Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China
Abstract
:1. Introduction
2. Results and Discussion
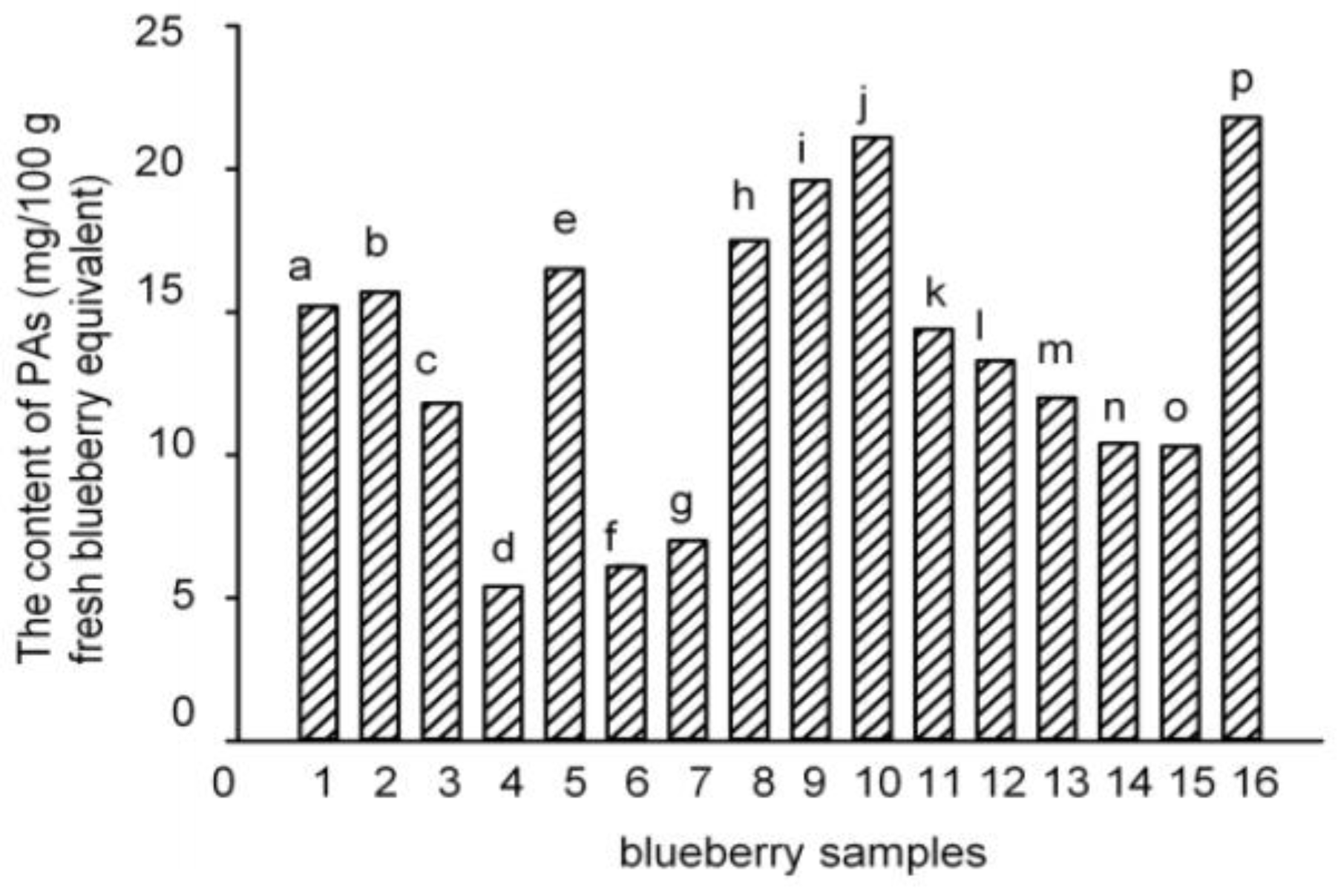
2.1. PAs Were Identified in Polyphenol-Enriched Fractions of 16 Blueberry Samples
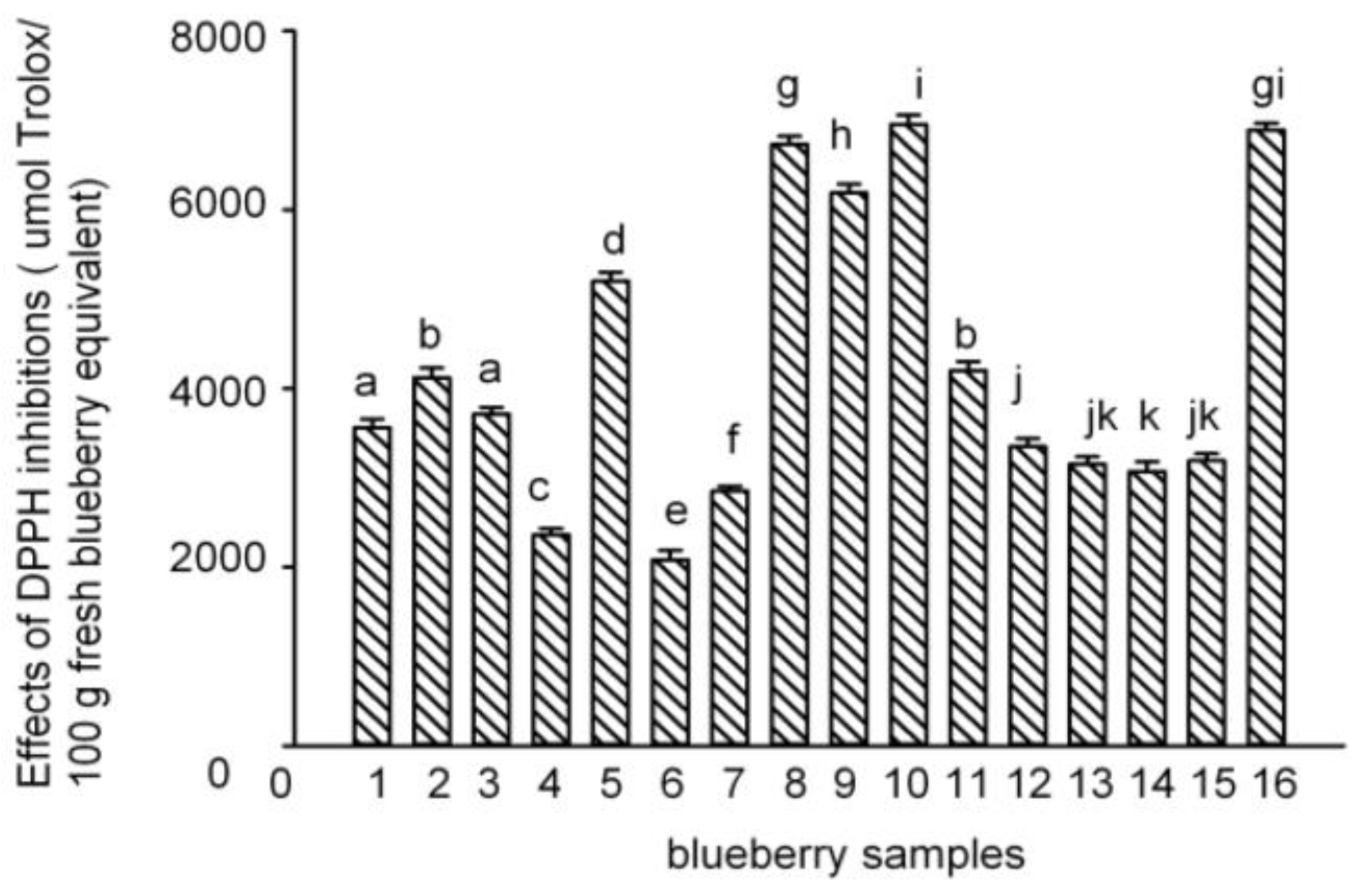
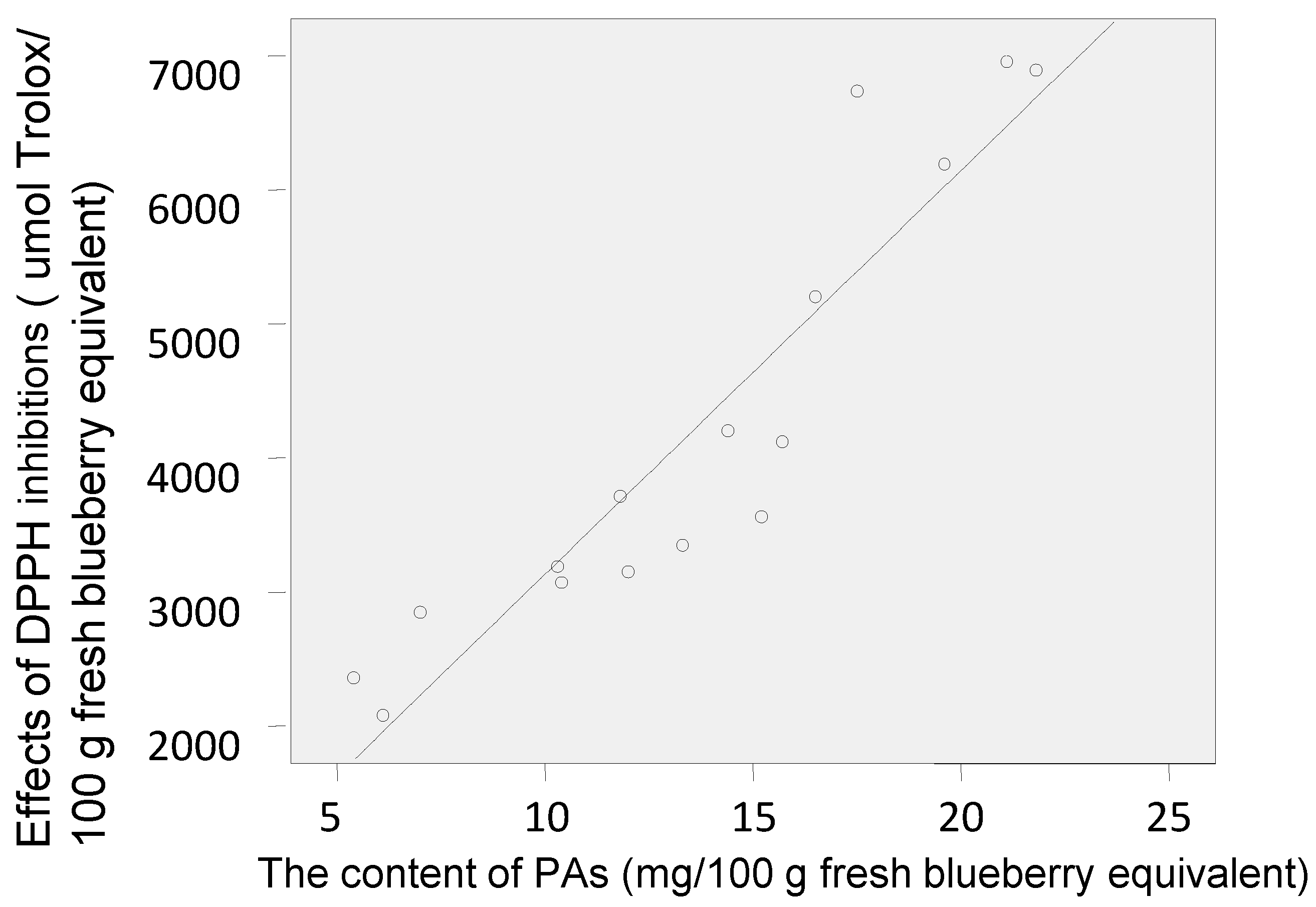
2.2. Antioxidant Activities of Polyphenol-Enriched Fractions of Blueberries
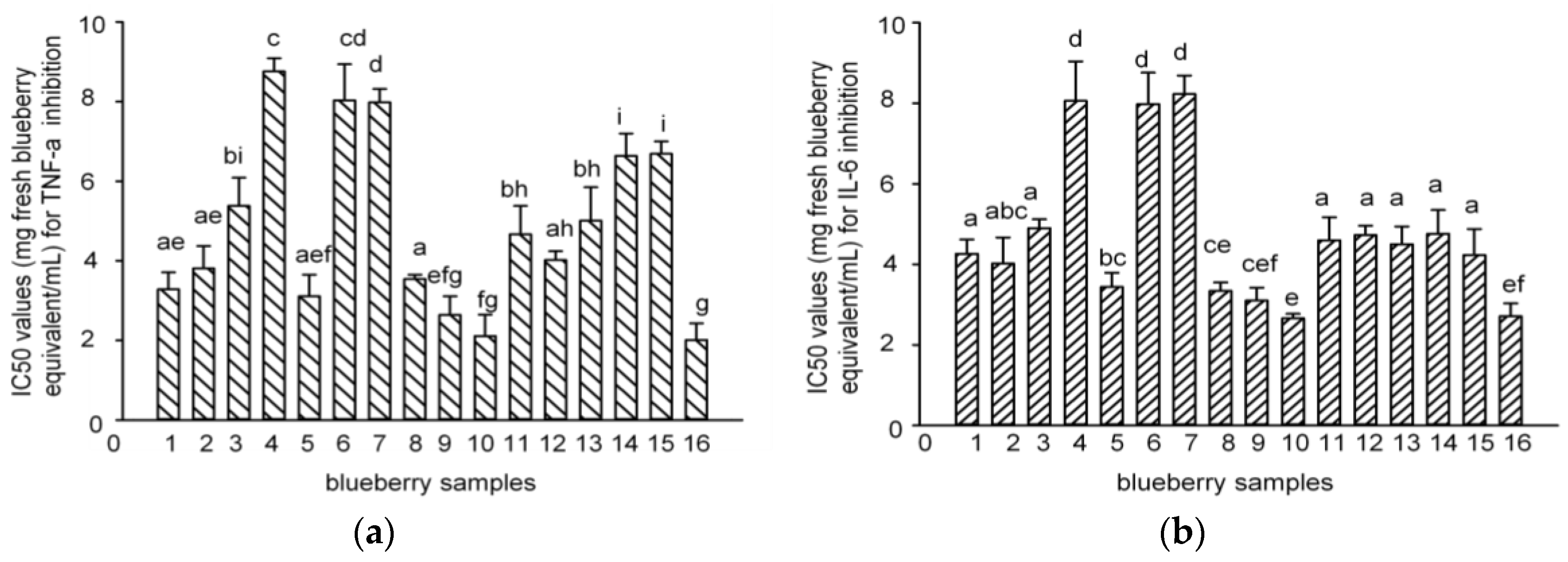
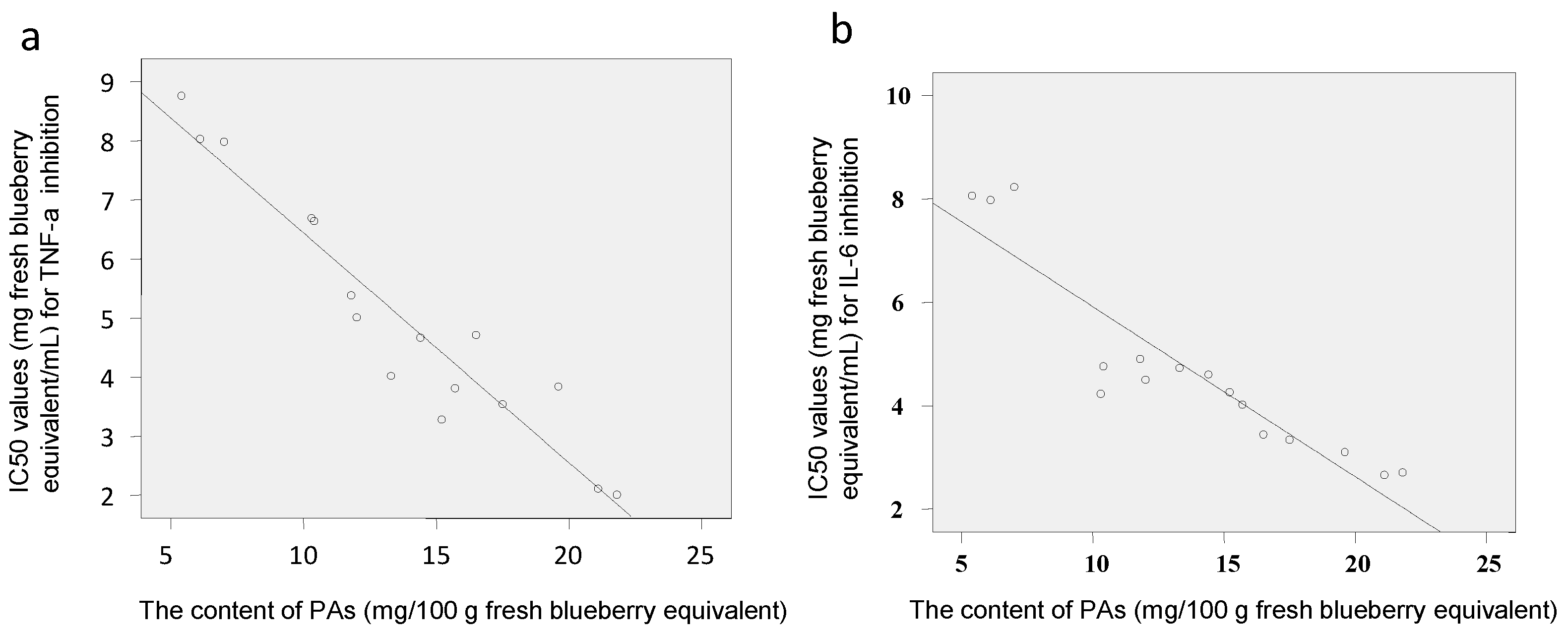
2.3. Anti-Inflammatory Effects of Polyphenol-Enriched Fractions of Blueberries
2.4. miR-21, miR-146a, and miR-125b Inhibition in Polyphenol-Enriched Fractions of Blueberries
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Polyphenol-Enriched Extraction Procedure
3.4. HPLC/MS2 Analysis
3.5. DPPH Antioxidant Assay
3.6. Enzyme-Linked Immunosorbent Assay Analysis of Interleukin-6 and Tumor Necrosis Factor-α Expression
3.7. Analysis of miR-21, miR-125b, and miR-146a Expression
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant capacity of black currant varies with organ, season, and cultivar. J. Agric. Food Chem. 2006, 54, 6271–6276. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Hellström, J.; Torronen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Taruscio, T.G.; Barney, D.L.; Exon, J. Content and profile of flavanoid and phenolic acid compounds in conjunction with the antioxidant capacity for a variety of Northwest Vaccinium berries. J. Agric. Food Chem. 2004, 52, 3169–3176. [Google Scholar] [CrossRef]
- Holiman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Analysis and health effects of flavonoids. Food Chem. 1996, 57, 43–46. [Google Scholar] [CrossRef]
- Forbes-Hernandez, T.U.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Rochette, N.; Da, S.V.M.; Nabavi, S.M.; Mota, E.F.; Nunes-Pinheiro, D.C.; Daglia, M.; De, M.D.F. Fruit as potent natural antioxidants and their biological effects. Curr. Pharm. Biotechnol. 2016, 17, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/beta-catenin canonical Wnt signaling. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Chen, J.R.; Nagarajan, S.; Badger, T.M.; Wu, X. Phenolic acids are in vivo atheroprotective compounds appearing in the serum of rats after blueberry consumption. J. Agric. Food Chem. 2011, 59, 10381–10387. [Google Scholar] [CrossRef] [PubMed]
- Montales, M.T.; Rahal, O.M.; Kang, J.; Rogers, T.J.; Prior, R.L.; Wu, X.; Simmen, R.C. Repression of mammosphere formation of humanbreast cancer cells by soy isoflavone genistein and blueberrypolyphenolic acids suggests diet-mediated targeting of cancer stemlike/progenitor cells. Carcinogenesis 2012, 33, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Thakali, K.M.; Jensen, G.S.; Wu, X. Phenolic acids of the two major blueberry species in the US market and their antioxidant and anti-inflammatory activities. Plant Foods Hum. Nutr. 2015, 70, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Kim, H.L.; Kim, S.J.; Park, K. Fruit quality, anthocyanin and total phenolic contents, and antioxidant activities of 45 blueberry cultivars grown in Suwon, Korea. J. Zhejiang Univ. Sci. B 2013, 14, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, P.J.; Wilson, C.B.; Wilson, L.D.; Nair, A.R.; Francis, J. The anti-Inflammatory effects of blueberries in an animal model of post-traumatic stress disorder (PTSD). PLoS ONE 2016, 11, e0160923. [Google Scholar] [CrossRef] [PubMed]
- Klimis-Zacas, D.; Vendrame, S.; Kristo, A.S. Wild blueberries attenuate risk factors of the metabolic syndrome. J. Berry Res. 2016, 6, 225–236. [Google Scholar] [CrossRef]
- Brown, E.; Gill, C.; Stewart, D.; McDougall, G. Lingoberries (Vacciniumvitis-idaea L.) and blueberries (Vacciniumcorymbosum) contain discrete epicatechin anthocyanin derivatives linked by ethyl bridges. J. Berry Res. 2016, 6, 13–23. [Google Scholar] [CrossRef]
- Michalska, A.; Lysiak, G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Cifuentes-Gomez, T.; Spencer, J.P.E. Bioavailability of wild blueberry (poly)phenols at different levels of intake. J. Berry Res. 2016, 6, 137–148. [Google Scholar] [CrossRef]
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S. Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J. Nutr. 2010, 140, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Ferguson, M.E.; Nagarajan, S.; Badger, T.M.; Wu, X. Blueberries reduce pro-inflammatory cytokine TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB activation and the MAPK pathway. Mol. Nutr. Food Res. 2011, 55, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Raitoharju, E.; Lyytikäinen, L.P.; Levula, M.; Oksala, N.; Mennadser, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kahonen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atheroslerosis 2011, 219, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Shuai, X.; Jia, Z.; Li, H.; Liang, X.; Su, D.; Guo, W. Ganodermalucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J. Transl. Med. 2015, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Sivachandran, N.; Lau, A.; Boudreau, E.; Zhao, J.L.; Baltimore, D.; Delgado-Olguin, P.; Cybulsky, M.; Fish, J.E. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013, 5, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, P.; Xiong, Q.; Song, X.; Yang, X.; Liu, L.; Yuan, W.; Rui, Y.C. MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J. Hypertens. 2010, 28, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Q.; Ding, X.P.; Qi, J.; Yu, H.; He, S.A.; Zhang, J.; Ge, H.X.; Yu, B.Y. Antioxidant anthocyanins screening through spectrum-effect relationships and DPPH-HPLC-DAD analysis on nine cultivars of introduced rabbiteye blueberry in China. Food Chem. 2012, 132, 759–765. [Google Scholar] [CrossRef]
- Li, C.; Feng, J.; Huang, W.Y.; An, X.T. Composition of polyphenols and antioxidant activity of rabbiteye blueberry (Vacciniumashei) in Nanjing. J. Agric. Food Chem. 2013, 61, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.; Tan, J.; Wang, B. Influence of harvest season on antioxidant activity and constituents of rabbiteye blueberry (Vacciniumashei) leaves. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar]
- Li, D.; Meng, X.; Li, B. Profiling of anthocyanins from blueberries produced in China using HPLC-DAD-MS and exploratory analysis by principal component analysis. J. Food Compos. Anal. 2015, 47, 1–7. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Wang, L.J.; Wu, J.; Wang, H.X.; Li, S.S.; Zheng, X.C.; Du, H.; Xu, Y.J.; Wang, L.S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Cordenunsi, B.R.; Oliveira do Nascimento, J.R.; Genovese, M.I.; Lajolo, F.M. Influence of cultivar on quality parameters and chemical composition of strawberry fruits grown in Brazil. J. Agric. Food Chem. 2002, 50, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Jimenez, Y.; Garcia-Moreno, M.V.; Igartuburu, J.M.; Garcia Barroso, C. Simplification of the DPPH assay for estimating the antioxidant activity of wine and wine by-products. Food Chem. 2014, 165, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yin, L.; Zhong, C.; Zhang, M.; Niu, Y. Advances in the DPPH Radical Scavenging assay for antioxidant activity evaluation. Shi Pin Ke Xue 2014, 35, 317–322. [Google Scholar]
- Zhu, H.; Xu, T.; Qiu, C.; Wu, B.; Zhang, Y.; Chen, L.; Xia, Q.; Li, C.; Zhou, B.; Liu, Z.; et al. Synthesis and optimization of novel allylated mono-carbonyl analogs of curcumin (MACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI) in rats. Eur. J. Med. Chem. 2016, 121, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Yan, H.; Han, C.; Wang, W.; Tian, Y.; Chen, X. Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264.7 macrophages. Int. J. Biol. Macromol. 2014, 69, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, M.Y.; Cho, Y.J.; Lee, J.H.; Yoo, C.G.; Lee, C.T.; Lee, S.M. Anti-inflammatory effect of erdosteine in lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation 2016, 39, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, N.; Zhang, J.; Liu, Z. MicroRNA-125b is involved in atherosclerosis obliterans in vitro by targeting podocalyxin. Mol. Med. Rep. 2015, 12, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-Espinoza, M.C. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem. 2016, 194, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the blueberries (1–16) are available from the authors.

| No. | Species | Cultivars | Companies | Collection Origins | Collection Time |
|---|---|---|---|---|---|
| 1 | North high | Reka | Jiawo | Dandong, Liaoning Province | April 2015 |
| 2 | North high | Patriot | Jiawo | Dandong, Liaoning Province | April 2015 |
| 3 | North high | Brigitta | Jiawo | Dandong, Liaoning Province | April 2015 |
| 4 | North high | Bluecrop | Jiawo | Dandong, Liaoning Province | April 2015 |
| 5 | North high | Bluecrop | Pulan | Lijiang, Yunnan Province | June 2015 |
| 6 | North high | Berkeley | Jiawo | Dandong, Liaoning Province | April 2015 |
| 7 | North high | Duke | Pulan | Lijiang, Yunnan Province | June 2015 |
| 8 | North high | Darrow | Pulan | Lijiang, Yunnan Province | June 2015 |
| 9 | Half-high | Northland | Jiawo | Dandong, Liaoning Province | April 2015 |
| 10 | Half-high | Northland | Pulan | Lijiang, Yunnan Province | June 2015 |
| 11 | Half-high | Northblue | Jiawo | Dandong, Liaoning Province | April 2015 |
| 12 | Half-high | Northcountry | Jiawo | Dandong, Liaoning Province | April 2015 |
| 13 | South high | Bluesource | Jiawo | Dandong, Liaoning Province | April 2015 |
| 14 | South high | Southgood | Jiawo | Dandong, Liaoning Province | April 2015 |
| 15 | South high | O’Neal | Pulan | Lijiang, Yunnan Province | June 2015 |
| 16 | South high | Misty | Pulan | Lijiang, Yunnan Province | June 2015 |
| No. | Phenolic Acids % | ||||||
|---|---|---|---|---|---|---|---|
| GA | 3,4-DHBA | VA | CGA | CA | SGA | FA | |
| 1 | - | - | 1.19 | 98.36 | - | 0.34 | 0.12 |
| 2 | - | - | 0.68 | 98.98 | - | 0.28 | 0.06 |
| 3 | - | - | 2.07 | 97.24 | - | 0.25 | 0.44 |
| 4 | - | - | 2.46 | 96.63 | - | 0.61 | 0.29 |
| 5 | 0.06 | 0.18 | 3.00 | 95.67 | 0.68 | 0.28 | 0.14 |
| 6 | - | - | 5.64 | 93.51 | - | 0.72 | 0.13 |
| 7 | - | 0.50 | 2.60 | 95.38 | 0.25 | 1.27 | - |
| 8 | 0.27 | 0.51 | 2.21 | 95.50 | 0.92 | 0.33 | 0.27 |
| 9 | 0.09 | - | 1.21 | 98.20 | 0.22 | 0.20 | 0.09 |
| 10 | 0.63 | 0.67 | 5.14 | 90.93 | 1.52 | 0.42 | 0.69 |
| 11 | - | 0.04 | 2.35 | 97.22 | - | 0.34 | 0.04 |
| 12 | 0.34 | 0.43 | 1.76 | 90.45 | 6.40 | 0.37 | 0.25 |
| 13 | 0.03 | 0.13 | 0.02 | 99.23 | 0.16 | 0.33 | 0.11 |
| 14 | - | - | 1.71 | 97.59 | 0.24 | 0.38 | 0.08 |
| 15 | 1.61 | 1.76 | 8.34 | 84.39 | 2.26 | 0.84 | 0.81 |
| 16 | 0.41 | 0.61 | 2.43 | 94.48 | 1.18 | 0.52 | 0.37 |
| No. | microRNA (miRNA) | ||
|---|---|---|---|
| miR-21 | miR-125b | miR-146a | |
| 1 | 49 | 34 | 12 |
| 2 | 50 | 26 | 13 |
| 3 | 52 | 18 | 14 |
| 4 | 50 | 30 | 13 |
| 5 | 53 | 27 | 16 |
| 6 | 43 | 28 | 15 |
| 7 | 53 | 31 | 15 |
| 8 | 48 | 32 | 12 |
| 9 | 52 | 28 | 15 |
| 10 | 60 | 37 | 22 |
| 11 | 61 | 41 | 23 |
| 12 | 55 | 33 | 21 |
| 13 | 41 | 38 | 6 |
| 14 | 46 | 34 | 7 |
| 15 | 44 | 23 | 8 |
| 16 | 50 | 36 | 13 |
| PAs | miRNA | ||
|---|---|---|---|
| miR-21 | miR-125b | miR-146a | |
| PAs content | 0.405 | 0.271 | 0.281 |
| No. | Name | M.W. | Pairs | tR(min) | Equation a | Linear Range (ng/mL) | RSD b (%) | LOD c (ng/mL) | LOQ d (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | GA | 170 | 169/125 | 1.33 | y = 0.0044x − 123.297 | 50–6000 | 1.6 | 10 | 3 |
| 2 | 3,4-DHBA | 154 | 153/109 | 2.59 | y = 0.0025x − 171.664 | 50–6000 | 1.5 | 9 | 3 |
| 3 | VA | 168 | 167/152 | 6.46 | y = 0.2240x − 63.617 | 500–6000 | 1.4 | 400 | 100 |
| 4 | CGA | 354 | 353/191 | 6.87 | y = 0.0080x − 100.648 | 50–6000 | 1.3 | 30 | 10 |
| 5 | CA | 180 | 179/135 | 6.99 | y = 0.0018x − 211.284 | 50–6000 | 0.7 | 20 | 6 |
| 6 | SGA | 198 | 197/182 | 8.05 | y = 0.1513x − 27.866 | 100–6000 | 0.7 | 60 | 25 |
| 7 | FA | 194 | 193/178 | 11.05 | y = 0.0856x − 19.518 | 200–6000 | 1.8 | 200 | 60 |
| 8 | benzoic acid-d5 | 126 | 126/82 | 11.33 | 1.8 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Zhang, J.; Wang, H.; Xu, J.; He, J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules 2017, 22, 312. https://doi.org/10.3390/molecules22020312
Su X, Zhang J, Wang H, Xu J, He J, Liu L, Zhang T, Chen R, Kang J. Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules. 2017; 22(2):312. https://doi.org/10.3390/molecules22020312
Chicago/Turabian StyleSu, Xianming, Jian Zhang, Hongqing Wang, Jing Xu, Jiuming He, Liying Liu, Ting Zhang, Ruoyun Chen, and Jie Kang. 2017. "Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China" Molecules 22, no. 2: 312. https://doi.org/10.3390/molecules22020312
APA StyleSu, X., Zhang, J., Wang, H., Xu, J., He, J., Liu, L., Zhang, T., Chen, R., & Kang, J. (2017). Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules, 22(2), 312. https://doi.org/10.3390/molecules22020312






