Abstract
Stems are the important residues of Trapa quadrispinosa Roxb., which are abundant in phenolic compounds. Ultrasonic-assisted enzymatic extraction (UAEE) is confirmed as a novel extraction technology with main advantages of enhancing extraction yield and physiological activities of the extracts from various plants. In this study, UAEE was applied to obtain the highest yield of phenolic content, strongest antioxidant, and antitumor activities and to optimize the extraction conditions using response surface methodology (RSM). The extracts from the stems of T. quadrispinosa were characterized by determination of their antioxidant activities through 2,2-azinobis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS), 1,1-Diphenyl-2-picrylhydrazxyl (DPPH) radical scavenging, total antioxidant capacity (TAC), ferric reducing antioxidant capacity (FRAC) methods and of their antitumor activity by MTT method. The selected key independent variables were cellulase concentration (X1: 1.5%–2.5%), extraction time (X2: 20–30 min) and extraction temperature (X3: 40–60 °C). The optimal extraction conditions for total phenolic content (TPC) value of the extracts were determined as 1.74% cellulase concentration, 25.5 min ultrasonic extraction time and 49.0 °C ultrasonic temperature. Under these conditions, the highest TPC value of 53.6 ± 2.2 mg Gallic acid equivalent (GAE)/g dry weight (DW) was obtained, which agreed well with the predicted value (52.596 mg GAE/g·DW. Furthermore, the extracts obtained from UAEE presented highest antioxidant activities through ABTS, DPPH, TAC and FRAC methods were of 1.54 ± 0.09 mmol Trolox equivalent (TE)/g·DW; 1.45 ± 0.07 mmol·TE/g·DW; 45.2 ± 2.2 mg·GAE/g·DW; 50.4 ± 2.6 μmol FeSO4 equivalent/g·DW and lowest IC50 values of 160.4 ± 11.6 μg/mL, 126.1 ± 10.8 μg/mL, and 178.3 ± 13.1 μg/mL against Hela, HepG-2 and U251 tumor cells, respectively. The results indicated that the UAEE was an efficient alternative to improve extraction yield and enhance the antioxidant and antitumor activities of the extracts. The phenolic extracts from the stems of T. quadrispinosa had significant antioxidant and antitumor activities, which could be used as a source of potential antioxidant and antitumor agents.
1. Introduction
Phenolic compounds, one of the most important secondary metabolites, are greatly distributed in all parts of higher plants [1]. More recently, phenolic compounds have attracted extensive attention due to their numerous biological activities, such as antioxidant, antibacterial, antitumor, anti-diabetic and anti-inflammatory activities [2,3,4,5,6]. In recent years, natural antioxidant phenolic compounds obtained from edible byproducts or residual sources have also become interesting because they could be recycled and developed into new medicinal resources [7,8,9,10].
Extraction of these bioactive phenolic compounds is the first step for their utilization and further research. Traditionally, heating, boiling or refluxing were the most used methods for obtaining these phenolic compounds from plant materials. However, the main drawback of these methods are the loss of phenolic compounds and their bioactivities due to oxidation, ionization and hydrolysis caused by long extraction time and high extraction temperature during extraction process [11,12]. In this context, other innovative extraction techniques, including ultrasonic assisted extraction (UAE), microwave assisted extraction (MAE), enzymatic assisted extraction (EAE) and pressurized assisted extraction (PAE), have been developed [13,14,15,16]. Among them, EAE is considered as a moderate, efficient, and environment-friendly extraction method, and has been proven to be effective in improving the yield of target compounds [15]. However, EAE is usually associated with longer extraction time, which could increase the processing cost [17]. As an alternative, UAE has proven to be a rapid, efficient, simple, and inexpensive method that could give higher reproducibility, greater extraction yields, higher purity of the final product, and little effect on the bioactivities of the products [18,19]. Thus, EAE coupled with ultrasound irradiation may be an effective method for target compounds extraction and many positive results for ultrasonic-assisted enzymatic extraction of target compounds have been reported [20,21,22].
In UAEE, the efficiency of extraction process is influenced by many parameters such as kinds and concentrations of enzymes, solvent composition, solvent to solid ratio, extraction temperature and extraction time. Thus, optimization of these parameters is very important for improving the extraction yields. Response surface methodology (RSM), an effective statistical technique, can investigate and optimize complex processes when the independent variables have combined effect on the response values [18]. The main advantage of RSM is to significantly reduce the number of experimental trials needed for evaluating multiple parameters and their interactions and generating a mathematical model to find the optimal values [23,24].
Trapa quadrispinosa also named Sijiaoling, belonging to Trapaceae, are regarded as a popular vegetable for its wonderful flavor and medical functions [25]. The pericarps and stems of T. quadrispinosa are commonly discarded as residues. Recent studies have reported that the pericarps exhibit remarkable antioxidant and anticancer activities on behalf of its excellent content of phenolic compounds [26,27]. However, the compositions and biological activities of the stem of T. quadrispinosa have rarely been examined.
In our previous study, ultrasonic-assisted extraction has proven to enhance the extraction yield and antioxidant activities of polysaccharides from T. quadrispinosa stems [28]. In our further study, phenolic compounds were detected in the stem of T. quadrispinosa. However, to the best of our knowledge, there have been no previous reports on the combined use of enzymes and ultrasonic-assisted extraction of phenolic compounds from the stem of T. quadrispinosa. Therefore, the aim of this current study was to investigate the feasibility of ultrasonic-assisted enzymatic extraction (UAEE) for the phenolic compounds from the stems of T. quadrispinosa and to evaluate their antioxidant and antitumor activities in vitro. Box-Behnken design (BBD) combined with response surface methodology (RSM) was employed to evaluate the effects of different operating parameters on extraction procedure and to optimize the processing conditions.
2. Results and Discussion
2.1. Single Factor Experiment
In this part, four key parameters including concentration of cellulase, ultrasonic temperature, ultrasonic time and liquid to solid ratio were picked out respectively for investigation.
2.1.1. Effect of Cellulase Concentration on Extraction of TPC
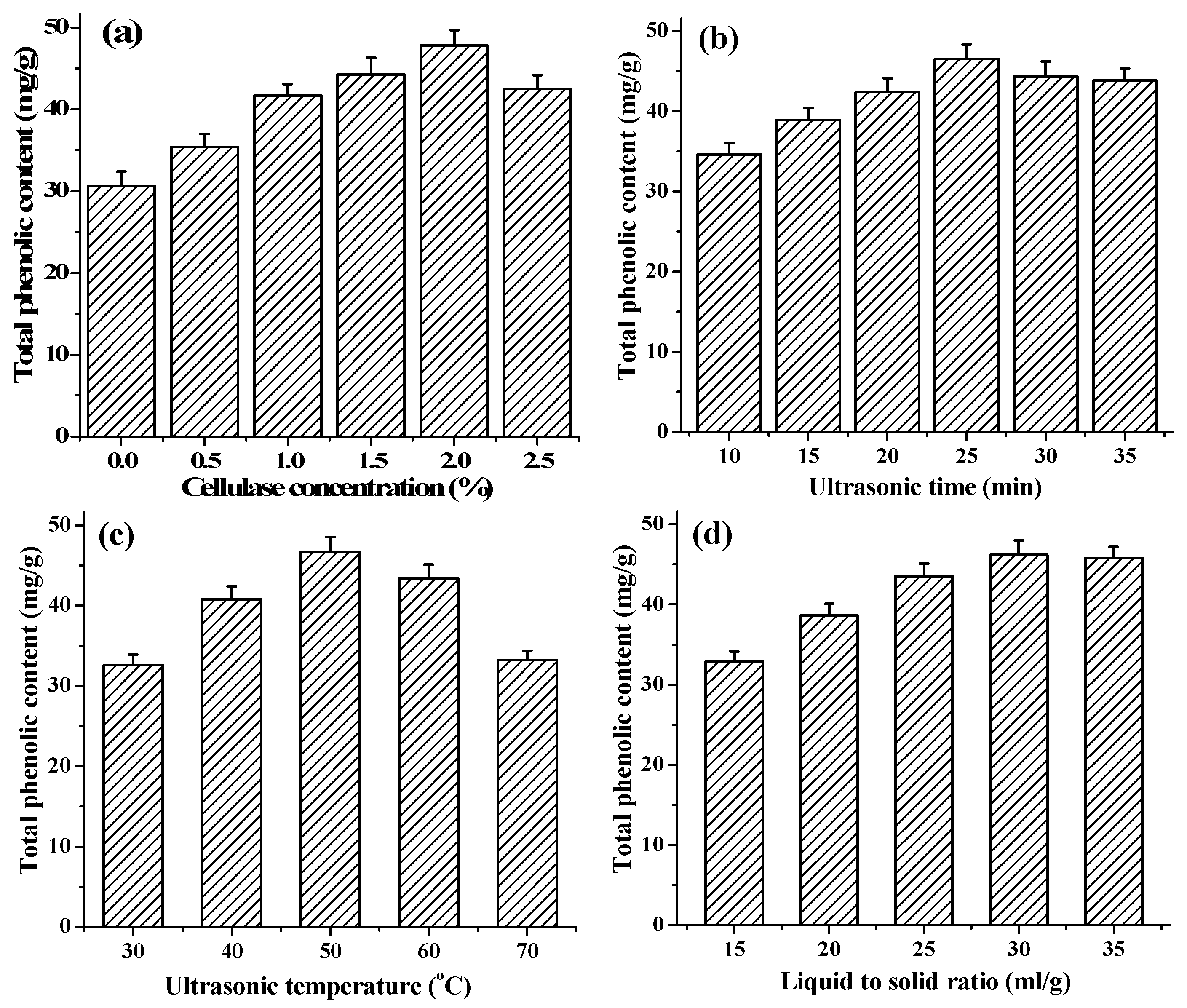
With UAEE, the enzyme degradation and destruction of the cell-wall matrix have been considered as a primary step to enhance the release of phenolic compounds, keep their stability and antioxidant activities [29]. In this study, with other extraction conditions fixed at ultrasonic time 20 min, ultrasonic temperature 50 °C and liquid to solid ratio 30 mL/g, the effect of cellulase concentrations at different levels (from 0% to 2.5%) on extraction of TPC was studied. As shown in Figure 1a, there was a notable increasing in TPC with increasing cellulase concentrations from 0% to 2.0%, beyond which the TPC value began to decrease. Similar findings were reported for flavonoid extraction from Illicium verum residues [30]. This result indicated that 2.0% cellulase could completely hydrolyze the cell walls of T. quadrispinosa stems to release the intracellular phenolic compounds. However, the viscous cellulase solution with high concentrations was not avail to the hydrolysis reaction process [30]. Therefore, 2.0% cellulase was considered the optimal enzyme concentration in further experiments.
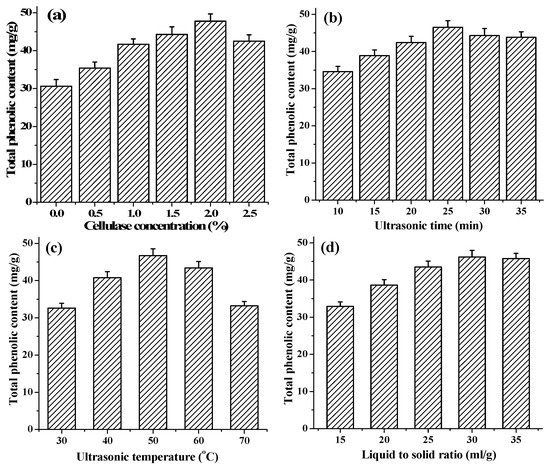
Figure 1.
Effects of different extraction parameters on the yield of phenolic extracts: (a) cellulase concentration (%); (b) extraction time (min); (c) extraction temperature (°C); and (d) liquid to solid ratio (mL/g).
2.1.2. Effect of Ultrasonic Time on Extraction of TPC
Extraction time is an important parameter that can influence the extraction efficiency. Numerous studies have demonstrated that a long extraction time presented a favorable effect on the production of phenolic compounds [31,32]. However, excessive lengthening of extraction time may induce the change of phenolic compounds molecule structure and bioactivities because of hydrolysis or oxidization [33]. To investigate the effect of ultrasonic time on extraction of TPC, different ultrasonic times, varying from 10 to 35 min, were investigated while other conditions were set as follows: cellulase concentrations 2.0%, ultrasonic temperature 50 °C and liquid to solid ratio 30 mL/g. As shown in Figure 1b, the extraction yield of TPC distinctly increased as the extraction time ascending from 10 to 25 min, the maximum yield of 46.5 ± 1.7 mg·GAE/g was obtained when the extraction time reached 25 min, and, after this point, it began to decrease slightly with a further extension of extraction time. This phenomenon might be due to the degradation of the extracted compounds with excessively lengthening of extraction time [34]. Therefore, extraction time of 25 min was adopted in the present work.
2.1.3. Effect of Ultrasonic Temperature on Extraction of TPC
Extraction temperature is another main important parameter affecting the extraction process. To investigate the effect of temperature on extraction of TPC, various temperatures within 30–70 °C were evaluated in the present study, while keeping the cellulase concentrations 2.0%, ultrasonic time 20 min, and liquid to solid ratio 30 mL/g. As shown in Figure 1c, the TPC value increased positively from 30 °C to 50 °C, and the maximum yield of 46.7 ± 1.8 mg·GAE/g was observed when extraction temperature was at 50 °C. However, the TPC value declined gradually when further increasing extraction temperature above 50 °C. This result was in line with other reports from in extracting resveratrol from Polygonum cuspidatum [35]. The decreasing the viscosity coefficient, increasing the diffusion coefficient and enhancing the solubility of phenolics and the better enzyme activities at higher temperatures caused the increase of the phenolic compounds releasing from the stems particles into extraction solution [36,37]. Furthermore, high temperature could decrease number of cavitation bubbles and weaken the impact of cavity collapse on homogenized samples [38]. Meanwhile, high temperatures could also cause thermo degradation or oxidization of phenolic compounds, and denaturalization of cellulase [37,39]. Thus, the optimal extraction temperature was adopted at 50 °C in the present work fixed as the central point for the RSM.
2.1.4. Effect of Liquid to Solid Ratio on Extraction of TPC
Different ratio of liquid to solid is another parameter that could alter the extraction yield of phenolic compounds in UAEE. In this present study, the effect of ratio of liquid to solid in the range of 15 to 35 mL/g on the extraction of TPC was investigated when other parameters were fixed as follows: extraction temperature 50 °C, extraction time 20 min, and cellulase concentration 2.0%, respectively. As shown in Figure 1d, the TPC increased with increasing of ratio of liquid to solid and reached the peak value of 46.2 ± 1.9 mg·GAE/g at liquid to solid ratio of 30 mL/g, and then no longer changed along with the extraction process. The effect of liquid to solid ratio on the yield was not significant (p > 0.05). Therefore, this factor was fixed at 30 mL/g and ignored in further experiment.
2.2. Response Surface Methodology
2.2.1. The Results in the BBD Experiments
The experimental and predicted values of TPC are listed in Table 1. Among the 15 experiments including three replicates, the TPC values ranged from 44.6 mg·GAE/g·DW to 52.7 mg·GAE/g·DW, and experiment 13 (cellulase concentration 2.0%, ultrasonic time 25 min and ultrasonic temperature 50 °C) has the greatest TPC (52.7 mg·GAE/g·DW) and experiment 8 (cellulase concentration 2.5%, ultrasonic time 25 min and ultrasonic temperature 60 °C) has the smallest (44.6 mg·GAE/g·DW).

Table 1.
Box-Behnken design with independent variables and its experimental response values.
2.2.2. Fitting the Model
Based on the experimental data, the obtained model, which shows the relationship between the TPC value of the extracts and the extraction parameters, is presented in the following equations:
YTPC = 52.33 − 0.82X1 + 0.33X2 − 0.90X3 + 0.10X1X2 − 0.55X1X3 − 0.30X2X3 − 0.74X12 − 1.591X22 − 4.54X32
The statistical significance of the regression model was checked by F-test and p-value, and the analysis of variance (ANOVA) for the response surface quadratic model, as shown in Table 2. According to the results, it was observed that quadratic polynomial models were highly significant with the low p-values of <0.0001 and the high F values of 74.90 which suggested that the fitness of this model was very high. The high value of determination coefficient (R2) and adjusted determination coefficient (0.9926 and 0.9794, respectively), very close to 1.0, indicated that there was a good correlation between the experimental and predicted values and only 2.06% of the total variations could not be explained by this model. A relatively low value of C.V. (the coefficient of variation) indicated a better reliability of the experiments values [40]. In this study, C.V. value of 0.78% suggested that the model was accurate and reproducible. Significance of the model was also determined by lack-of-fit test. As shown in Table 2, the F-value of 0.80 and p-value of 0.5986 suggested that it was not significant and a 59.86% chance could occur due to noise.

Table 2.
Analysis of variables for regression model of response in extraction conditions.
The p-value was used as a tool to examine the significance of each coefficient, and the smaller of the p-value was, the more significant the corresponding coefficient was [41]. It can be seen very clearly in Table 2 that the TPC value was significantly influenced by two linear coefficients (X1 and X3), three quadratic coefficients (X12, X22, and X32) and one cross product coefficient (X1X3), with small p-values less than 0.05. However, other term coefficients did not significantly influence the TPC value with higher p-values (p values > 0.05).
2.2.3. Analysis of Response Surfaces and Contours
Response surface plots (3D) and contour plots (2D) were represented to evaluate the effects of the independent variables and their interactions on the extraction of TPC. The shapes of the contour plots (circular or elliptical) indicate whether the mutual interactions between the variables were significant. A circular contour plot indicated that the interactions between the corresponding variables were negligible, and an elliptical contour plot indicated that the interactions between the corresponding variables were significant [42].
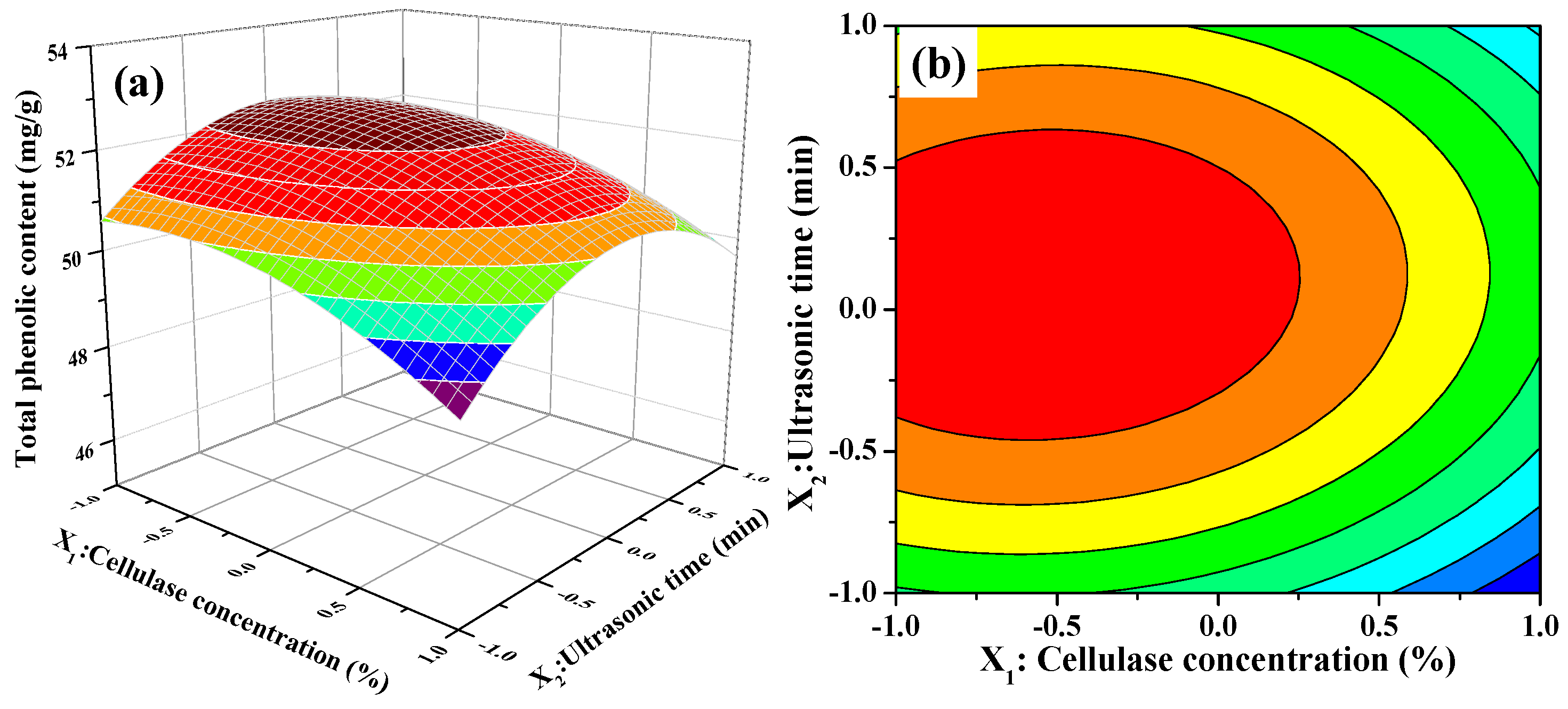
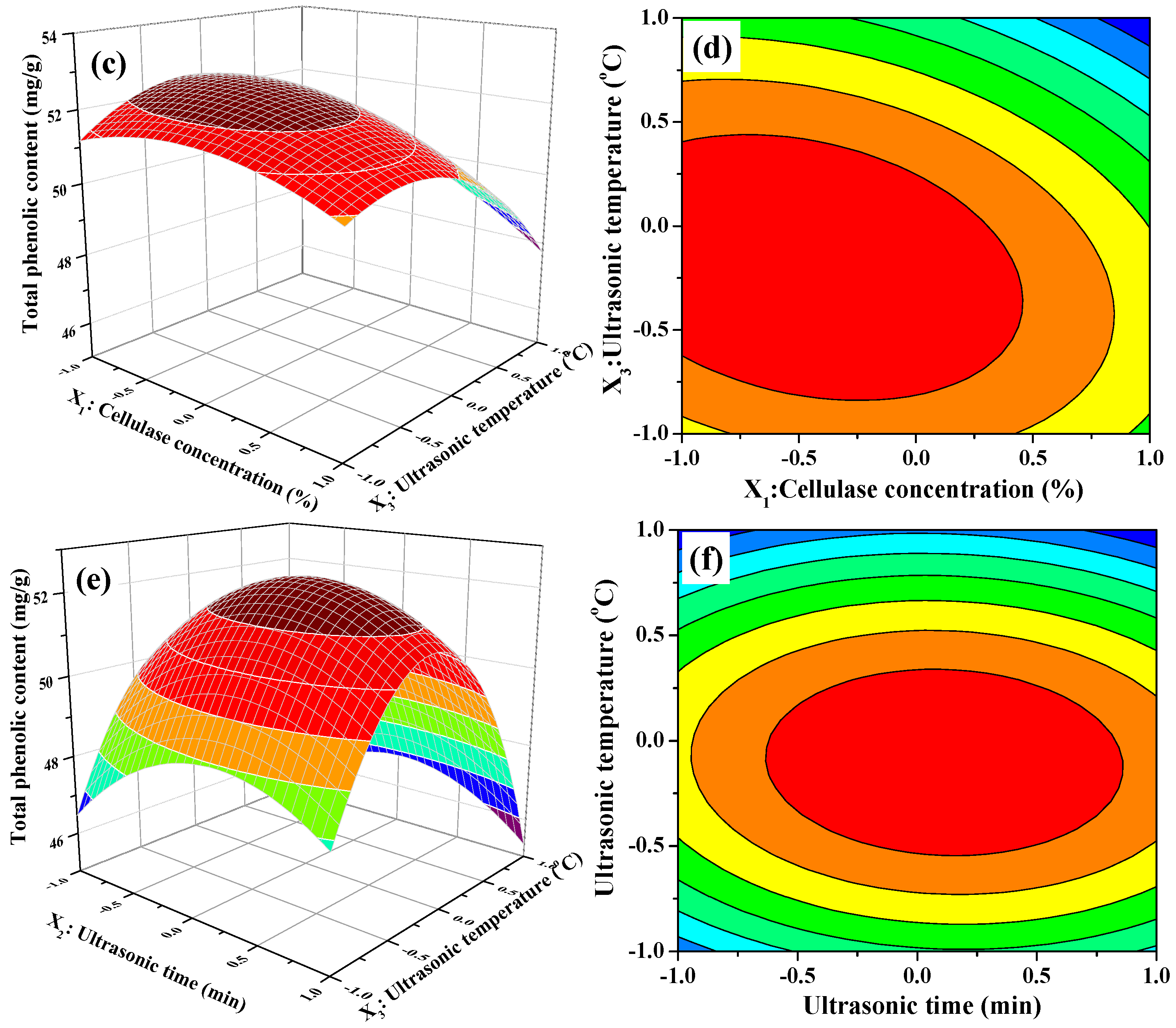
The 3D response surface and 2D contour plots about the relationship between extraction parameters and the value of TPC are presented in Figure 2. Figure 2a,b illustrates the effect of cellulase concentration and ultrasonic time on the TPC value when ultrasonic temperature was fixed at 50 °C (0 level). The extraction of TPC increased rapidly with increasing cellulase concentration from 1.5% to 1.75%, increased slowly with increasing of ultrasonic time from 20 to 26 min, and then followed by a decline with further increase. In Figure 2b, we also observe that the curved surface of cellulase concentration was steeper than the curved surface of the ultrasonic time. The results indicated that both cellulase concentration and ultrasonic time had quadratic effects on TPC value and the influence of cellulase concentration on the extraction of TPC was greater than that of ultrasonic time. Figure 2c,d displays the interaction between cellulase concentration and ultrasonic temperature on the TPC value when ultrasonic time was fixed at 25 min (0 level). When extraction temperature was kept at a lower level, the TPC value initially increased and then decreased slowly with increasing cellulase concentration. Nevertheless, the TPC value significantly decreased when extraction temperature was more than 50 °C. Furthermore, the response value of TPC was influenced significantly by the interaction between the cellulase concentration and ultrasonic temperature because of the elliptical contour shape of the 2-D contour plot. Figure 2e,f describes the interaction between ultrasonic temperature and ultrasonic time on the TPC value at a fixed cellulase concentration of 2.0% (0 level). As presented in Figure 2e, the 3D response surface, the TPC value was very relate to the quadratic effects of ultrasonic temperature and ultrasonic time. The TPC value increased with increasing of ultrasonic temperature or ultrasonic time. However, beyond 0 level, the TPC value decreased obviously with increasing ultrasonic temperature or ultrasonic time when the other one was set. It meant that too low or too high level of ultrasonic temperature or ultrasonic time could decrease the extraction yield and the moderate level of these two extraction parameters resulted in high TPC value. On the other hand, the mutual interaction between ultrasonic temperature and ultrasonic time was not significant demonstrated by the 2-D contour plot shown in Figure 2f.
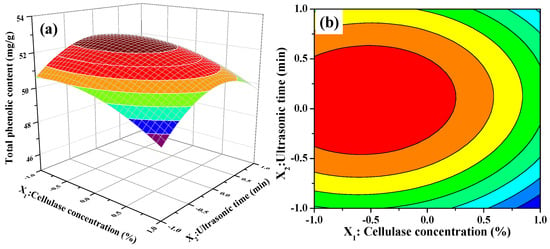
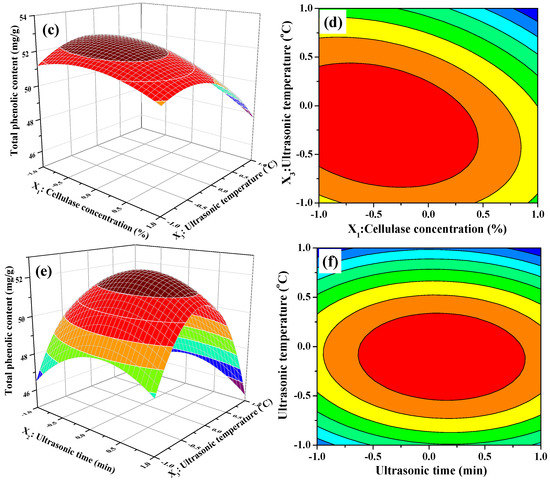
Figure 2.
Contour (a,c,e); and response surface (b,d,f) plots for interactions between three independent extraction parameters on the extraction yields of phenolic extracts (X1: Cellulase Concentration; X2: Ultrasonic Time; and X3: Ultrasonic Temperature).
2.2.4. Verification Experiments
Base on the built mathematical models, the optimal experimental conditions of UAEE for extraction of TPC from the stem of T. quadrispinosa was obtained as follows: concentration of cellulase, 1.74%; extraction time, 25.45 min; and extraction temperature, 49.3 °C. Under the optimal parameters, the predictive value of extraction yields was 52.596 mg·GAE/g·DW. Because the values of extraction time and extraction temperature were difficult to operate in the actual extraction experiments, the actual optimal parameters were carried out with slight modifications: concentration of cellulase, 1.74%; extraction time, 25.5 min; and extraction temperature, 49.0 °C. The experimental value of 53.6 ± 2.2 mg·GAE/g·DW agreed well with the predicted values, which confirmed that the response model was adequate to reflect the expected optimization.
2.3. Analysis of Microscopic Changes
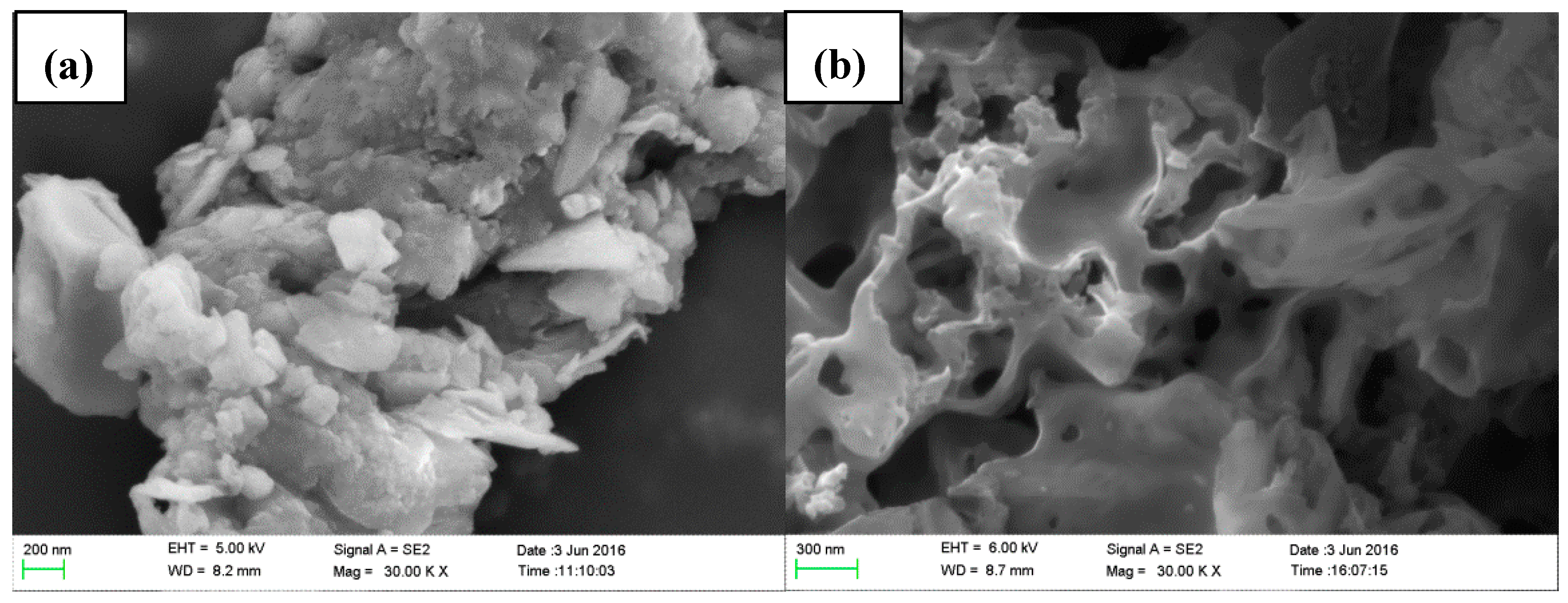
In order to study the influence of UAEE on the physical changes of the extracted tissue surface and structural features, SEM analysis was applied for observing the microscopic changes. As shown in Figure 3a, it was obvious that there was a complete parenchyma and no destroys on cell walls for the untreated samples. However, samples subjected to UAEE had broken and damaged tissues and many large perforations on the external surfaces were observed (see Figure 3b). These changes could be attributed to the combined effects of cavitation effect brought by ultrasonic vibration and enzymolysis effect provided by cellulase [20].

Figure 3.
Scanning electron microscopic images of residues in the extraction of T. quadrispinosa stems: (a) untreated; and (b) treated by UAEE.
2.4. Comparison of UAEE with Other Extraction Methods
2.4.1. Total Phenolic Content
The efficiency of the TPC value by UAEE and other extraction methods were compared, and the extraction conditions and TPC values used are shown in Table 3. As shown in Table 3, the UAEE method had the highest extraction yield of TPC (53.6 ± 2.2 mg·GAE/g·DW), and the HE had a lowest extraction yield of TPC (42.4 ± 1.3 mg·GAE/g·DW). Compared with HE, UAE and EAE, UAEE had lowest extraction temperature and shortest extraction time with highest extraction yield. The order of yield of TPC extracted with different methods was similar to the results of former studies, which demonstrated that UAEE is more efficient than the three other methods, and is a promising extraction method [21,43].

Table 3.
Comparison of experimental results obtained from different extraction methods.
2.4.2. Antioxidant Activity of Phenolic Extracts
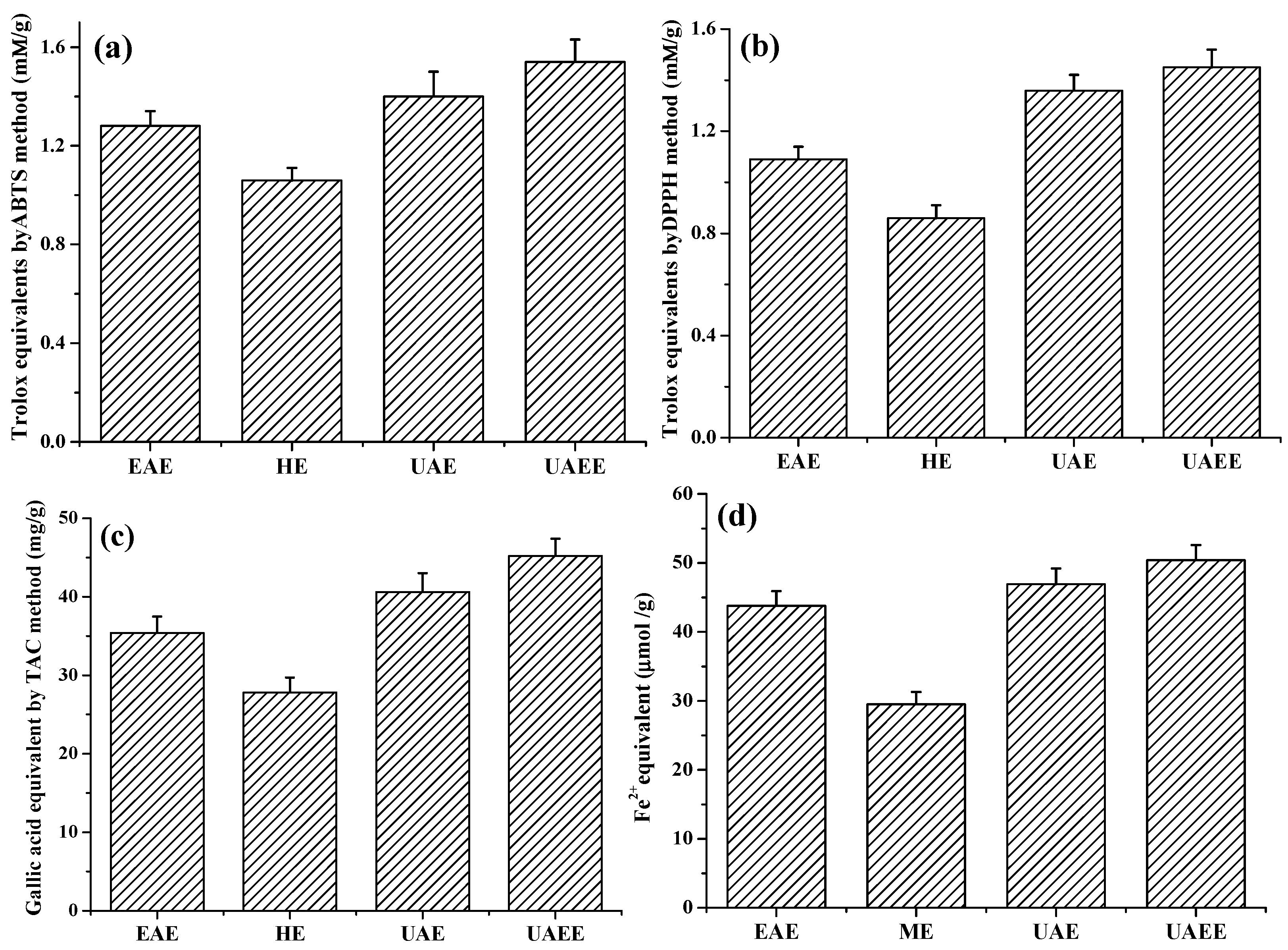
Four methods, ABTS, DPPH TAC and FRAC, were chosen to determine the antioxidant capacity of extracts obtained from different extraction methods. The results are shown in Figure 4. As shown in Figure 4, all of the four values exhibited the similar behaviors: UAEE yielded the highest antioxidant activities of 1.54 ± 0.09 mmol·TE/g·DW, 1.45 ± 0.07 mmol·TE/g·DW, 45.2 ± 2.2 mg·GAE/g·DW and 50.4 ± 2.6 μmol FeSO4 equivalent/g·DW by ABTS, DPPH, TAC, and FRAP methods respectively. Conversely, HE method yielded the lowest antioxidant activities of 1.28 ± 0.06 mmol·TE/g·DW, 1.09 ± 0.05 mmol·TE/g·DW, 27.8 ± 1.9 mg·GAE/g·DW and 29.5 ± 1.8 μmol FeSO4 equivalent/g·DW, respectively.
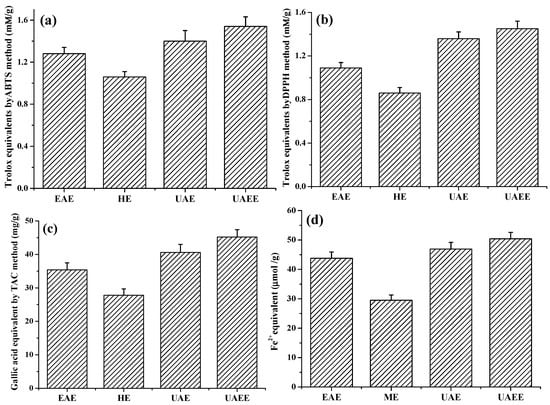
Figure 4.
Antioxidant activity of phenolic extracts obtained from different methods: (a) ABTS (mmol Trolox equivalent/g dry weight); (b) DPPH (mmol Trolox equivalent/g dry weight); (c) FRAP (mg Gallic acid equivalent/g dry weight); and (d) FRAC (μmol FeSO4 equivalent/g dry weight).
The above results indicated that the increasing of antioxidant activities of extracts by UAEE process was attributed to the enhancement of the extraction efficiency of phenolic compounds from T. quadrispinosa stem residues.
2.4.3. Antitumor Activity of Phenolic Extracts
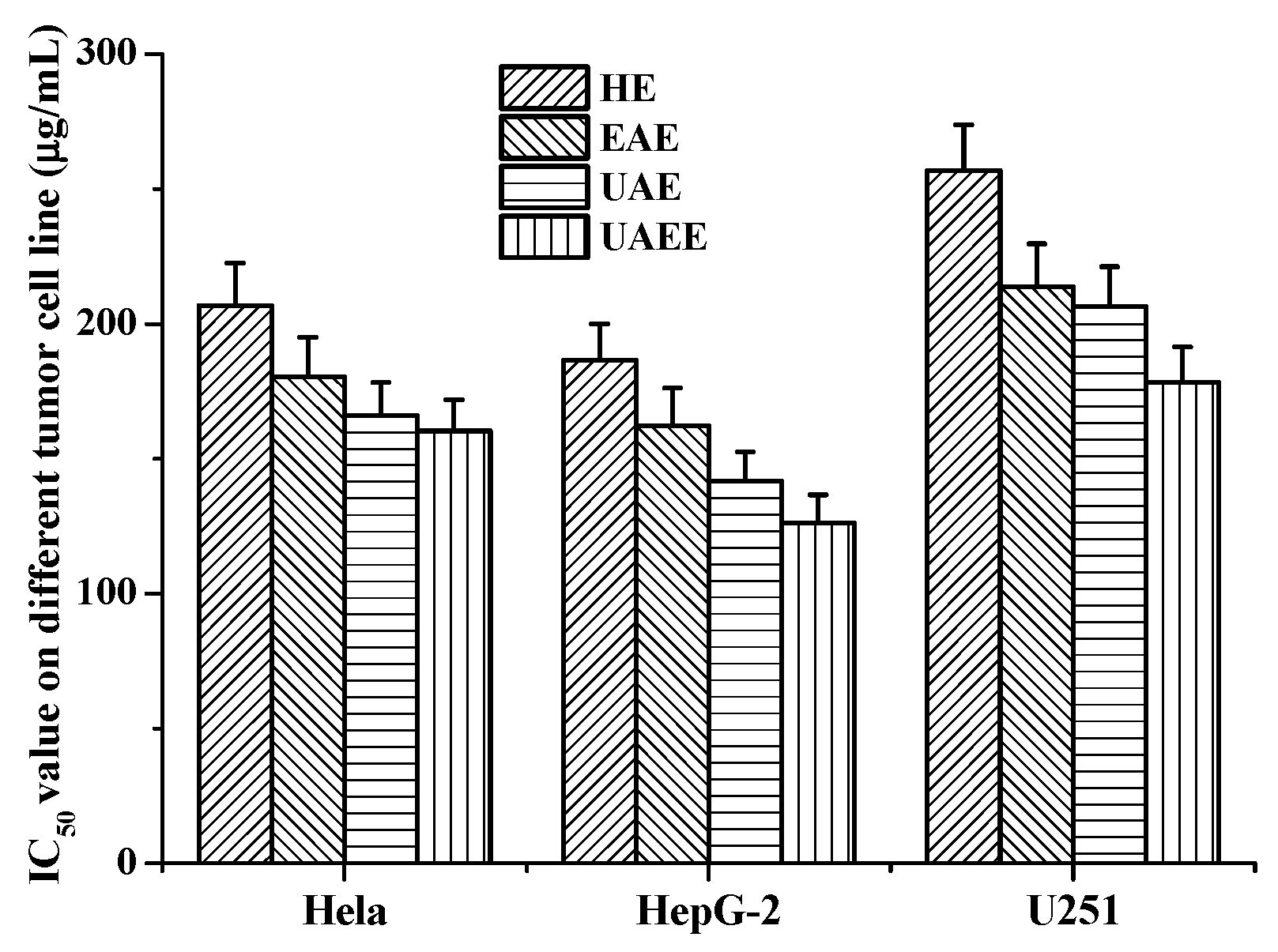
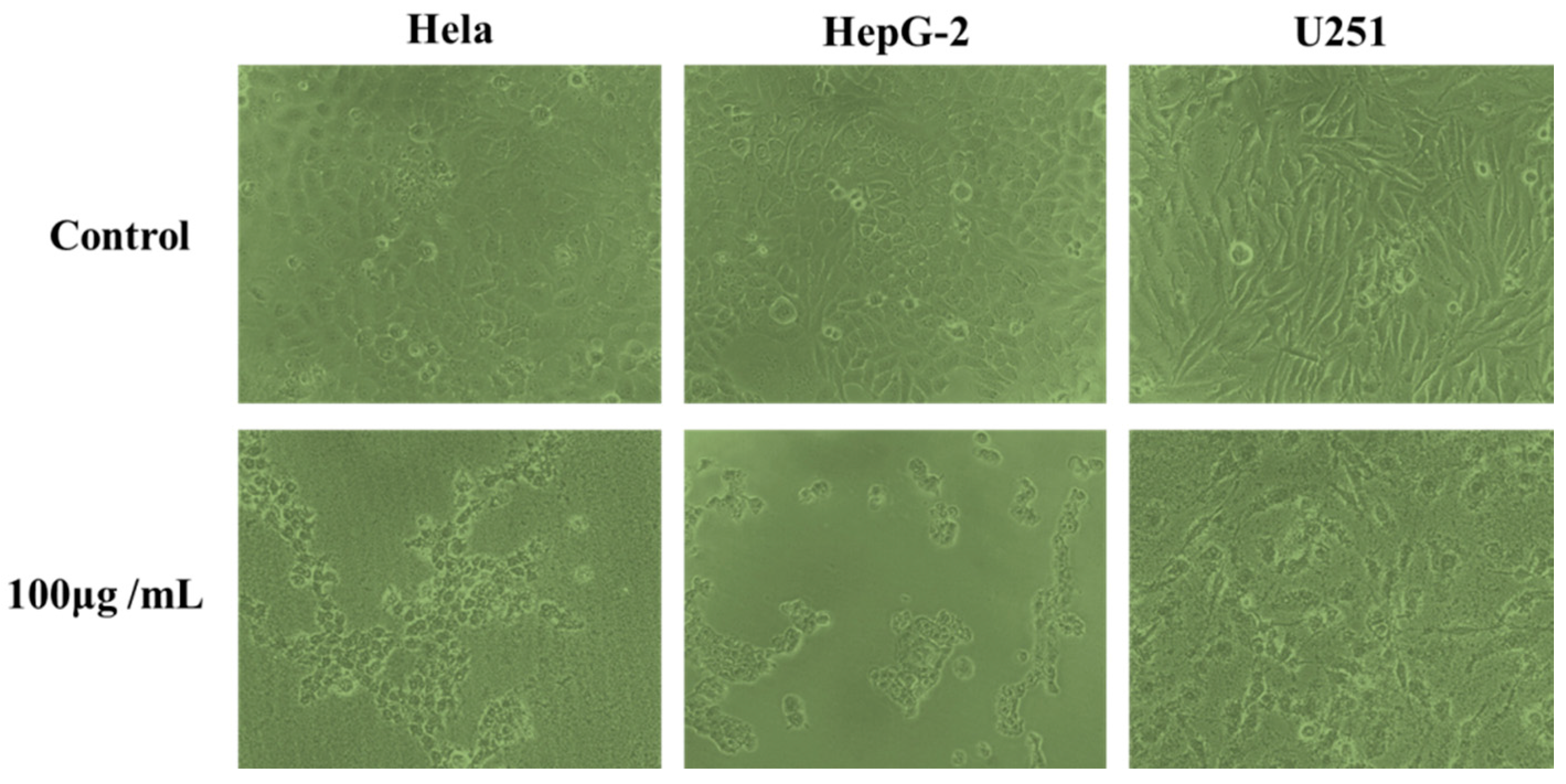
The effects of phenolic extracts obtained from different methods on the growth of Hela, HepG-2 and U251cells were investigated using the MTT assay. The results of growth inhibitory effects of different phenolic extracts, with IC50 values, are shown in Figure 5, which very clearly indicates all phenolic extracts exhibited high growth inhibitory effects on cell viability of Hela, HepG-2 and U251 cells. Among them, the phenolic extracts obtained from UAEE had greatest inhibitory effect on the growth of cells compared to other methods with the lowest IC50 values of 160.4 ± 11.6 μg/mL, 126.1 ± 10.8 μg/mL, 178.3 ± 13.1 μg/mL against Hela, HepG-2 and U251 tumor cells, respectively. However, phenolic extracts obtained from HE had weakest inhibitory effect on the growth of cells with the highest IC50 values of 206.8 ± 15.6 μg/mL, 186.5 ± 13.5 μg/mL, and 256.9 ± 16.9 μg/mL against Hela, HepG-2 and U251 tumor cells, respectively. To further confirm the proliferation inhibitory effects on Hela, HepG-2 and U251 cells, the morphology changes induced by phenolic extracts (100 μg/mL) obtained from UAEE were investigated. As illustrated in Figure 6, the morphology of the control untreated cell was regular and intact, whereas cells treated with phenolic extracts exhibited obvious signs of growth inhibition, including decreased cell number, diminished cell volume, and increased cell shrinkage. At the same time, some cells gathered together and dead cells or apoptotic cells appeared. This revealed that phenolic extracts of T. quadrispinosa stems could affect cell proliferation and survival which was consistent with the cell viability assay results.
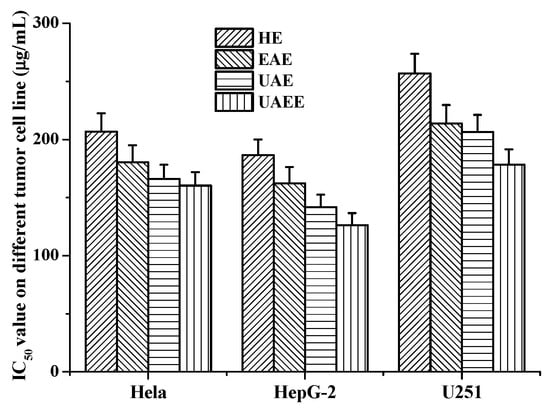
Figure 5.
Antitumor activities (IC50 μg/mL) of phenolic extracts obtained from different extraction methods on the Hela, HepG-2 and U251 cells.
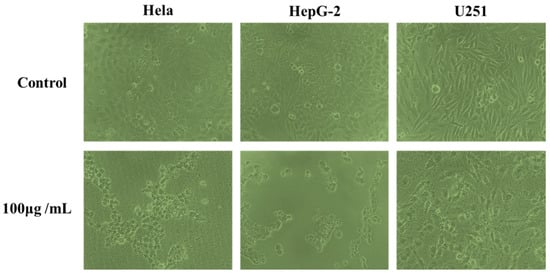
Figure 6.
Morphological changes of Hela, HepG-2 and U251 cells treated for 24 h with 100 μg/mL of phenolic extracts of T. quadrispinosa stem obtained by UAEE.
3. Material and Methods
3.1. Plant Material and Chemical Reagents
The stems of T. quadrispinosa were collected from Weishan Lake, Weishan County, Shandong Province, China. The air-dried stems were ground and screened through a 40 mesh sieve for further extraction.
1,1-Diphenyl-2-picrylhydrazxyl (DPPH), 2,2-azinobis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and Folin–Ciocalteu’s phenol reagent were obtained from Sigma Chemicals Co. (St. Louis, MO, USA). Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), Penicillin and Streptomycin were purchased from Gibco Laboratories (Grand Island, NY, USA). Cellulase (EC 3.2.1.1, 400 U/mg), Gallic acid, Sodium carbonate, Iron sulfate heptahydrate, Iron chloride hexahydrate, Ammonium molybdate, Sodium phosphate, Potassium ferricyanide and 1,10-phenanthroline were provided from TianKe Co., Ltd. (Suzhou, China). Ethanol and methanol were obtained from Kelong Chemical Factory (Chengdu, China). All other chemical reagents used in this experiment were of analytical grade and doubly distilled water was used throughout the experiment.
3.2. Ultrasonic-Assisted Enzymatic Extraction
The UAEE method has been previously described in the study on the extraction of polyphenols from waste peanut shells [44]. Extractions were carried out in a temperature and time controlled ultrasonic cleaner (KQ-250DB, Kunshan Ultrasonic Co. Ltd., Kunshan, China) with a fixed frequency of 40 kHz and an alterable electric powder range from 100 W to 250 W. The internal tank of the ultrasonic cleaner was 300 mm × 240 mm × 150 mm (L × W × H). In this study, 30% ethanol with pH value of 5.0 adjusted by HCl was chosen as extraction solvent. The stem powders (1.0 g) were placed into 100 mL Erlenmeyer flask. Next, the flask was fixed in the same position in water (the water depth fixed as 100 mm) in the ultrasonic cleaner at 40 kHz, 250 W and extracted according to the experiment design. The designed extraction temperature was adjusted by the temperature controller, and the real temperature of the solution was monitored by a thermometer. After extraction, the mixtures were centrifuged at 4000 rpm for 10 min, and the supernatants were collected for the total phenolic content and further antioxidant and antitumor activity determinations.
3.3. Experimental Design
3.3.1. Single-Factor Experiment
To evaluate the effect of each factor on the extraction yield from the T. quadrispinosa stems, different cellulase concentration (from 0% to 2.5%), ultrasonic time (from 10 to 35 min), ultrasonic temperature (from 30 to 70 °C) and ratio of liquid to solid (from 15 to 35 mL/g) were investigated as single factors.
3.3.2. Response Surface Methodology Experiment
According the single-factor experimental results, a Box-Behnken Design (BBD) with response surface methodology (RSM) was applied to further determine the optimal UAEE conditions. The levels of the three independent variables were confirmed cellulase concentration (X1, 1.5%–2.5%), ultrasonic time (X2, 20–30 min) and ultrasonic temperature (X3, 40–60 °C) related to the response yield of total phenolic content (mg Gallic acid equivalent/g dried weight). The coded and uncoded (actual) levels of the independent variables were given in Table 4. A quadratic polynomial model performed based on experimental data from CCD was explained by the following quadratic equation:
where Y is response, A0 is intercept, Ai is coefficient of variable for linear, Aii is coefficient of variable for quadratic, and Aii is coefficient of variable for interaction term. Xi and Xj are independent variables.

Table 4.
Independent variables and their levels used for the Box-Behnken design.
The experimental data were analyzed using a statistical package, Design-Expert version 8.0.5b, (Stat-Ease Inc., Minneapolis, MN, USA). The adequacy of the established model and statistical significance of the regression coefficients was evaluated by the lack of fit, coefficient of determination (R2), and Fisher test value (F-value) generated from the ANOVA analysis (p < 0.05).
3.4. Scanning Electron Microscopy Analysis
In order to investigate the effect of UAEE on the microstructure of samples, the untreated sample as well as residue after extraction of phenolics were collected and air-dried for the scanning electron microscopy (SEM) analysis. The powders were fixed on the aluminum stubs with adhesive tape and covered with gold as a sputter coater. The shape and the surface characters of the powders were examined with a Sigma 500/VP SEM, under high vacuum conditions at a voltage of 10.0 kV.
3.5. Comparison with Other Extraction Procedures
3.5.1. Heat Extraction (HE)
T. quadrispinosa stem powders (1.0 g) was mixed with 30 mL of 30% ethanol in a 100 mL Erlenmeyer flask. For the extraction, the flask was placed into a water-bath and connected with cooling water, and then allowed to reflux extraction for 120 min at 80 °C.
3.5.2. Ultrasonic-Assisted Extraction (UAE)
T. quadrispinosa stem powders (1.0 g) was mixed with 30 mL of 30% ethanol and put into a 100 mL Erlenmeyer flask and was then extracted in an ultrasonic cleaner for 30 min at 50 °C.
3.5.3. Enzyme-Assisted Extraction (EAE)
T. quadrispinosa stem powders (1.0 g) was mixed with cellulase solution (2.0%) dispersed in 30 mL of 30% ethanol with pH value of 5.0 in a 100 mL Erlenmeyer flask. For the extraction, the flask was placed into a water-bath, and then allowed to incubation extraction 180 min at 50 °C.
3.6. Determination of Total Phenolic Content
The total phenolic content of the extracts was determined by using Folin–Ciocalteu’s reagent described previously [45] with Gallic acid as a standard. Briefly, 200 μL of each suitable diluted extracts was mixed with 0.5 mL of Folin–Ciocalteu’s reagent in 10 mL glass tube. After incubation for 2 min at room temperature, 2 mL of 20% sodium carbonate solution was added and mixed thoroughly. The reaction solution was then brought up to a final volume of 10 mL with distilled water and incubated for 120 min in dark at room temperature. The absorbance of samples was measured at 760 nm. The total phenolic content was expressed as mg of Gallic acid equivalent per gram of dry weight (mg·GAE/g·DW) based on the calibration curve (Y = 0.0148X − 0.0039, R2 = 0.9993).
3.7. Evaluation of Antioxidant Capacity
ABTS, DPPH TAC and FRAC methods, utilizing the same single electron transfer mechanism, are commonly applied to determine the antioxidant capacity of biological compounds. These four methods were adopted in this study to assess the antioxidant activity of the phenolic extracts from the stems of T. quadrispinosa.
3.7.1. ABTS Method
The capacity of the extracts scavenging ABTS•+ was carried out according to the procedure described by Sariburun E. [46] with small modification. The radical of ABTS•+ was prepared by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulfate in dark conditions at room temperature for 12–16 h. The ABTS•+ working solution was obtained by diluting the stock solution with deionized water to an absorbance of 0.7 ± 0.02 at 754 nm. In experiment, 100 μL of suitable diluted extracts was mixed thoroughly with 2.9 mL of diluted ABTS solution, the absorbance of the mixture was measured at 754 nm against blank control after incubation for 6 min at room temperature. Trolox was used as the standard and the results were expressed as mmol Trolox equivalents per gram of dry weight (mmol·TE/g·DW) based on the calibration curve (Y = 0.01102X − 0.0222, R2 = 0.9989).
3.7.2. DPPH Method
The DPPH radical scavenging capacity of the extracts was performed using a method described elsewhere [47] with slight modification. A 100 μL of suitable diluted extracts was taken into 10 mL glass tubes and mixed with 2.9 mL of freshly prepared 0.1 mmol DPPH methanol solution. With the mixtures being stood for 30 min at room temperature in dark, the absorbance was measured at 517 nm. The antioxidant capacity was expressed as mmol Trolox equivalents per gram of dry weight (mmol·TE/g·DW) based on the calibration curve (Y = 0.00878X − 0.0006, R2 = 0.9998).
3.7.3. TAC Method
The total antioxidant captivity (TAC) of the extracts were evaluated by the phosphomolybdenum method according to the procedure described previously [48] with some modification. A 200 μL of each suitable diluted extracts was mixed with 3.0 mL of complex reagent solution (including 0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) in 10 mL glass tubes. The reaction solution were incubated at 95 °C for 90 min and then cooled to room temperature. Finally, the absorbance of the solution was measured at 695 nm. The total antioxidant captivity was expressed as mg Gallic acid equivalents per gram of dry weight (mg·GAE/g·DW) based on the calibration curve (Y = 0.009X + 0.06, R2 = 0.9992).
3.7.4. FRAC Method
The Ferric reducing antioxidant capacity of extract was determined according to the method of 1,10-phenanthroline [49] with minor modification. Briefly, 200 μL of each suitable diluted extracts was mixed with 0.5 mL of 0.5% 1,10-phenanthroline methanol solution and 1 mL of 0.2% FeCl3 methanol solution in 10 mL glass tubes. The reaction solution was then brought up to a final volume of 10 mL with methanol and kept at room temperature in dark for 20 min. Finally, the absorbance of the solution was measured at 510 nm. The result was expressed as μmol of Fe(II) per gram of weight material (μmol·Fe2+/g·DW) based on the calibration curve of FeSO4 (Y = 0.01054X − 0.0097, R2 = 0.9993).
3.8. Evaluation of Antitumor Capacity
3.8.1. Cell Culture
The cell lines of Hela, HepG-2 and U251 used in this study were kindly provided by Prof. Jing Gao (School of Pharmacy, Jiangsu University, Su Zhou, China). The cells were cultured in DMEM mediums containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin under 37 °C in a humidified atmosphere with 5% CO2. Cells in log phase were used for experiments.
3.8.2. MTT Cell Proliferation Assay
The cells viability was assessed by MTT colorimetric assay [50]. Briefly, cells were plated into 96-well cell culture plates at an optimum density of 1 × 105 cells/well and incubated for 24 h, and then the cells were treated with serial concentration of the phenolic extracts (50–1000 μg/mL) obtained from different extraction methods for another 24 h. Following treatment, 10 μL of MTT (5 mg/mL) in physiological buffered saline (PBS) was added into each well, and incubated for further 4 h. After adding 150 μL of DMSO to dissolve formed formazan crystals, the absorbance of each well was measured at 570 nm using ELISA reader. The percentage cell viability was calculated using the formulae below: cell viability (%) = OD570(Treated sample)/OD570(Untreated control) × 100. The IC50 values were determined using Graph Pad Prism software (Graph Pad software, 5.0.1, San Diego, CA, USA).
3.8.3. Morphological Evaluation
The cells were incubated in 6-well cell culture plates at a cell density of 2 × 105 cells per well for 24 h. After treatments with 100 μg/mL of the phenolic extracts obtained from UAEE for the subsequent 24 h, the images of the cells were visualized at a 20× magnification using a Nikon microscope (Tokyo, Japan) fitted with a Leica digital camera. Untreated cells were set as a negative control.
3.9. Statistical Analysis
All results were subjected to statistical analyses. All data were expressed as means ± SD obtained from triplicate experiments. Statistical analysis was performed using the software of Statistical Analysis System Version 8.0 (SAS 8.0, Stat-Ease Inc., Minneapolis, MN, USA). Differences were considered statistically significant at p < 0.05.
4. Conclusions
In this study, an ultrasonic-assisted enzymatic extraction method was developed for the extraction of phenolic compounds from the stems of T. quadrispinosa, and the optimal extraction conditions were obtained by response surface methodology. The high correlation (R2 = 0.9926) of the model indicated that the second-order polynomial model could successfully express the influence of independent variables on the response. The optimal UAEE conditions (cellulase concentration of 1.74%, ultrasonic extraction time of 25.5 min and ultrasonic temperature of 49.0 °C) for the extraction process resulted the yield of 53.6 ± 2.2 mg·GAE/g·DW. The extracts obtained from UAEE presented highest antioxidant activities through ABTS, DPPH, TAC and FRAC methods, and were 1.54 ± 0.09 mmol Trolox equivalent (TE)/g·DW, 1.45 ± 0.07 mmol·TE/g·DW, 45.2 ± 2.2 mg·GAE/g·DW, and 50.4 ± 2.6 μmol FeSO4 equivalent/g·DW, respectively, and had the lowest IC50 values of 160.4 ± 11.6 μg/mL, 126.1 ± 10.8 μg/mL, and 178.3 ± 13.1 μg/mL against Hela, HepG-2 and U251 tumor cells, respectively, compared with other extraction methods including heat extraction, ultrasonic-assisted extraction and enzyme-assisted extraction. The results evidence that UAEE combined with RSM is an effective technique for extraction of phenolic compounds from the stems of Trapa quadrispinosa Roxb. The knowledge obtained from this study should be helpful for further exploitation and application of this resource.
Acknowledgments
The financial support for this investigation was given by National Natural Science Foundation of China (81402840); Natural Science Foundation of Jiangsu Province, China (BK20130495); Natural Science Foundation of Jiangsu province administration of traditional Chinese, China (LZ13244); Social Development Foundation of Zhenjiang City, China (SSH20140147); Doctoral Innovation Project of Jiangsu province, China (CXLX13-686); and Undergraduate Student Innovation Project (2015A154) of Jiangsu University, China.
Author Contributions
X.-Q.X. and L.-P.Q. conceived and designed the experiments; F.L., Y.-D.M. and Y.-F.W. performed the experiments; A.R. and X.-Q.X. analyzed the data and wrote the paper; X.-Q.X. and A.R. contributed to valuable discussion and revising manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ananga, A.; Georgiev, V.; Tsolova, V. Manipulation and engineering of metabolic and biosynthetic pathway of plant polyphenols. Curr. Pharm. Des. 2013, 19, 6186–6206. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi, S.; Jayalakshmi, S.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification and their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xie, H.; Xu, X.; Liang, Y.; Wei, X. Phenolic constituents from isodon lophanthoides var. Graciliflorus and their antioxidant and antibacterial activities. J. Funct. Foods 2014, 6, 492–498. [Google Scholar] [CrossRef]
- Yi, J.; Wang, Z.; Bai, H.; Yu, X.; Jing, J.; Zuo, L. Optimization of purification, identification and evaluation of the in vitro antitumor activity of polyphenols from pinus koraiensis pinecones. Molecules 2015, 20, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Nishi, K.; Yasunaga, S.; Muranaka, A.; Maeyama, K.; Kadota, A.; Sugahara, T. Nobiletin, a polymethoxy flavonoid, exerts anti-allergic effect by suppressing activation of phosphoinositide 3-kinase. J. Funct. Foods 2014, 6, 606–614. [Google Scholar] [CrossRef]
- Nahar, P.P.; Driscoll, M.V.; Li, L.; Slitt, A.L.; Seeram, N.P. Phenolic mediated anti-inflammatory properties of a maple syrup extract in raw 264.7 murine macrophages. J. Funct. Foods 2014, 6, 126–136. [Google Scholar] [CrossRef]
- Gómez-García, R.; Martínez-Ávila, G.C.; Aguilar, C.N. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis Vinifera L.) residues. 3 Biotech 2012, 2, 297–300. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Michel, I.; Tessaro, I.C.; Marczak, L.D.F. Optimization of phenolics extraction from sesame seed cake. Sep. Purif. Technol. 2014, 122, 506–514. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus Fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Q.; Zhang, C.; Cao, W.; Fan, D.; Yang, H. Extraction optimization of polyphenols from waste kiwi fruit seeds (Actinidia Chinensis Planch.) and evaluation of its antioxidant and anti-inflammatory properties. Molecules 2016, 21, 832. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, B.; Yao, S. Application of ultrasonic technique for extracting chlorogenic acid from eucommia ulmodies oliv.(e. Ulmodies). Ultrason. Sonochem. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Odabaş, H.İ.; Koca, I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind. Crops Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.; Wong-Paz, J.E.; Belmares-Cerda, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Ultrasound-assisted extraction of phenolic compounds from laurus nobilis l. And their antioxidant activity. Ultrason. Sonochem. 2013, 20, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yan, J.; Liu, R.; Marcone, M.F.; Aisa, H.A.; Tsao, R. Optimization of microwave-assisted extraction of phenolics from potato and its downstream waste using orthogonal array design. Food Chem. 2012, 133, 1292–1298. [Google Scholar] [CrossRef]
- Huynh, N.T.; Smagghe, G.; Gonzales, G.B.; Van Camp, J.; Raes, K. Enzyme-assisted extraction enhancing the phenolic release from cauliflower (Brassica Oleracea L. Var. Botrytis) outer leaves. J. Agric. Food Chem. 2014, 62, 7468–7476. [Google Scholar] [CrossRef] [PubMed]
- Joo, C.G.; Lee, K.H.; Park, C.; Joo, I.W.; Choe, T.B.; Lee, B.C. Correlation of increased antioxidation with the phenolic compound and amino acids contents of camellia sinensis leaf extracts following ultra high pressure extraction. J. Ind. Eng. Chem. 2012, 18, 623–628. [Google Scholar] [CrossRef]
- Le, H.V.; Le, V.V.M. Comparison of enzyme-assisted and ultrasound-assisted extraction of vitamin c and phenolic compounds from acerola (Malpighia Emarginata DC.) fruit. Int. J. Food Sci. Technol. 2012, 47, 1206–1214. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Zhang, Q.; Li, Y.-F.; Dong, L.-L.; Liu, S.-L. Optimization of ultrasound extraction of alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohydr. Polym. 2015, 119, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Ultrasound-assisted extraction of natural antioxidants from the flower of limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Zhong, J.; Ye, X.; Lu, S.; Wang, W.; Zhang, R.; Xu, J.; Chen, S.; Liu, D. Ultrasonic-assisted enzymatic extraction of polysaccharide from corbicula fluminea: Characterization and antioxidant activity. LWT-Food Sci. Technol. 2015, 60, 1113–1121. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Engmann, F.N.; Zhang, H. Ultrasound-assisted enzymatic extraction (uaee) of phytochemical compounds from mulberry (Morus Nigra) must and optimization study using response surface methodology. Ind. Crops Prod. 2015, 63, 214–225. [Google Scholar] [CrossRef]
- Dang, B.; Huynh, T.; Le, V. Simultaneous treatment of acerola mash by ultrasound and pectinase preparation in acerola juice processing: Optimization of the pectinase concentration and pectolytic time by response surface methodology. Int. Food Res. J. 2012, 19, 509–513. [Google Scholar]
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015, 172, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q.; Wu, Q. Optimisation of ultrasound assisted extraction of phenolic compounds from sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013, 20, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.-Y.; Li, P.-H.; Huang, C.-C.; Wang, C.-C. Changes in functional characteristics of starch during water caltrop (Trapa Quadrispinosa Roxb.) growth. Food Chem. 2007, 104, 376–382. [Google Scholar] [CrossRef]
- Yasuda, M.; Yasutake, K.; Hino, M.; Ohwatari, H.; Ohmagari, N.; Takedomi, K.; Tanaka, T.; Nonaka, G.-I. Inhibitory effects of polyphenols from water chestnut (Trapa Japonica) husk on glycolytic enzymes and postprandial blood glucose elevation in mice. Food Chem. 2014, 165, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Hwang, J.-W.; Han, Y.-K.; Kwon, H.-J.; Hong, H.; Kim, E.-H.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Antioxidant activity and protective effects of trapa japonica pericarp extracts against tert-butylhydroperoxide-induced oxidative damage in chang cells. Food Chem. Toxicol. 2014, 64, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Li, F.; Xu, X.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2017, 94, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.N.; Zhou, X.L.; Si, J.; Gong, X.M.; Wang, S. Studies on cellulase-ultrasonic assisted extraction technology for flavonoids from Illicium verum residues. Chem. Cent. J. 2016, 10, 2–9. [Google Scholar] [CrossRef]
- Heleno, S.A.; Diz, P.; Prieto, M.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus Bisporus L. By response surface methodology and comparison with conventional soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zhou, Y.; Zheng, J.; Li, S.; Li, A.N.; Li, H.B. Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Jatropha integerrima by Response Surface Methodology. Molecules 2016, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Kuo, C.-H.; Chen, B.-Y.; Li, Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Shao, D.; Yuan, Y.; Wang, Z.; Qiang, C. Ultrasound-assisted extraction, hplc analysis, and antioxidant activity of polyphenols from unripe apple. J. Sep. Sci. 2012, 35, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhu, J.X.; Diao, W.C.; Wang, C.R. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Yuan, J.F.; Zhang, Z.Q. Microwave-assisted extraction optimised with response surface methodology and antioxidant activity of polyphenols from hawthorn (Crataegus Pinnatifida Bge.) fruit. Int. J. Food Sci. Technol. 2010, 45, 2400–2406. [Google Scholar] [CrossRef]
- Majd, M.H.; Rajaei, A.; Bashi, D.S.; Mortazavi, S.A.; Bolourian, S. Optimization of ultrasonic-assisted extraction of phenolic compounds from bovine pennyroyal (phlomidoschema parviflorum) leaves using response surface methodology. Ind. Crops Prod. 2014, 57, 195–202. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Bhatti, H.N.; Asghar, M. Rsm based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus Lanatus Thunb.) rind. J. Food Sci. Technol. 2015, 52, 5048–5056. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chen, D.; Li, X.; Li, T.; Yuan, M.; Zhou, Y.; Ding, C. Optimization of extraction process and antioxidant activity of polysaccharides from leaves of paris polyphylla. Carbohydr. Polym. 2014, 104, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Gao, T.; Yang, H.; Du, Y.; Li, C.; Wei, L.; Zhou, T.; Lu, J.; Bi, H. Simultaneous microwave/ultrasonic-assisted enzymatic extraction of antioxidant ingredients from nitraria tangutorun bobr. Juice by-products. Ind. Crops Prod. 2015, 66, 229–238. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, M.; He, L.; Fu, P.; Wang, L.; Zhou, J. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioprod. Proc. 2013, 91, 158–168. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus Amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hou, Z.; Gao, Y.; Xue, Y.; Liu, X.; Liu, G. Optimization of extraction parameters of bioactive components from defatted marigold (Tagetes Erecta L.) residue using response surface methodology. Food Bioprod. Proc. 2012, 90, 9–16. [Google Scholar] [CrossRef]
- Hammi, K.M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of ultrasound-assisted extraction of antioxidant compounds from tunisian zizyphus lotus fruits using response surface methodology. Food Chem. 2015, 184, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Saibu, G.; Katerere, D.; Rees, D.; Meyer, M. In vitro cytotoxic and pro-apoptotic effects of water extracts of tulbaghia violacea leaves and bulbs. J. Ethnopharmacol. 2015, 164, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).