer | 14.9 ± 0.1 (2.6 ± 0.0%) | 30.0 ± 0.5 (5.2 ± 0.1%) | |||
| EGC |  |  | EGC | 531.6 ± 9.4 (92.5 ± 1.6%) | 521.6 ± 7.1 (90.7 ± 1.2%) |
| Epimer | UD | UD | |||
| GC |  |  | GC | 557.5 ± 3.3 (97.0 ± 3.2%) | 546.7 ± 1.8 (95.1 ± 0.3%) |
| Epimer | UD | UD | |||
| ECg |  |  | ECg | 567.7 ± 1.2 (98.7 ± 0.2%) | 544.1 ± 4.1 (94.6 ± 0.7%) |
| Epimer | UD | UD | |||
| Cg | 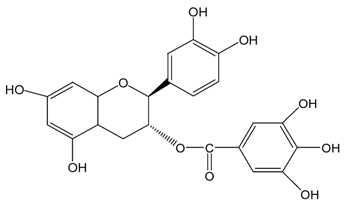 |  | Cg | 569.8 ± 3.0 (99.1 ± 0.5%) | 536.9 ± 3.8 (93.4 ± 0.7%) |
| Epimer | UD | UD | |||
| EGCg | 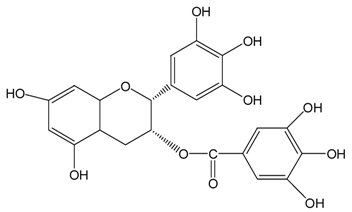 |  | EGCg | 572.4 ± 2.6 (99.6 ± 0.5%) | 525.3 ± 0.7 (91.4 ± 0.1%) |
| Epimer | UD | UD | |||
| GCg | 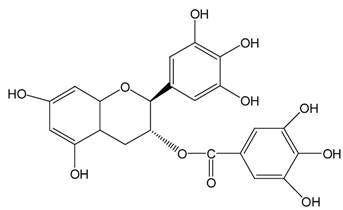 |  | GCg | 566.2 ± 0.0 (98.5 ± 0.0%) | 524.0 ± 2.8 (91.1 ± 0.5%) |
| Epimer | UD | UD |
| Treatment | EC | C | EGC | GC | ECg | Cg | EGCg | GCg |
|---|---|---|---|---|---|---|---|---|
| Before UVB radiation | 1219 ± 49 | 967 ± 48 | 1376 ± 27 | 1285 ± 7 | 1836 ± 28 | 1736 ± 43 | 2064 ± 29 | 1996 ± 53 |
| After UVB radiation | 1136 ± 33 | 921 ± 30 | 1363 ± 12 | 1274 ± 30 | 1808 ± 31 | 1745 ± 23 | 2064 ± 22 | 2026 ± 21 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Nie, Y.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R.; Ye, J.-H. Ultraviolet B (UVB) Photosensitivities of Tea Catechins and the Relevant Chemical Conversions. Molecules 2016, 21, 1345. https://doi.org/10.3390/molecules21101345
Shi M, Nie Y, Zheng X-Q, Lu J-L, Liang Y-R, Ye J-H. Ultraviolet B (UVB) Photosensitivities of Tea Catechins and the Relevant Chemical Conversions. Molecules. 2016; 21(10):1345. https://doi.org/10.3390/molecules21101345
Chicago/Turabian StyleShi, Meng, Ying Nie, Xin-Qiang Zheng, Jian-Liang Lu, Yue-Rong Liang, and Jian-Hui Ye. 2016. "Ultraviolet B (UVB) Photosensitivities of Tea Catechins and the Relevant Chemical Conversions" Molecules 21, no. 10: 1345. https://doi.org/10.3390/molecules21101345
APA StyleShi, M., Nie, Y., Zheng, X.-Q., Lu, J.-L., Liang, Y.-R., & Ye, J.-H. (2016). Ultraviolet B (UVB) Photosensitivities of Tea Catechins and the Relevant Chemical Conversions. Molecules, 21(10), 1345. https://doi.org/10.3390/molecules21101345