Phytochemical Study of Tapirira guianensis Leaves Guided by Vasodilatory and Antioxidant Activities
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis of MET
2.2. Vasodilatory Effect of MET
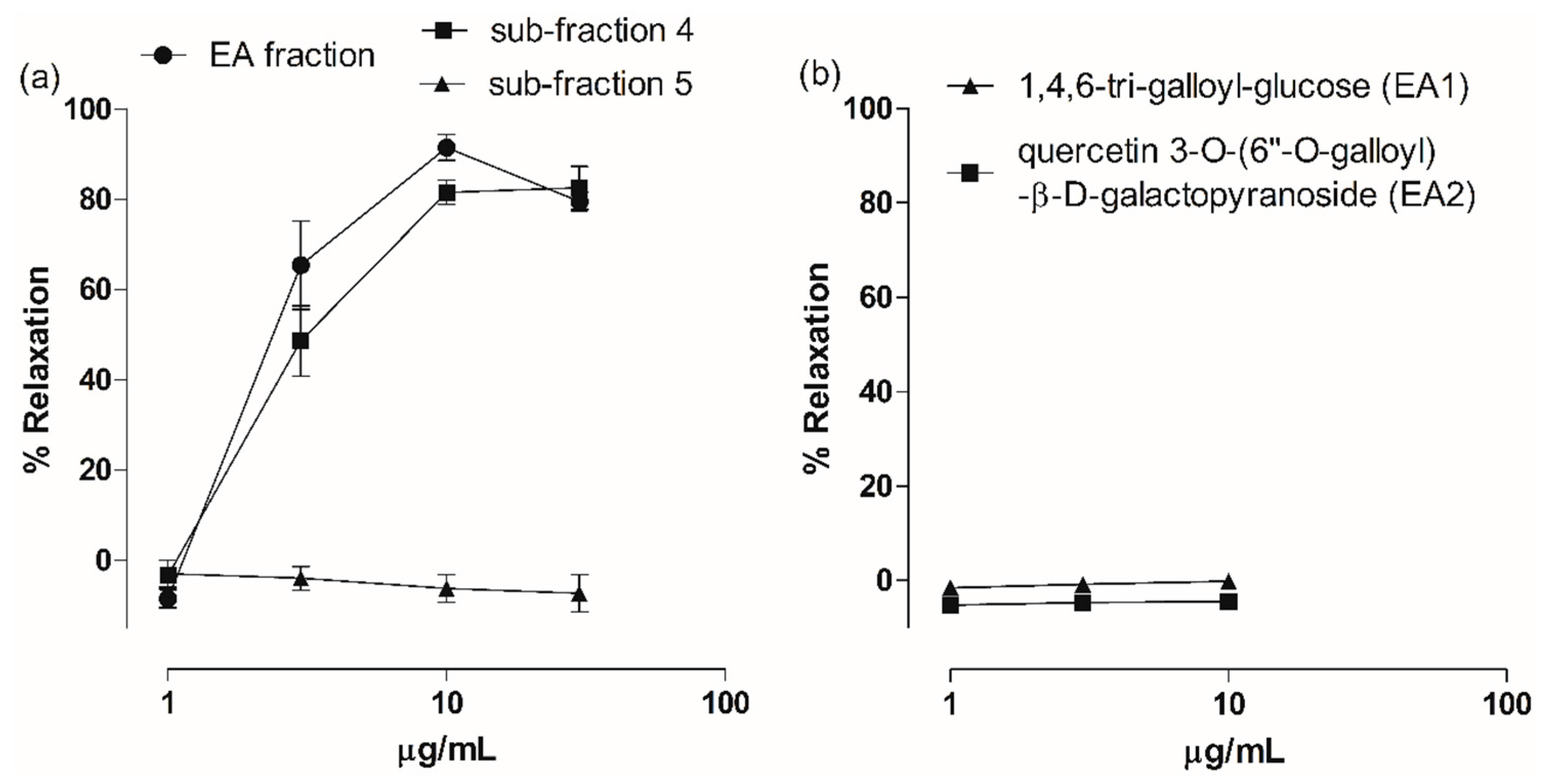
2.3. Vasodilatory Effect of Fractions, Sub-Fractions and Isolated Compounds
2.4. Antioxidant Effect of MET and Fractions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of Crude Extract and Fractions
4.2. Chromatographic Separation of EA Fraction
4.3. LC-MS Analyses of EA Fraction
4.4. Preparation of Rat Aortic Rings for iSometric Tension Recording
4.5. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. A Global Brief on Hypertension. Document Number: WHO/DCO/WHD/2013.2. Available online: http://ish-world.com/downloads/pdf/globalbrief hypertension.pdf (accessed on 23 October 2016).
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Sander, G.E.; Nossaman, B.D.; Kadowitz, P.J. Impaired vasodilatation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J. Clin. Hypertens. 2012, 14, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Samuel, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Biochem. Physiol. 2012, 90, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.A.; Tong, X.Y. Vascular oxidative stress: The common link in hypertensive and diabetic vascular disease. J. Cardiovasc. Pharmacol. 2010, 55, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar] [PubMed]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sánchez, M.A. Vasodilator compounds derived from plants and their mechanisms of action. Molecules 2013, 18, 5814–5857. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.W.; Xu, H.X.; Dong, H.; Fiscus, R.R.; But, P.P. Role of nitric oxide in the vasorelaxant and hypotensive effects of extracts and purified tannins from Geum japonicum. J. Ethnopharmacol. 2007, 109, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Tapas, A.R.; Sakarkar, D.M.; Kakde, R.B. Flavonoids as nutraceuticals. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Annapurna, A.; Reddy, C.S.; Akondi, R.B.; Rao, S.R. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 2009, 61, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Ferri, C. Flavonoids: Antioxidants against atherosclerosis. Nutrients 2010, 2, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic effects of plant flavonoids. BioMed Res. Int. 2014, 2014, 480258. [Google Scholar] [CrossRef] [PubMed]
- Roos, C.M.; Zhang, B.; Palmer, A.K.; Ogrodnik, M.B.; Pirtskhalava, T.; Thalji, N.M.; Hagler, M.; Jurk, D.; Smith, L.A.; Casaclang-Verzosa, G.; et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016, 15, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 5 May 2015).
- David, J.M.; Chávez, J.P.; Chai, H.B.; Pezzuto, J.M.; Cordell, G.A. Two new cytotoxic compounds from Tapirira guianensis. J. Nat. Prod. 1998, 61, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Coelho-ferreira, M. Medicinal knowledge and plant utilization in an Amazonian coastal community of Marudá, Pará State (Brazil). J. Ethnopharmacol. 2009, 126, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.J.; David, J.M.; Da Silva, L.P.; David, J.P.; Lopes, L.M.X.; Guedes, M.L.S. Flavonoides, norisoprenóides e outros terpenos das folhas de Tapirira guianensis. Quim. Nova 2008, 31, 2056–2059. [Google Scholar] [CrossRef]
- Roumy, V.; Fabre, N.; Portet, B.; Bourdy, G.; Acebey, L.; Vigor, C.; Valentin, A.; Moulis, C. Four anti-protozoal and anti-bacterial compounds from Tapirira guianensis. Phytochemistry 2009, 70, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; Hasegawa, M.; Rodríguez, M.; Estrada, O.; Méndez, J.; Castillo, A.; Gonzalez-Mujica, F.; Motta, N.; Vásquez, J.; Romero-Vecchione, E. Biological screening of plants of the Venezuelan Amazons. J. Ethnopharmacol. 2001, 77, 77–83. [Google Scholar] [CrossRef]
- Greenham, J.; Harborne, J.B.; Williams, C.A. Identification of lipophilic flavones and flavonols by comparative HPLC, TLC and UV spectral analysis. Phytochem. Anal. 2003, 14, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar] [PubMed]
- Shimokawa, H. 2014 Willians Harvey lecture: Importance of coronary vasomotion abnormalities—From bench to bedside. Eur. Heart J. 2014, 35, 3180–3193. [Google Scholar] [CrossRef] [PubMed]
- Urakami-Harasawa, L.; Shimokawa, H.; Nakashima, M.; Egashira, K.; Takeshita, A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J. Clin. Investig. 1997, 100, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Busse, R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pérez-Vizcaíno, F.; Zarzuelo, A.; Jiménez, J.; Tamargo, J. Vasodilator effects of quercetin isolated rat vascular smooth muscle. Eur. J. Pharmacol. 1993, 239, 1–7. [Google Scholar] [CrossRef]
- Ajay, M.; Gilani, A.U.; Mustafa, M.R. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003, 74, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Satoh, H. Possible involvement of Ca2+ activated K+ channels, SK channel, in the quercetin-induced vasodilation. Korean J. Physiol. Pharmacol. 2009, 13, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Shimada, Y.; Akechi, Y.; Kohta, K.; Hattori, M.; Terasawa, K. Endothelium-dependent vasodilator effect of extract prepared from of roots of Paeonia lactiflora on isolated rat aorta. Planta Med. 1996, 62, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Moon, M.K.; Choi, D.H.; Lee, J.K.; Kwon, T.O.; Lee, H.S. Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-β-d-glucose (PGG) via a nitric oxide-cGMP pathway. Eur. J. Pharmacol. 2005, 524, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, Z.W.; Guo, Y.; Wang, Q.H.; Song, B. Cellular mechanisms underlying hyperin-induced relaxation of rat basilar artery. Fitoterapia 2011, 82, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Phillipson, D.; Greengrass, P.M.; Bowery, N.E.; Cai, Y. Plants polyphenols: biologically active compounds or non-selective binders to proteins? Phytochemistry 1997, 44, 441–447. [Google Scholar] [CrossRef]
- Lee, T.H.; Liu, D.Z.; Hsu, F.L.; Wu, W.C.; Hou, W.C. Structure-activity relationships of five myricetin galloylglycosides from leaves of Acacia confusa. Bot. Stud. 2006, 47, 37–43. [Google Scholar]
- Zhang, B.; Chen, Y.; Shen, Q.; Liu, G.; Ye, J.; Sun, G.; Sun, X. Myricitrin Attenuates High Glucose-Induced Apoptosis through Activating Akt-Nrf2 Signaling in H9c2 Cardiomyocytes. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Luo, Y.; Meng, X.B.; Wang, M.; Wang, H.W.; Song, S.Y.; Ye, J.X.; Pan, R.L.; Yao, F.; Wu, P.; et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathway. Vasc. Pharmacol. 2015, 70, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Zidane, A.; Tits, M.; Angenot, L.; Wauters, J.N.; Frederich, M.; Dib, I.; Mekhfi, H.; Aziz, M.; Bnouham, M.; Legssyer, A.; et al. Phytochemical analysis of Tetraclinis articula in relation to its vasorelaxant property. J. Mater. 2014, 5, 1368–1375. [Google Scholar]
- Lum, H.; Roebuck, K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001, 280, C719–C741. [Google Scholar] [PubMed]
- Cai, Y.Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.F.; Xu, M.; Yang, C.R.; Xu, M.; Zhang, Y.J. Phenolic antioxidants from the leaves of Camellia pachyandra Hu. J. Agric. Food Chem. 2010, 58, 8820–8824. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.P.; Wong, D.Z.; Ling, S.K.; Chuah, C.H.; Kadir, H.A. Neuroprotective activity of galloylated cyanogenic glucosides and hydrolysable tannins isolated from leaves of Phyllagathis rotundifolia. Fitoterapira 2012, 83, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.; Lopez-Sepulveda, R.; Romero, M.; Toral, M.; Cogolludo, A.; Perez-Vizcaino, F.; Duarte, J. Quercetin and its metabolites inhibit the membrane NADPH oxidase activity in vascular smooth muscle cells from normotensive and spontaneously hypertensive rats. Food Funct. 2015, 6, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.A.M.; Hussein, S.A.M.; Merfort, I. NMR spectral analysis of polyphenols from Punica granatum. Phytochemistry 1994, 36, 793–798. [Google Scholar] [CrossRef]
- Braca, A.; Politi, M.; Sanogo, R.; Sanou, H.; Morelli, I.; Pizza, C.; De Tommasi, N. Chemical composition and antioxidant activity of phenolic compounds from wild and cultivated Sclerocarya birrea (Anacardiaceae) leaves. J. Agric. Food Chem. 2003, 51, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wan, C.; Zhou, S. Quercetin—A flavonoid compound from Sarcopyramis bodinieri var delicate with potential apoptotic activity in HepG2 liver cancer cells. Trop. J. Pharm. Res. 2013, 12, 529–533. [Google Scholar] [CrossRef]
- Ferreira, L.L.D.M.; Gomes, M.V.; Paes, B.M.; Do Carmo, P.L.; Konno, T.U.; Esteves, F.A.; Lopes, N.P.; Tomaz, J.C.; Leal, I.C.; Guimarães, D.O.; et al. The hydroalcoholic extract of leaves of Mandevilla moricandiana induces NO-mediated vascular relaxation. Planta Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, J.M.; Trindade, A.P.; Velozo, L.S.; Kaplan, M.A.; Sudo, R.T.; Zapata-Sudo, G. The lignan eudesmin extracted from Piper truncatum induced vascular relaxation via activation of endothelial histamine H1 receptors. Eur. J. Pharmacol. 2009, 606, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.C.; Lage, L.F.O.; Camargos, C.R.D.; Amaral, J.C.; Costa, L.M.; De Souza, A.N.; Oliveira, F.Q. Antioxidant determination activity by DPPH method and assay for total flavonoids in leaves extracts of Bauhinia variegata L. Rev. Bras. Farm. 2011, 92, 327–332. [Google Scholar]
- Sample Availability: Not available.
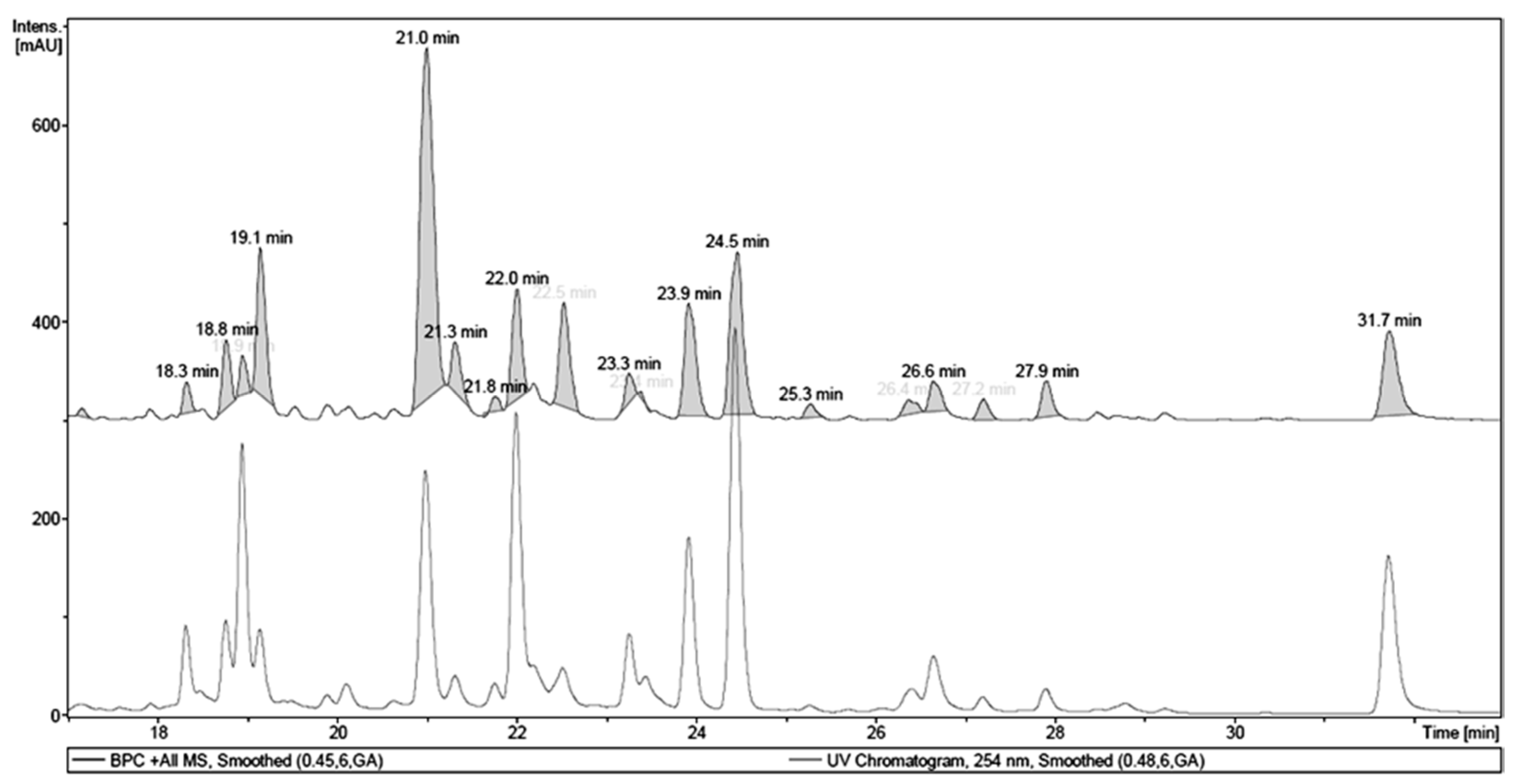
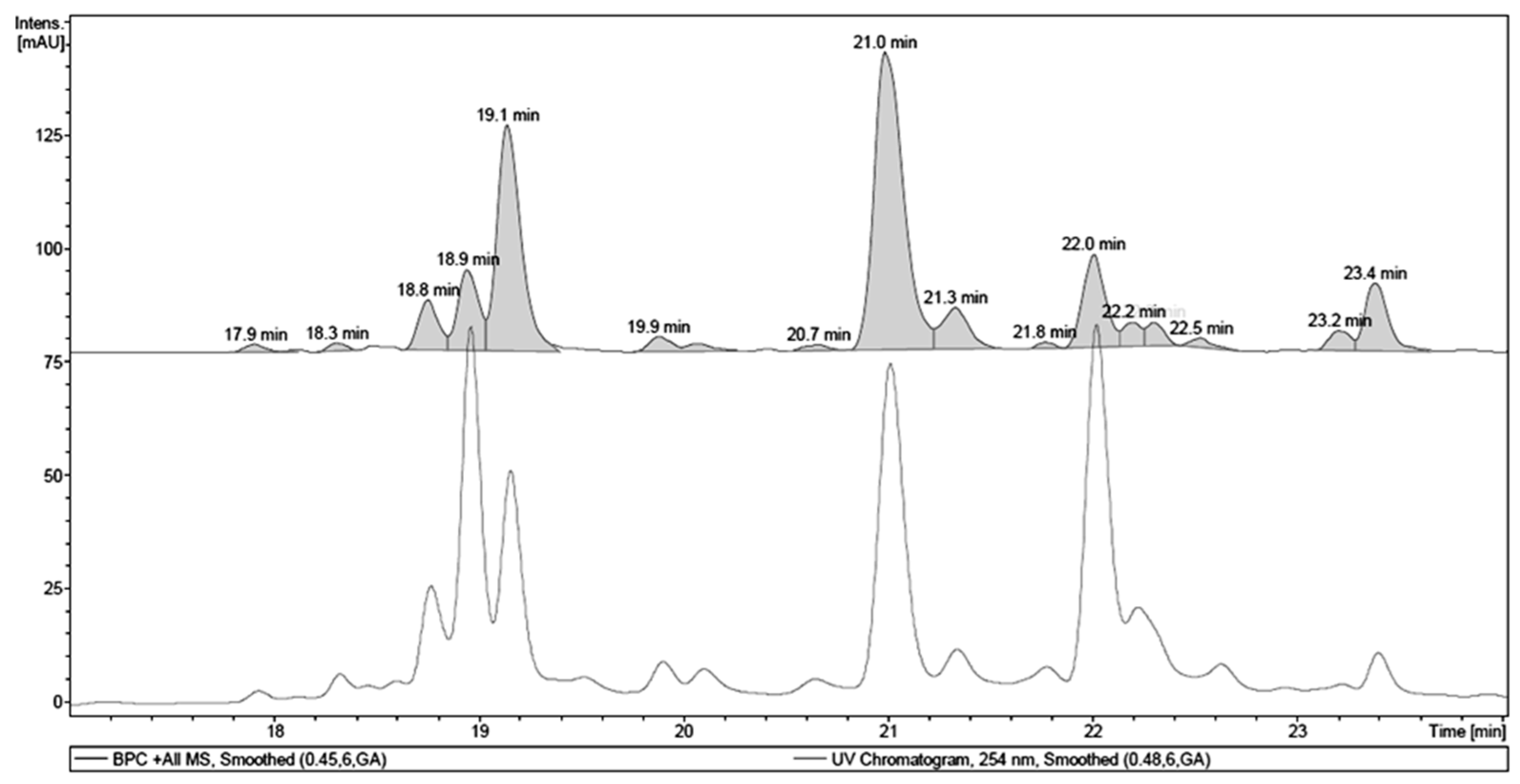

| Retention Time (tR, min) | Identity | Pseudomolecular Ion m/z | Fragment m/z (Loss) | λMAX (nm) |
|---|---|---|---|---|
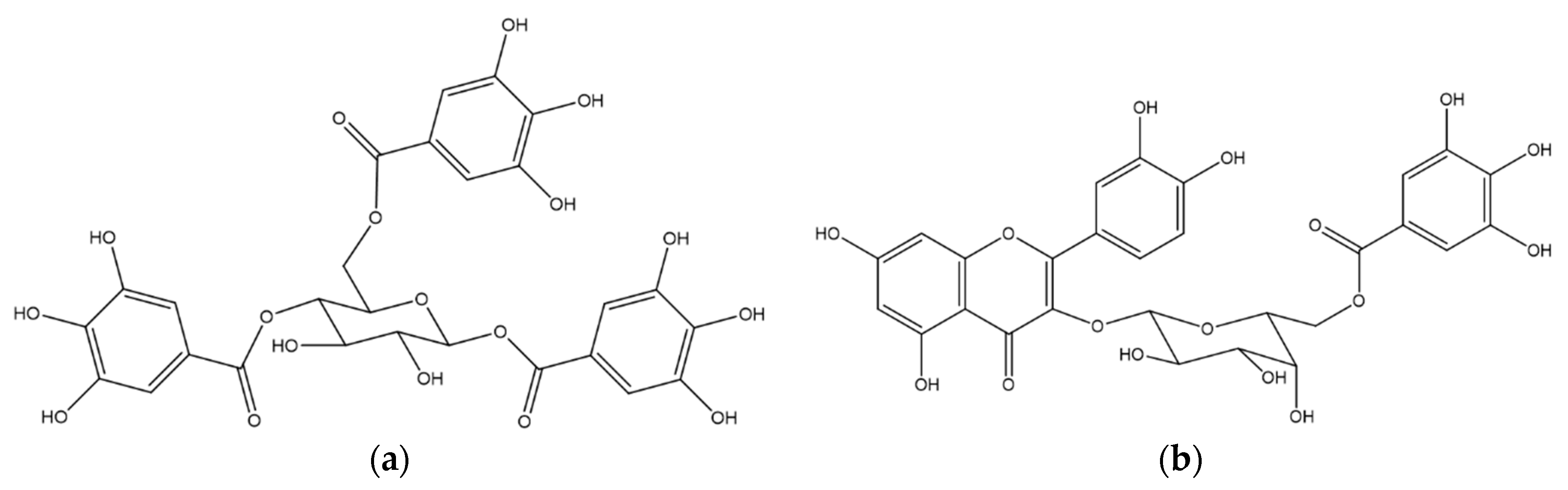
| 18.9 | 1,4,6-tri-O-galloyl-β-d-glucose a | - | 467.12 (170) | 276 |
| 21.0 | quercetin 3-O-(6″-O-galloyl)-β-d-galactopyranoside a | 617.17 [M + H]+ | - | 263, 352 |
| 22.0 | myricetin deoxyhexoside | 465.13 [M + H]+ | 319.06 (146) | 257, 350 |
| 23.9 | quercetin pentoside | 435.12 [M + H]+ | 303.05 (132) | 256, 352 |
| 24.5 | quercetin dideoxyhesoside | 595.20 [M + H]+ | 449.13 (146) 303.06 (146) | 255, 348 |
| 31.7 | Quercetin a | 303.05 [M + H]+ | - | 255, 369 |
| Retention Time (tR, min) | Identity | Pseudomolecular Ion m/z | Fragment m/z (Loss) | λMAX (nm) |
|---|---|---|---|---|
| 18.9 | 1,4,6-tri-O-galloyl-β-d-glucose a | - | 467.08 (170) | 277 |
| 19.1 | galloyl-HHDP-hexoside | 633.10 [M + H]+ | - | 266 |
| 21.0 | quercetin 3-O-(6″-O-galloyl)-β-d-galactopyranoside a | 617.17 [M + H]+ | - | 263, 352 |
| 22.0 | myricetin deoxyhexoside | 465.13 [M + H]+ | 319.06 (146) | 261, 350 |
| Samples | EC50 (µg/mL) |
|---|---|
| MET | 3.12 ± 0.20 a |
| HN fraction | 40.30 ± 0.39 c |
| DCM fraction | 19.83 ± 0.90 d |
| EA fraction | 5.33 ± 0.16 b |
| BT fraction | 6.05 ± 0.19 b |
| Aq fraction | 14.33 ± 0.15 e |
| Egb 761® | 22.91 ± 0.66 f |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.M.G.; Guimarães, D.O.; Konno, T.U.P.; Tinoco, L.W.; Barth, T.; Aguiar, F.A.; Lopes, N.P.; Leal, I.C.R.; Raimundo, J.M.; Muzitano, M.F. Phytochemical Study of Tapirira guianensis Leaves Guided by Vasodilatory and Antioxidant Activities. Molecules 2017, 22, 304. https://doi.org/10.3390/molecules22020304
Rodrigues AMG, Guimarães DO, Konno TUP, Tinoco LW, Barth T, Aguiar FA, Lopes NP, Leal ICR, Raimundo JM, Muzitano MF. Phytochemical Study of Tapirira guianensis Leaves Guided by Vasodilatory and Antioxidant Activities. Molecules. 2017; 22(2):304. https://doi.org/10.3390/molecules22020304
Chicago/Turabian StyleRodrigues, Amélia M. G., Denise O. Guimarães, Tatiana U. P. Konno, Luzineide W. Tinoco, Thiago Barth, Fernando A. Aguiar, Norberto P. Lopes, Ivana C. R. Leal, Juliana M. Raimundo, and Michelle F. Muzitano. 2017. "Phytochemical Study of Tapirira guianensis Leaves Guided by Vasodilatory and Antioxidant Activities" Molecules 22, no. 2: 304. https://doi.org/10.3390/molecules22020304
APA StyleRodrigues, A. M. G., Guimarães, D. O., Konno, T. U. P., Tinoco, L. W., Barth, T., Aguiar, F. A., Lopes, N. P., Leal, I. C. R., Raimundo, J. M., & Muzitano, M. F. (2017). Phytochemical Study of Tapirira guianensis Leaves Guided by Vasodilatory and Antioxidant Activities. Molecules, 22(2), 304. https://doi.org/10.3390/molecules22020304







