Cytotoxic Compounds Derived from Marine Sponges. A Review (2010–2012)
Abstract
:1. Introduction
1.1. Taxonomy of Marine Sponges
1.2. Research and Methodology
2. Porifera Involved in the Biosynthesis of Cytotoxic Metabolites
2.1. Class: Demospongiae
2.1.1. Order: Agelasida
Family: Agelasidae
2.1.2. Order: Axinellida
Family: Axinellidae
Family: Raspailiidae
2.1.3. Order: Bubarida
Family: Dictyonellidae
2.1.4. Order: Dictyoceratida
Family: Dysideidae
Family: Irciniidae
Family: Spongiidae
Family: Thorectidae
2.1.5. Order: Haplosclerida
Family: Callyspongiidae
Family: Niphatidae
Family: Petrosiidae
2.1.6. Order: Homosclerophorida
Family: Plakinidae
2.1.7. Order: Poecilosclerida
Family: Acarnidae
Family: Coelosphaeridae
Family: Crambeidae
Family: Hymedesmiidae
Family: Latrunculiidae
Family: Podospongiidae
2.1.8. Order: Scopalinida
Family: Scopalinidae
2.1.9. Order: Suberitida
Family: Halichondriidae
2.1.10. Order: Tetractinellida
Family: Ancorinidae
Family: Calthropellidae
Family: Geodiidae
2.1.11. Order: Verongida
Family: Aplysinellidae
Family: Ianthellidae
Family: Pseudoceratinidae
2.2. Miscellaneous
3. Final Considerations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, A.C.; Blum, J.E.; Pawlik, J.R. Testing for defensive synergy in Caribbean sponges: Bad taste or glass spicules? J. Exp. Mar. Biol. Ecol. 2005, 322, 67–81. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Cassiano, K.M.; Cavalcanti, D.N.; Teixeira, V.L.; Pereira, R.C. Isolated and synergistic effects of chemical and structural defenses of two species of Tethya (Porifera: Demospongiae). J. Sea Res. 2012, 68, 57–62. [Google Scholar] [CrossRef]
- Andavan, G.S.B.; Lemmens-Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M. Cytotoxic terpene quinones from marine sponges. Mar. Drugs 2010, 8, 2849–2870. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Novel antitumor agents: Marine sponge alkaloids, their synthetic analogs and derivatives. Mini-Rev. Med. Chem. 2005, 5, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Frota, M.J.; Silva, R.B.; Mothes, B.; Henriques, A.T.; Moreira, J.C. Current status on natural products with antitumor activity from Brazilian marine sponges. Curr. Pharm. Biotechnol. 2012, 13, 235–244. [Google Scholar] [PubMed]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed]
- Gomes Filho, S.M.; Cardoso, J.D.; Anaya, K.; Silva do Nascimento, E.; de Lacerda, J.T.; Mioso, R.; Santi Gadelha, T.; de Almeida Gadelha, C.A. Marine sponge lectins: Actual status on properties and biological activities. Molecules 2015, 20, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.H.; Blunt, J.W.; Dumdey, E.J.; Hickford, S.J.H.; Lill, R.E.; Li, S.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Chang, W.-B.; Chen, H.-M.; El-Shazly, M.; Du, Y.-C.; Kung, T.-H.; Chen, Y.-C.; Sung, P.-J.; Ho, Y.-S.; Kuo, F.-W.; et al. 10-Acetylirciformonin B, a sponge furanoterpenoid, induces DNA damage and apoptosis in leukemia cells. Molecules 2012, 17, 11839–11848. [Google Scholar] [CrossRef] [PubMed]
- Ekwall, B.; Silano, V.; Paganuzzi-Stammati, A.; Zucco, F. Toxicity tests with mammalian cell cultures. In Short-Term Toxicity Tests from Non-Genotoxic Effects; Bourdeau, P., Somers, E., Richardson, G.M., Hickman, J.R., Eds.; John Wiley & Sons: London, UK, 1990; pp. 75–97. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L. Cytotoxicity testing: Measuring viable cells, dead cells, and detecting mechanism of cell death. Methods Mol. Biol. 2011, 740, 103–114. [Google Scholar] [PubMed]
- Sumantran, V.N. Cellular chemosensitivity assays: An overview. Methods Mol. Biol. 2011, 731, 219–236. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. Methods Mol. Biol. 2013, 1055, 191–205. [Google Scholar] [PubMed]
- Mayer, A.M.; Gustafson, K.R. Marine pharmacology in 2005–2006: Antitumour and cytotoxic compounds. Eur. J. Cancer 2008, 44, 2357–2387. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Sahebkar, A. Anti-cancer products from marine sponges: Progress and promise. Recent Pat. Drug Deliv. Formul. 2015, 9, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Van Treeck, P.; Eisinger, M.; Müller, J.; Paster, M.; Schuhmacher, H. Mariculture trials with Mediterranean sponge species. The exploitation of an old natural resource with sustainable and novel methods. Aquaculture 2003, 218, 439–455. [Google Scholar] [CrossRef]
- Sipkema, D.; Osinga, R.; Schatton, W.; Mendola, D.; Tramper, J.; Wijffels, R.H. Large-scale production of pharmaceuticals by marine sponges: Sea, cell, or synthesis? Biotechnol. Bioeng. 2005, 90, 201–222. [Google Scholar] [CrossRef] [PubMed]
- De Caralt, S.; Uriz, M.J.; Wijffels, R.H. Cell culture from sponges: Pluripotency and immortality. Trends Biotechnol. 2007, 25, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Rane, R.; Sahu, N.; Shah, C.; Karpoormath, R. Marine bromopyrrole alkaloids: Synthesis and diverse medicinal applications. Curr. Top. Med. Chem. 2014, 14, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Schnekenburger, M.; Dicato, M.; Diederich, M. Epigenetic modulators from “The Big Blue”: A treasure to fight against cancer. Cancer Lett. 2014, 351, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Rady, H. Sponge mesohyl induces anti-proliferation activity and cell cycle arrest in colon cancer in vitro. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1070–1075. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Gaudêncio, S.P.; Pereira, F. Dereplication: Racing to speed up the natural products discovery process. Nat. Prod. Rep. 2015, 32, 779–810. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.; Butler, S.K. Eribulin mesylate: A novel halichondrin B analogue for the treatment of metastatic breast cancer. Am. J. Health Syst. Pharm. 2012, 69, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Venkatesan, J.; Manivasagan, P.; Kim, S.K. Antiangiogenic effects of marine sponge derived compounds on cancer. Environ. Toxicol. Pharmacol. 2013, 36, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Palkar, M.B.; Rane, R.A.; Thapliyal, N.; Shaikh, M.S.; Alwan, W.S.; Jain, K.S.; Karunanidhi, S.; Patel, H.M.; Hampannavar, G.A.; Karpoormath, R. An insight into purine, tyrosine and tryptophan derived marine antineoplastic alkaloids. Anticancer Agents Med. Chem. 2015, 15, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Foltz, K.R.; Adams, N.L.; Runft, L.L. Echinoderm eggs and embryos: Procurement and culture. Methods Cell Biol. 2004, 74, 39–74. [Google Scholar] [PubMed]
- Wu, C. An important player in brine shrimp lethality bioassay: The solvent. J. Adv. Pharm. Technol. Res. 2014, 5, 57–58. [Google Scholar] [PubMed]
- Bohlin, L.; Bruhn, J.G. Bioassay Methods in Natural Product Research and Drug Development. In Proceedings of the Phytochemical Society of Europe, Bordeaux, France, 14–16 April 1999; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; Volume 43. [Google Scholar]
- Geran, R.I.; Greenberg, N.H.; Macdolnald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972, 3, 1–103. [Google Scholar]
- Guzmán, E.; Johnson, J.D.; Linley, P.A.; Gunasekera, S.E.; Wright, A.E. A novel activity from an old compound: Manzamine A reduces the metastatic potential of AsPC-1 pancreatic cancer cells and sensitizes them to TRAIL-induced apoptosis. Investig. New Drugs 2011, 29, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2010. Molecules 2011, 30, 5629–5646. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B., III; Smits, H.; Kim, D.-S. Spirastrellolide studies. Synthesis of the C1-C25 southern hemispheres of spirastrellolides A and B, exploiting anion relay chemistry. Tetrahedron 2010, 66, 6597–6605. [Google Scholar] [CrossRef] [PubMed]
- Shearman, J.W.; Myers, R.M.; Brenton, J.D.; Ley, S.V. Total synthesis of subereamollines A and B. Org. Biomol. Chem. 2011, 9, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, S.; Pereira-Cameselle, R.; de Lera, A.R. First total synthesis of dioxepine bastadin 3. Org. Biomol. Chem. 2012, 10, 6945–6950. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhou, F.; Al-Kareef, A.M.; Wang, H. Anticancer agents from marine sponges. J. Asian Nat. Prod. Res. 2015, 17, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Sulaiman, M.; Khedr, A.; El Sayed, K.A. Bioactive alkaloids from the Red Sea marine Verongid sponge Pseudoceratina arabica. Tetrahedron 2015, 71, 7837–7841. [Google Scholar] [CrossRef]
- Hooper, J.N.A.; van Soest, R.W.M. Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1–1101. [Google Scholar]
- Van Soest, R.W.M.; Boury-Esnault, N.; Hooper, J.N.A.; Rutzler, K.; de Voogd, N.J.; Alvarez de Glasby, B.; Hajdu, E.; Pisera, A.B.; Manconi, R.; Schoenberg, C.; et al. World Porifera Database. Available online: http://www.marinespecies.org/porifera (accessed on 14 July 2015).
- Morrow, C.; Cárdenas, P. Proposal for a revised classification of the Demospongiae (Porifera). Front. Zool. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M. Family Agelasidae Verrill, 1907. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 819–823. [Google Scholar]
- Calcul, L.; Tenney, K.; Ratnam, J.; McKerrow, J.H.; Crews, P. Structural variations to the 9-N-methyladeninium diterpenoid hybrid commonly isolated from Agelas sponges. Aust. J. Chem. 2010, 63, 915–921. [Google Scholar] [CrossRef]
- Proszenyák, Á.; Brændvang, M.; Charnock, C.; Gundersen, L.-L. The first synthesis of ent-Agelasine F. Tetrahedron 2009, 65, 194–199. [Google Scholar] [CrossRef]
- Stout, E.P.; Yu, L.C.; Molinski, T.F. Antifungal diterpene alkaloids from the Caribbean sponge Agelas citrina: Unified configurational assignments of agelasidines and agelasines. Eur. J. Org. Chem. 2012, 27, 5131–5135. [Google Scholar] [CrossRef] [PubMed]
- Asao, K.; Iio, H.; Tokoroyama, T. Total synthesis of (+)-agelasidine C, a physiologically active marine diterpenoid with hypotaurocyamine group. Chem. Lett. 1989, 10, 1813–1814. [Google Scholar] [CrossRef]
- Tilvi, S.; Moriou, C.; Martin, M.-T.; Gallard, J.-F.; Sorres, J.; Patel, K.; Petek, S.; Debitus, C.; Ermolenko, L.; Al-Mourabit, A. Agelastatin E, agelastatin F, and benzosceptrin C from the marine sponge Agelas dendromorpha. J. Nat. Prod. 2010, 73, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Stout, E.; Choi, M.Y.; Castro, J.E.; Molinski, T.F. Potent fluorinated agelastatin analogues for chronic lymphocytic leukemia: Design, synthesis, and pharmacokinetic studies. J. Med. Chem. 2014, 57, 5085–5093. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ueoka, R.; Takada, K.; Okada, S.; Ohtsuka, S.; Ise, Y.; Matsunaga, S. Isolation of spirastrellolides A and B from a marine sponge Epipolasis sp. and their cytotoxic activities. J. Nat. Prod. 2012, 75, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Lamoral-Theys, D.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Kiss, R.; Costantino, V. Evaluation of the antiproliferative activity of diterpene isonitriles from the sponge Pseudoaxinella flava in apoptosis-sensitive and apoptosis-resistant cancer cell lines. J. Nat. Prod. 2011, 74, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- Ueoka, R.; Ise, Y.; Ohtsuka, S.; Okada, S.; Yamori, T.; Matsunaga, S. Yaku’amides A and B, cytotoxic linear peptides rich in dehydroamino acids from the marine sponge Ceratopsion sp. J. Am. Chem. Soc. 2010, 132, 17692–17694. [Google Scholar] [CrossRef] [PubMed]
- Kuranaga, T.; Mutoh, H.; Sesoko, Y.; Goto, T.; Matsunaga, S.; Inoue, M. Elucidation and total synthesis of the correct structures of tridecapeptides yaku’amides A and B. Synthesis-driven stereochemical reassignment of four amino acid residues. J. Am. Chem. Soc. 2015, 137, 9443–9451. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Jimbo, M.; Gill, M.B.; Lash-Van Wyhe, L.L.; Murata, M.; Nonomura, K.; Swanson, G.T.; Sakai, R. Isolation, amino acid sequence and biological activities of novel long-chain polyamine-associated peptide toxins from the sponge Axinyssa aculeata. ChemBioChem 2011, 12, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jang, K.H.; Lee, Y.-J; Lee, H.-S.; Sim, C.J.; Oh, K.-B.; Shin, J. Triterpene galactosides of the pouoside class and corresponding aglycones from the sponge Lipastrotethya sp. J. Nat. Prod. 2011, 74, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jeon, J.-E.; Lee, Y.-J.; Lee, H.-S.; Sim, C.J.; Oh, K.-B.; Shin, J. Nortriterpene glycosides of the sarasinoside class from the sponge Lipastrotethya sp. J. Nat. Prod. 2012, 75, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.C.; Bergquist, P.R. Order Dictyoceratida Minchin, 1900. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1021–1066. [Google Scholar]
- Hooper, J.N.A.; van Soest, R.W.M. Family Dysideidae Gray, 1867. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1061–1066. [Google Scholar]
- Govindam, S.V.S.; Choi, B.-K.; Yoshioka, Y.; Kanamoto, A.; Fujiwara, T.; Okamoto, T.; Ojika, M. Novel cytotoxic polyoxygenated steroids from an okinawan sponge Dysidea sp. Biosci. Biotechnol. Biochem. 2012, 76, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.-H.; Huang, X.-J.; Yang, J.-S.; Yang, F.; Piao, S.-J.; Gao, H.; Li, J.; Ye, W.-C.; Yao, X.-S.; Chen, W.-S.; et al. Dysidavarones A–D, new sesquiterpene quinones from the marine sponge Dysidea avara. Org. Lett. 2012, 14, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Schmalzbauer, B.; Herrmann, J.; Müller, R.; Menche, D. Total synthesis and antibacterial activity of dysidavarone A. Org. Lett. 2013, 15, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Narita, K.; Katoh, T. Enantioselective total synthesis of dysidavarone A, a novel sesquiterpenoid quinone from the marine sponge Dysidea avara. Chem. Eur. J. 2014, 20, 2436–2439. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.C.; Bergquist, P.R. Family Irciniidae Gray, 1867. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1022–1027. [Google Scholar]
- Balansa, W.; Islam, R.; Fontaine, F.; Piggott, A.M.; Zhang, H.; Webbb, T.I.; Gilbert, D.F.; Lynch, J.W.; Capon, R.J. Ircinialactams: Subunit-selective glycine receptor modulators from Australian sponges of the family Irciniidae. Bioorg. Med. Chem. 2010, 18, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Abed, C.; Legrave, N.; Dufies, M.; Robert, G.; Guerineau, V.; Vacelet, J.; Auberger, P.; Amade, P.; Mehiri, M. A new hydroxylated nonaprenylhydroquinone from the mediterranean marine sponge Sarcotragus spinosulus. Mar. Drugs 2011, 9, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Tseng, S.-W.; Lu, M.-C.; Liu, L.-L.; Chou, Y.; Sung, P.-J. Cytotoxic C21 and C22 terpenoid-derived metabolites from the sponge Ircinia sp. J. Nat. Prod. 2011, 74, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.C.; Bergquist, P.R. Family Spongiidae Gray, 1867. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1051–1060. [Google Scholar]
- Bae, J.-M.; Jeon, J.-E.; Lee, Y.-J.; Lee, H.-S.; Sim, C.-J.; Oh, K.-B.; Shin, J.-H. Sesterterpenes from the tropical sponge Coscinoderma sp. J. Nat. Prod. 2011, 74, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.-J.; Zhang, H.-J.; Lu, H.-Y.; Yang, F.; Jiao, W.-H.; Yi, Y.-H.; Chen, W.-S.; Lin, H.-W. Hippolides A–H, acyclic manoalide derivatives from the marine sponge Hippospongia lachne. J. Nat. Prod. 2011, 74, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.C.; Bergquist, P.R. Family Thorectidae Bergquist, 1978. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1028–1050. [Google Scholar]
- Whitson, E.L.; Pluchino, K.M.; Hall, M.D.; McMahon, J.B.; McKee, T.C. New candidaspongiolides, tedanolide analogs that selectively inhibit melanoma cell growth. Org. Lett. 2011, 13, 3518–3521. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yamori, T.; Kobayashi, M.; Duan, H. Antiproliferative and antiangiogenic activities of smenospongine, a marine sponge sesquiterpene aminoquinone. Mar. Drugs 2011, 9, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. Sesquiterpene benzoxazoles and sesquiterpene quinones from the marine sponge Dactylospongia elegans. J. Nat. Prod. 2011, 74, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Bishara, A.; Rudi, A.; Aknin, M.; Neumann, D.; Ben-Califa, N.; Kashman, Y. Salarins D–J, seven new nitrogenous macrolides from the Madagascar sponge Fascaplysinopsis sp. Tetrahedron 2010, 66, 4339–4345. [Google Scholar] [CrossRef]
- Zhang, H.; Khalil, Z.G.; Capon, R.J. Fascioquinols A–F: Bioactive meroterpenes from a deep-water southern Australian marine sponge, Fasciospongia sp. Tetrahedron 2011, 67, 2591–2595. [Google Scholar] [CrossRef]
- Inman, W.D.; Bray, W.M.; Gassner, N.C.; Lokey, R.S.; Tenney, K.; Shen, Y.Y.; Tendyke, K.; Suh, T.; Crews, P. A β-carboline alkaloid from the Papua New Guinea marine sponge Hyrtios reticulatus. J. Nat. Prod. 2010, 73, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Yamanokuchi, R.; Imada, K.; Miyazaki, M.; Kato, H.; Watanabe, T.; Fujimuro, M.; Saeki, Y.; Yoshinaga, S.; Terasawa, H.; Iwasaki, N.; et al. Hyrtioreticulins A–E, indole alkaloids inhibiting the ubiquitin-activating enzyme, from the marine sponge Hyrtios reticulatus. Bioorg. Med. Chem. 2012, 20, 4437–4442. [Google Scholar] [CrossRef] [PubMed]
- Rothmeier, A.S.; Schneiders, U.M.; Wiedmann, R.M.; Ischenko, I.; Bruns, C.J.; Rudy, A.; Zahler, S.; Vollmar, A.M. The marine compound spongistatin 1 targets pancreatic tumor progression and metastasis. Int. J. Cancer 2010, 127, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huang, K.-C.; Tendyke, K.; Marsh, J.; Liu, J.; Qiu, D.; Littlefield, B.A.; Nomoto, K.; Atasoylu, O.; Risatti, C.A.; et al. In vitro and in vivo anticancer activity of (+)-spongistatin 1. Anticancer Res. 2011, 31, 2773–2780. [Google Scholar] [PubMed]
- Smith, A.B., III; Zhu, W.; Shirakami, S.; Sfouggatakis, C.; Doughty, V.A.; Bennett, C.S.; Sakamoto, Y. Total synthesis of (+)-spongistatin 1. An effective second-generation construction of an advanced EF Wittig salt, fragment union, and final elaboration. Org. Lett. 2003, 5, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.B.; Mahdi, F.; Liu, Y.; Coothankandaswamy, V.; Jekabsons, M.B.; Johnson, T.A.; Sashidhara, K.V.; Crews, P.; Nagle, D.G.; Zhou, Y.-D. The marine sponge metabolite mycothiazole: A novel prototype mitochondrial complex I inhibitor. Bioorg. Med. Chem. 2010, 18, 5988–5994. [Google Scholar] [CrossRef] [PubMed]
- Le Flohic, A.; Meyer, C.; Cossy, J. Total synthesis of (±)-mycothiazole and formal enantioselective approach. Org. Lett. 2005, 7, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Prawat, H.; Mahidol, C.; Kaweetripob, W.; Wittayalai, S.; Ruchirawat, S. Iodo-sesquiterpene hydroquinone and brominated indole alkaloids from the Thai sponge Smenospongia sp. Tetrahedron 2012, 68, 6881–6886. [Google Scholar] [CrossRef]
- Sperry, J. Concise synthesis of 5,6-dibromotryptamine and 5,6-dibromo-N,N-dimethyltryptamine en route to the antibiotic alternatamide. Tetrahedron Lett. 2011, 52, 4042–4044. [Google Scholar] [CrossRef]
- Nakamura, M.; Suzuki, A.; Nakatani, M.; Fuchikami, T.; Inoue, M.; Katoh, T. An efficient synthesis of (+)-aureol via boron trifluoride etherate-promoted rearrangement of (+)-arenarol. Tetrahedron Lett. 2002, 43, 6929–6932. [Google Scholar] [CrossRef]
- Kuan, K.K.W.; Pepper, H.P.; Bloch, W.M.; George, J.H. Total synthesis of (+)-aureol. Org. Lett. 2012, 14, 4710–4713. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.; Muñoz-Bascón, J.; Roldan-Molina, E.; Rivas-Bascón, N.; Padial, N.M.; Rodríguez-Maecker, R.; Rodríguez-García, I.; Enrique Oltra, J. Synthesis of (±)-aureol by bioinspired rearrangements. J. Org. Chem. 2015, 80, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M.; Hooper, J.N.A. Order Haplosclerida Topsent, 1928. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 831–832. [Google Scholar]
- Desqueyroux-Faúndez, R.; Valentine, C. Family Callyspongiidae de Laubenfels, 1936. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 835–851. [Google Scholar]
- Umeyama, A.; Matsuoka, N.; Mine, R.; Nakata, A.; Arimoto, E.; Matsui, M.; Shoji, N.; Arihara, S.; Takei, M.; Hashimoto, T. Polyacetylene diols with antiproliferative and driving Th1 polarization effects from the marine sponge Callyspongia sp. J. Nat. Med. 2010, 64, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Gung, B.W.; Craft, D.T.; Truelove, J. A short synthesis of (S)-(+)-siphonodiol. Tetrahedron Asymmetry 2007, 18, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Min, C.C.; Teuscher, F.; Ebel, R.; Kakoschke, C.; Lin, W.; Wray, V.; Edrada-Ebel, R.-A.; Proksch, P. Callyaerins A–F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg. Med. Chem. 2010, 18, 4947–4956. [Google Scholar] [CrossRef] [PubMed]
- Desqueyroux-Faúndez, R.; Valentine, C. Family Niphatidae Van Soest, 1980. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 874–889. [Google Scholar]
- Kura, K.; Kubota, T.; Fromont, J.; Kobayashi, J. Pyrinodemins E and F, new 3-alkylpyridine alkaloids from sponge Amphimedon sp. Bioorg. Med. Chem. Lett. 2011, 21, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Meimetis, L.G.; Williams, D.E.; Mawji, N.R.; Banuelos, C.A.; Lal, A.A.; Park, J.J.; Tien, A.H.; Fernandez, J.G.; de Voogdt, N.J.; Sadar, M.D.; et al. Niphatenones, glycerol ethers from the sponge Niphates digitalis block androgen receptor transcriptional activity in prostate cancer cells: Structure elucidation, synthesis, and biological activity. J. Med. Chem. 2012, 55, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Desqueyroux-Faúndez, R.; Valentine, C. Family Petrosiidae Van Soest, 1980. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 906–917. [Google Scholar]
- Wei, X.; Nieves, K.; Rodriguez, A.D. Neopetrosiamine A, biologically active bis-piperidine alkaloid from the Caribbean Sea sponge Neopetrosia proxima. Bioorg. Med. Chem. Lett. 2010, 20, 5905–5908. [Google Scholar] [CrossRef] [PubMed]
- Hitora, Y.; Takada, K.; Okada, S.; Matsunaga, S. Miyakosynes A–F, cytotoxic methyl branched acetylenes from a marine sponge Petrosia sp. Tetrahedron 2011, 67, 4530–4534. [Google Scholar] [CrossRef]
- Hitora, Y.; Takada, K.; Okada, S.; Ise, Y.; Matsunaga, S. (−)-Duryne and its homologues, cytotoxic acetylenes from a marine sponge Petrosia sp. J. Nat. Prod. 2011, 74, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Gung, B.W.; Omollo, A.O. Total synthesis of (+)- and (−)-duryne: A potent anticancer agent from the marine sponge Cribrochalina dura. Establishment of the central double bond geometry and the absolute configuration of the chiral centers. J. Org. Chem. 2008, 73, 1067–7100. [Google Scholar] [CrossRef] [PubMed]
- Jakubec, P.; Hawkins, A.; Felzmann, W.; Dixon, D.J. Total synthesis of manzamine A and related alkaloids. J. Am. Chem. Soc. 2012, 134, 17482–17485. [Google Scholar] [CrossRef] [PubMed]
- Muricy, G.; Diaz, M.C. Order Homosclerophorida Dendy, 1905. Family Plakinidae Schulze, 1880. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 71–82. [Google Scholar]
- Mohammed, R.; Peng, J.; Kelly, M.; Yousaf, M.; Winn, E.; Odde, S.; Bie, Z.; Xie, A.; Doerksen, R.J.; Hamann, M.T. Polyketide-peroxides from a species of Jamaican Plakortis (Porifera: Demospongiae). Aust. J. Chem. 2010, 63, 877–885. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Tian, X.-Y.; Li, Z.-W.; Peng, X.-S.; Wong, H.N.C. Total synthesis of plakortide E and biomimetic synthesis of plakortone B. Chem. Eur. J. 2011, 17, 5874–5880. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Song, Y.-L.; Zhang, H.-J.; Yang, F.; Yu, H.-B.; Jiao, W.-H.; Piao, S.-J.; Chen, W.-S.; Lin, H.-W. Simplextones A and B, unusual polyketides from the marine sponge Plakortis simplex. Org. Lett. 2011, 13, 3154–3157. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Charman, S.A.; Charman, W.N.; White, K.L.; Shackleford, D.M.; Edstein, M.D.; Andrews, K.T.; et al. Antimalarial activity of pyrroloiminoquinones from the Australian marine sponge Zyzzya sp. J. Med. Chem. 2012, 55, 5851–5858. [Google Scholar] [CrossRef] [PubMed]
- Harayama, Y.; Kita, Y. Pyrroloiminoquinone alkaloids. Discorhabdins and makaluvamines. Curr. Org. Chem. 2005, 9, 1567–1588. [Google Scholar] [CrossRef]
- Ushiyama, S.; Umaoka, H.; Kato, H.; Suwa, Y.; Morioka, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; et al. Manadosterols A and B, sulfonated sterol dimers inhibiting the Ubc13-Uev1A interaction, solated from the marine sponge Lissodendryx fibrosa. J. Nat. Prod. 2012, 75, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.G.; Wilke, D.V.; Jimenez, P.C.; de Oliveira, J.R.; Pessoa, O.D.L.; Silveira, E.R.; Viana, F.A.; Pessoa, C.; Odorico de Moraes, M.; Hajdu, E.; et al. Guanidine alkaloids from Monanchora arbuscula: Chemistry and antitumor potential. Chem. Biodivers. 2011, 8, 1433–1445. [Google Scholar] [CrossRef]
- Shen, K.; Livinghouse, T. A stereocontrolled synthesis of (±)-ptilocaulin via a Rh(I)-catalyzed intramolecular [4 + 2] cycloaddition. Synlett 2010, 2, 247–249. [Google Scholar]
- Guzii, A.G.; Makarieva, T.N.; Denisenko, V.A.; Dmitrenok, P.S.; Kuzmich, A.S.; Dyshlovoy, S.A.; Krasokhin, V.B.; Stonik, V.A. Monanchocidin: A new apoptosis-inducing polycyclic guanidine alkaloid from the marine sponge Monanchora pulchra. Org. Lett. 2010, 12, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Tabakmaher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Shubina, L.K.; Kuzmich, A.S.; Lee, H.-S.; Stonik, V.A. Monanchocidins B–E: Polycyclic guanidine alkaloids with potent antileukemic activities from the sponge Monanchora pulchra. J. Nat. Prod. 2011, 74, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pierce, J.G. Synthesis of the 5,6-Dihydroxymorpholin-3-one. Fragment of monanchocidin A. Org. Lett. 2015, 17, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Tabakmaher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Kuzmich, A.S.; Lee, H.-S.; Stonik, V.A. Monanchomycalins A and B, unusual guanidine alkaloids from the sponge Monanchora pulchra. Tetrahedron Lett. 2012, 53, 4228–4231. [Google Scholar] [CrossRef]
- Parr, B.T. Enantioselective Synthesis of Monanchocidin A. Available online: https://grantome.com/grant/NIH/F32-GM110898-01 (accessed on 14 July 2016).
- Wang, W.; Lee, Y.; Lee, T.G.; Mun, B.; Giri, A.G.; Lee, J.; Kim, H.; Hahn, D.; Yang, I.; Chin, J.; et al. Phorone A and isophorbasone A, sesterterpenoids isolated from the marine sponge Phorbas sp. Org. Lett. 2012, 14, 4486–4489. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, H.; Hwang, H.; Kang, H.; Rho, J.-R. Gukulenins A and B, cytotoxic tetraterpenoids from the marine sponge Phorbas gukulensis. J. Nat. Prod. 2010, 73, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Na, Z.; Jung, M.; Lee, H.-S.; Sim, C.J.; Nahm, K.; Oh, K.-B.; Shin, J. Discorhabdins from the Korean marine sponge Sceptrella sp. J. Nat. Prod. 2010, 73, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Chou, K.-J.; Wang, G.-H.; Wu, Y.-C.; Wang, L.-H.; Chen, J.-P.; Sheu, J.-H.; Sung, P.-J. Norterpenoids and related peroxides from the Formosan marine sponge Negombata corticata. J. Nat. Prod. 2010, 73, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.A.; Debbab, A.; Wray, V.; Muller, W.E.G.; Proksch, P. New bioactive alkaloids from the marine sponge Stylissa sp. Tetrahedron 2012, 68, 10176–10179. [Google Scholar] [CrossRef]
- Saleem, R.S.Z.; Tepe, J. A concise total synthesis of hymenialdisine. Tetrahedron Lett. 2015, 56, 3011–3013. [Google Scholar] [CrossRef]
- Ohno, O.; Chiba, T.; Todoroki, S.; Yoshimura, H.; Maru, N.; Maekawa, K.; Imagawa, H.; Yamada, K.; Wakamiya, A.; Suenaga, K.; et al. Halichonines A, B, and C, novel sesquiterpene alkaloids from the marine sponge Halichondria okadai Kadota. Chem. Commun. 2011, 47, 12453–12455. [Google Scholar] [CrossRef] [PubMed]
- Uriz, M.J. Family Ancorinidae Schmidt, 1870. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 108–126. [Google Scholar]
- Barnes, E.C.; Said, N.A.B.M.; Williams, E.D.; Hooper, J.N.A.; Davis, R.A. Ecionines A and B, two new cytotoxic pyridoacridine alkaloids from the Australian marine sponge, Ecionemia geodides. Tetrahedron 2010, 66, 283–287. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. A new diketopiperazine, cyclo-(4-S-hydroxy-R-proline-R-isoleucine), from an Australian specimen of the sponge Stelletta sp. Mar. Drugs 2011, 9, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M.; Hooper, J.N.A. Family Calthropellidae Lendenfeld, 1907. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 127–133. [Google Scholar]
- Sirirak, T.; Kittiwisut, S.; Janma, C.; Yuenyongsawad, S.; Suwanborirux, K.; Plubrukarn, A. Kabiramides J and K, trisoxazole macrolides from the sponge Pachastrissa nux. J. Nat. Prod. 2011, 74, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Uriz, M.J. Family Geodiidae Gray, 1867. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 134–140. [Google Scholar]
- Cárdenas, P.; Rapp, H.T.; Schander, C.; Tendal, O.S. Molecular taxonomy and phylogeny of the Geodiidae (Porifera, Demospongiae, Astrophorida)—Combining phylogenetic and Linnaean classification. Zool. Scr. 2010, 39, 89–106. [Google Scholar]
- Cárdenas, P.; Xavier, J.R.; Reveillaud, J.; Schander, C.; Rapp, H.T. Molecular phylogeny of the Astrophorida (Porifera, Demospongiae) reveals an unexpected high level of spicule homoplasy. PLoS ONE 2015, 6, e18318. [Google Scholar]
- Liu, W.K.; Ho, J.C.; Che, C.T. Apoptotic activity of isomalabaricane triterpenes on human promyelocytic leukemia HL60 cells. Cancer Lett. 2005, 230, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Che, C.T. Isomalabaricane-type nortriterpenoids and other constituents of the marine sponge Geodia japonica. J. Nat. Prod. 2001, 64, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Cheung, F.W.K.; Li, C.; Che, C.-T.; Liu, B.P.L.; Wang, L.; Liu, W.-K. Geoditin A induces oxidative stress and apoptosis on human colon HT29 cells. Mar. Drugs 2010, 8, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Cheung, F.W.; Guo, J.; Ling, Y.H.; Che, C.T.; Liu, W.K. Anti-melanogenic property of geoditin A in murine B16 melanoma cells. Mar. Drugs 2012, 10, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Lyakhova, E.G.; Kolesnikova, S.A.; Kalinovsky, A.I.; Afiyatullov, S.S.; Dyshlovoy, S.A.; Krasokhin, V.B.; Minh, C.V.; Stonik, V.A. Bromine-containing alkaloids from the marine sponge Penares sp. Tetrahedron Lett. 2012, 53, 6119–6122. [Google Scholar] [CrossRef]
- Prior, A.; de Koning, C.B. Progress towards the total synthesis of marine sponge alkaloid 3,11-dibromo-13H-indolo[3,2-k]phenanthridine. In Proceedings of the SACI-ACS Binational Organic Chemistry Conference (BOCC), Stellenbosch, South Africa, 30 November–4 December 2014.
- Li, J.; Xu, B.; Cui, J.; Deng, Z.; de Voogd, N.J.; Proksch, P.; Lin, W. Globostelletins A–I, cytotoxic isomalabaricane derivatives from the marine sponge Rhabdastrella globostellata. Bioorg. Med. Chem. 2010, 18, 4639–4647. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, H.; Ren, J.; Deng, Z.; de Voogd, N.J.; Proksch, P.; Lin, W. Globostelletins J–S, isomalabaricanes with unusual cyclopentane sidechains from the marine sponge Rhabdastrella globostellata. Tetrahedron 2012, 68, 559–565. [Google Scholar] [CrossRef]
- Hirashima, M.; Tsuda, K.; Hamada, T.; Okamura, H.; Furukawa, T.; Akiyama, S.; Tajitsu, Y.; Ikeda, R.; Komatsu, M.; Doe, M.; et al. Cytotoxic isomalabaricane derivatives and a monocyclic triterpene glycoside from the sponge Rhabdastrella globostellata. J. Nat. Prod. 2010, 73, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, P.R.; Cook, S.C. Order Verongida Bergquist, 1980. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, p. 1081. [Google Scholar]
- Ciminiello, P.; Costantino, V.; Fattorusso, E.; Magno, S.; Mangoni, A.; Pansini, M. Chemistry of Verongida Sponges, II. Constituents of the Caribbean Sponge Aplysina fistularis forma fulva. J. Nat. Prod. 1994, 57, 705–712. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Cook, S.C. Family Aplysinellidae Bergquist, 1980. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1082–1085. [Google Scholar]
- Shaker, K.H.; Zinecker, H.; Ghani, M.A.; Imhoff, J.F.; Schneider, B. Bioactive metabolites from the sponge Suberea sp. Chem. Biodiv. 2010, 7, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Ogamino, T.; Nishiyama, S. A new ring-opening access to aeroplysinin-1, a secondary metabolite of Verongia aerophoba. Tetrahedron 2003, 59, 9419–9423. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Cook, S.C. Family Ianthellidae Hyatt, 1875. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1089–1093. [Google Scholar]
- Calcul, L.; Inman, W.D.; Morris, A.A.; Tenney, K.; Ratnam, J.; McKerrow, J.H.; Valeriote, F.A.; Crews, P. Additional insights on the bastadins: Isolation of analogues from the sponge Ianthella cf. reticulata and exploration of the oxime configurations. J. Nat. Prod. 2010, 73, 365–372. [Google Scholar] [PubMed]
- Decamps, C.; Hantson, A.-L.; Niemirowski, L.; Capiau, E.; De Meyer, M. Synthesis of molecules of biological interest. Contribution to the synthesis of bastadins. Biotechnol. Agron. Soc. Environ. 2010, 14, 593–602. [Google Scholar]
- Bergquist, P.R.; Cook, S.C. Family Pseudoceratinidae Carter, 1885. In Systema Porifera: A Guide to the Classification of Sponges, 1st ed.; Hooper, J.N.A., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume I, pp. 1086–1088. [Google Scholar]
- Shaala, L.A.; Youssef, D.T.A.; Sulaiman, M.; Behery, F.A.; Foudah, A.I.; El Sayed, K.A. Subereamolline A as a potent breast cancer migration, invasion and proliferation inhibitor and bioactive dibrominated alkaloids from the Red Sea sponge Pseudoceratina arabica. Mar. Drugs 2012, 10, 2492–2508. [Google Scholar] [CrossRef] [PubMed]
- Ueoka, R.; Ise, Y.; Okada, S.; Matsunaga, S. Cell differentiation inducers from a marine sponge Biemna sp. Tetrahedron 2011, 67, 6679–6681. [Google Scholar] [CrossRef]
- Oku, N.; Takada, K.; Fuller, R.W.; Wilson, J.A.; Peach, M.L.; Pannell, L.K.; McMahon, J.B.; Gustafson, K.R. Isolation, structural elucidation, and absolute stereochemistry of enigmazole A, a cytotoxic phosphomacrolide from the Papua New Guinea marine sponge Cinachyrella enigmatica. J. Am. Chem. Soc. 2010, 132, 10278–10285. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Conte, M.; Capon, R.J. Mirabilins revisited: Polyketide alkaloids from a Southern Australian marine sponge, Clathria sp. Org. Biomol. Chem. 2010, 8, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jang, K.H.; Jeon, J.-E.; Yang, W.-Y.; Sim, C.J.; Oh, K.-B.; Shin, J. Cyclic bis-1,3-dialkylpyridiniums from the sponge Haliclona sp. Mar. Drugs 2012, 10, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-E.; Bae, J.-M.; Lee, K.-J.; Oh, K.-B.; Shin, J.-H. Scalarane sesterterpenes from the sponge Hyatella sp. J. Nat. Prod. 2011, 74, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, F.; Wielgosz-Collin, G.; Robic, A.; Debitus, C.; Malleter, M.; Roussakis, C.; Kornprobst, J.-M.; Barnathan, G. Antiproliferative activity against human non-small cell lung cancer of two O-alkyl-diglycosylglycerols from the marine sponges Myrmekioderma dendyi and Trikentrion laeve. Eur. J. Med. Chem. 2012, 49, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.H.; Lee, Y.; Sim, C.J.; Oh, K.-B.; Shin, J. Bioactive lipids from the sponge Spirastrella abata. Bioorg. Med. Chem. Lett. 2012, 22, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- De Marino, S.; Festa, C.; D’Auria, M.V.; Cresteil, T.; Debitus, C.; Zampella, A. Swinholide J, a potent cytotoxin from the marine sponge Theonella swinhoei. Mar. Drugs 2011, 9, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Skepper, C.K.; Quach, T.; Molinski, T.F. Total synthesis of enigmazole A from Cinachyrella enigmatica. Bidirectional bond constructions with an ambident 2,4-disubstituted oxazole synthon. J. Am. Chem. Soc. 2010, 132, 10286–10292. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyasu, K.; Fumiyasu, T.; Akinori, K. Synthesis of cystodamine, a pentacyclic aza-aromatic alkaloid. Tetrahedron Lett. 1997, 38, 4441–4442. [Google Scholar]
- Chabner, B.A. NCI-60 cell line screening: A radical departure in its time. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yamori, T. JFCR39, a panel of 39 human cancer cell lines, and its application in the discovery and development of anticancer drugs. Bioorg. Med. Chem. 2012, 20, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
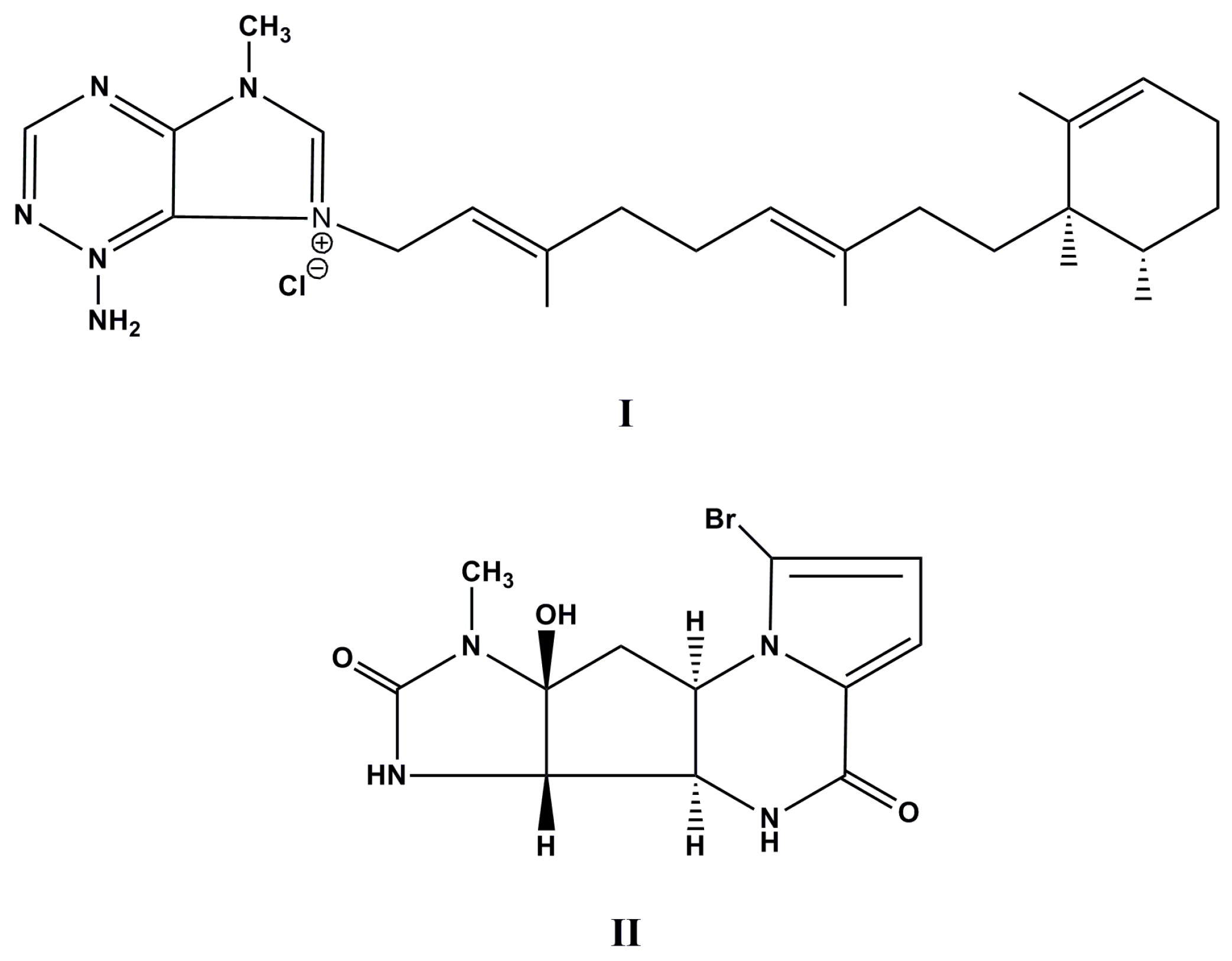
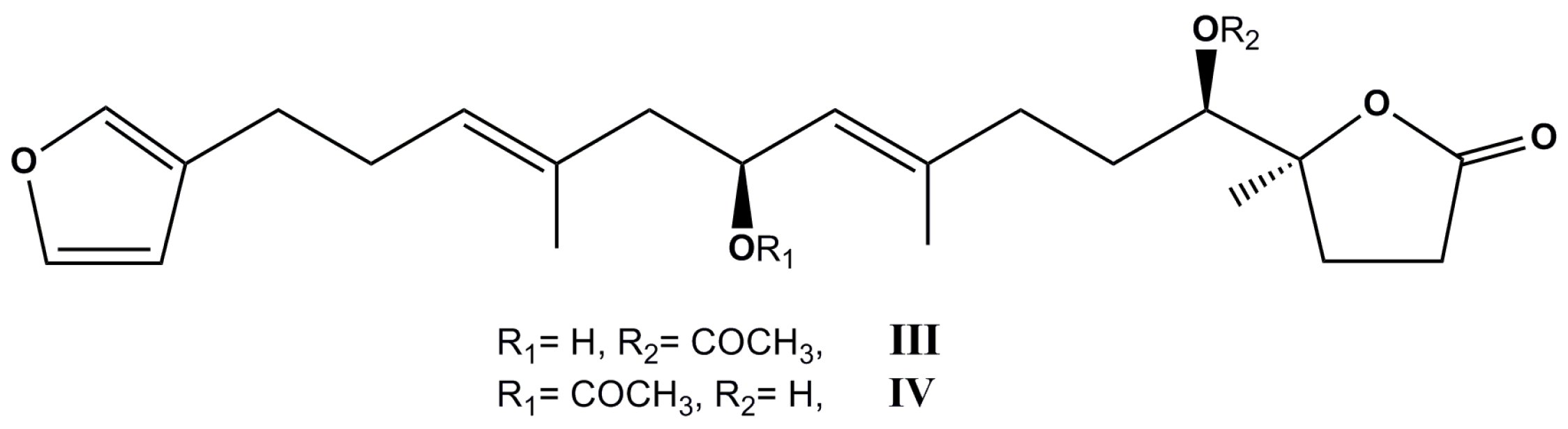
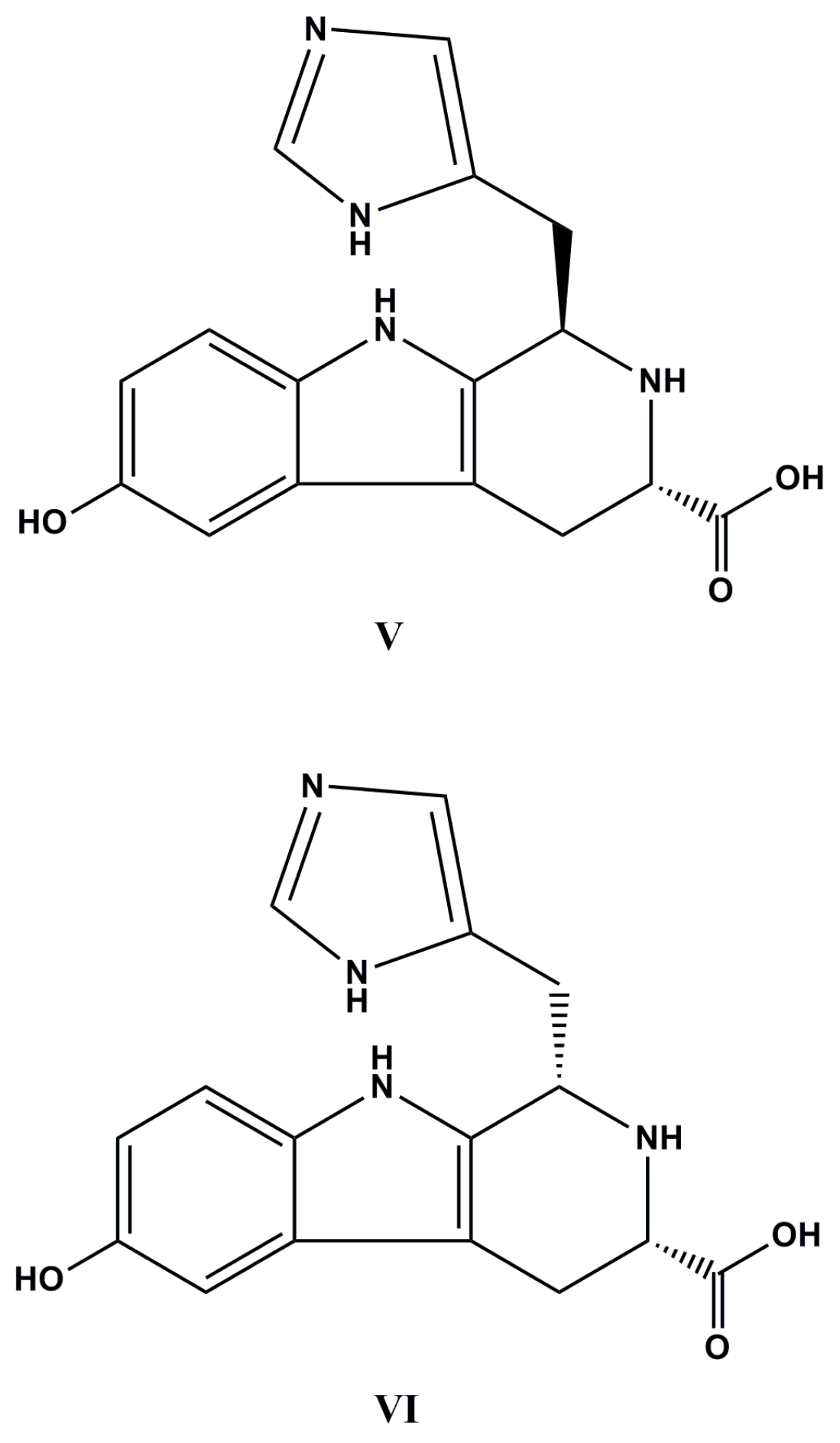
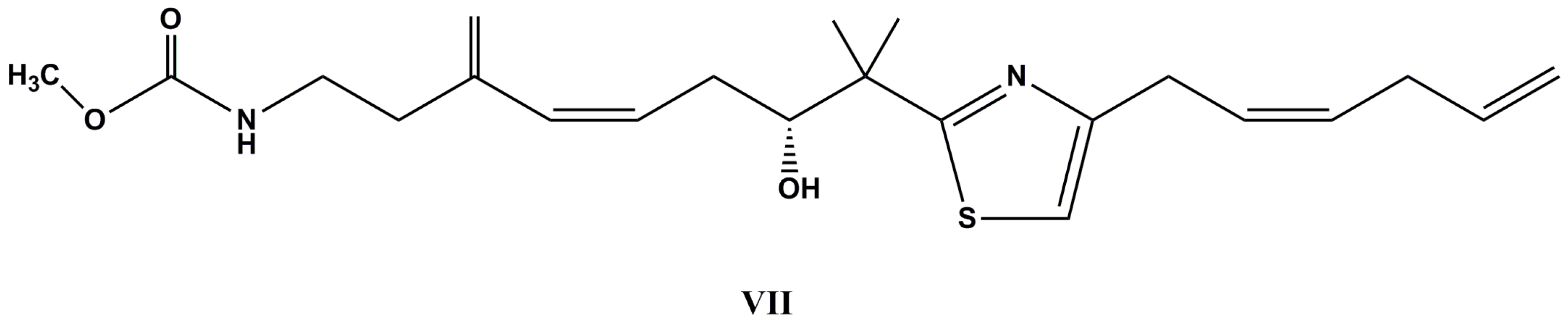
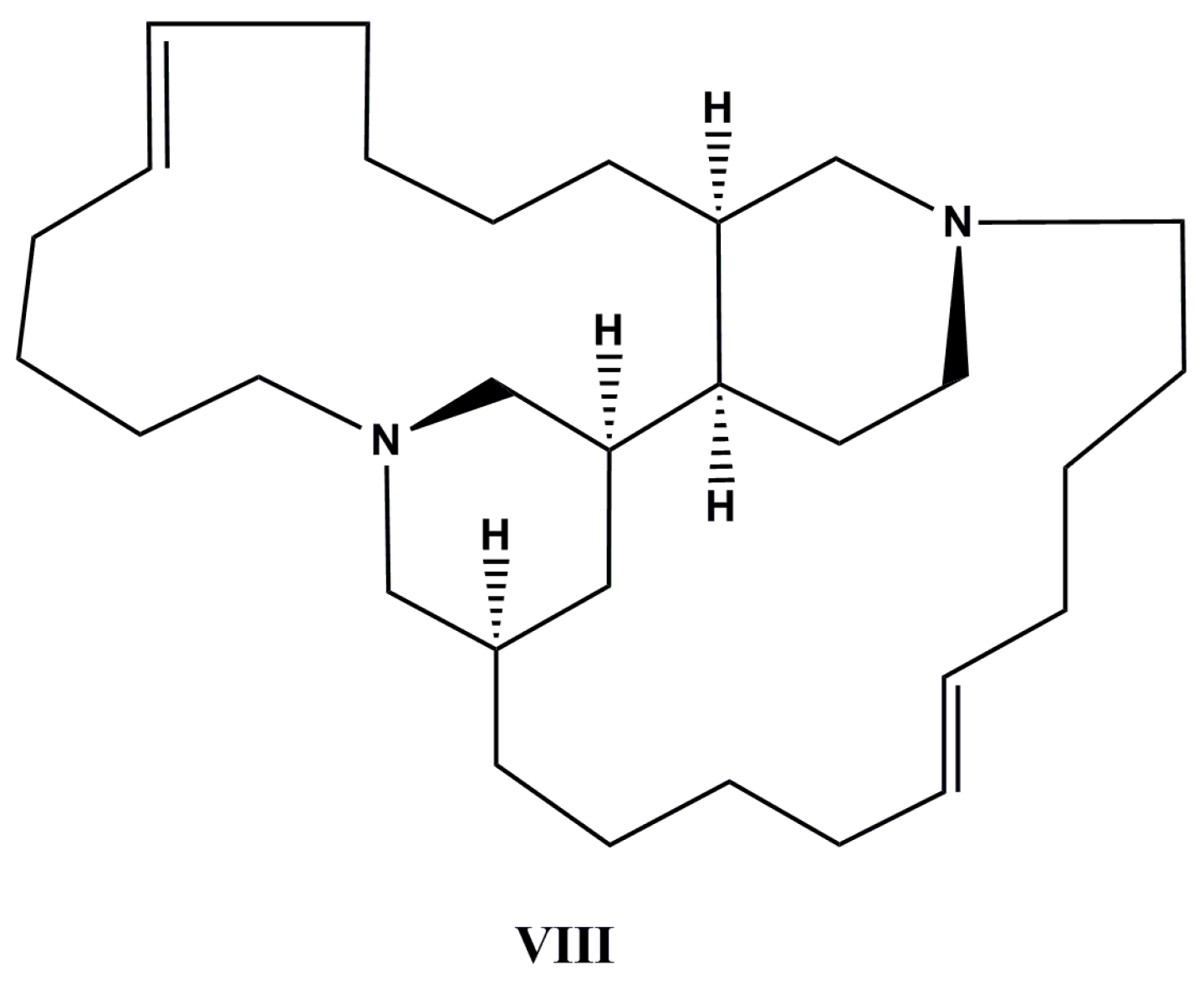

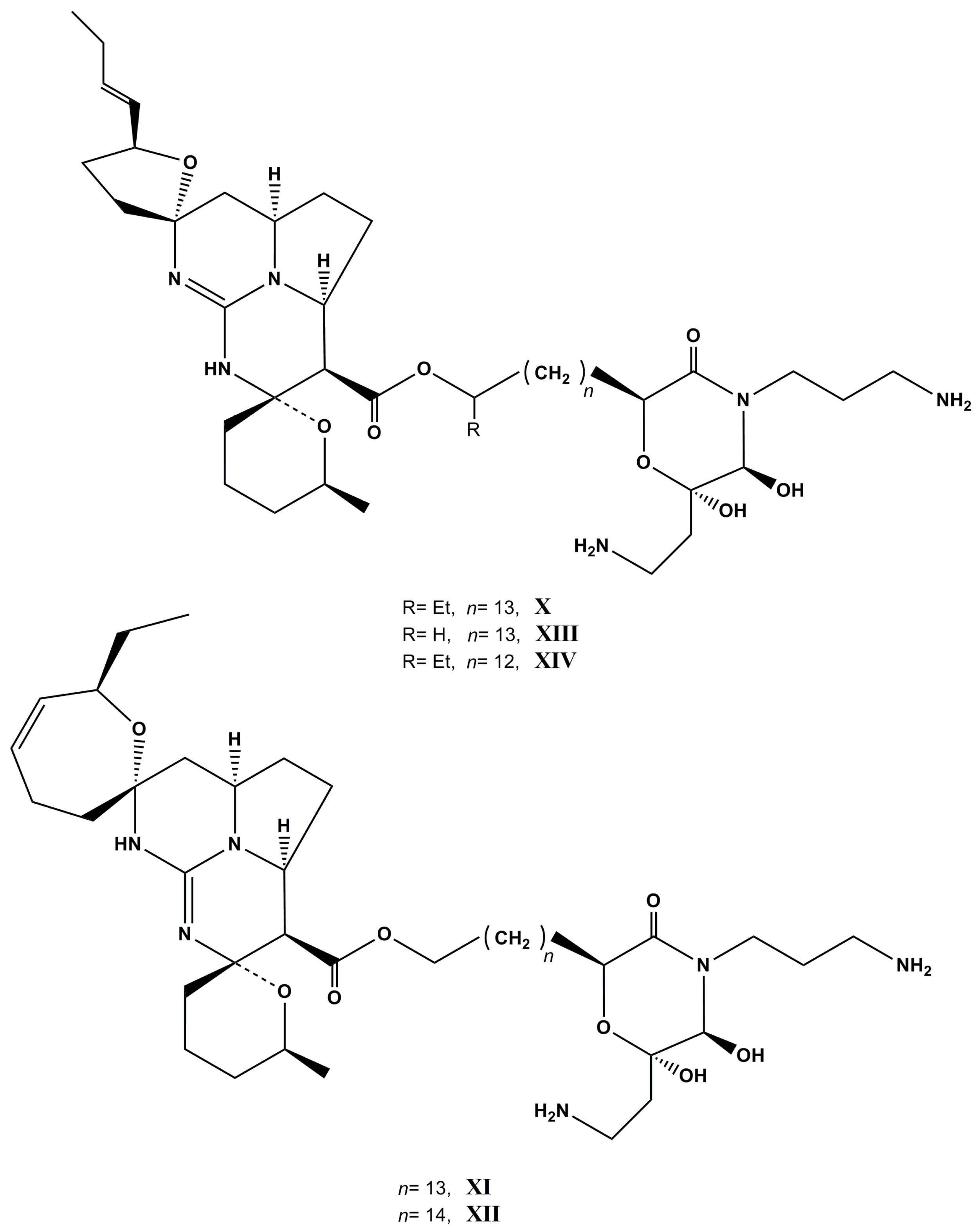
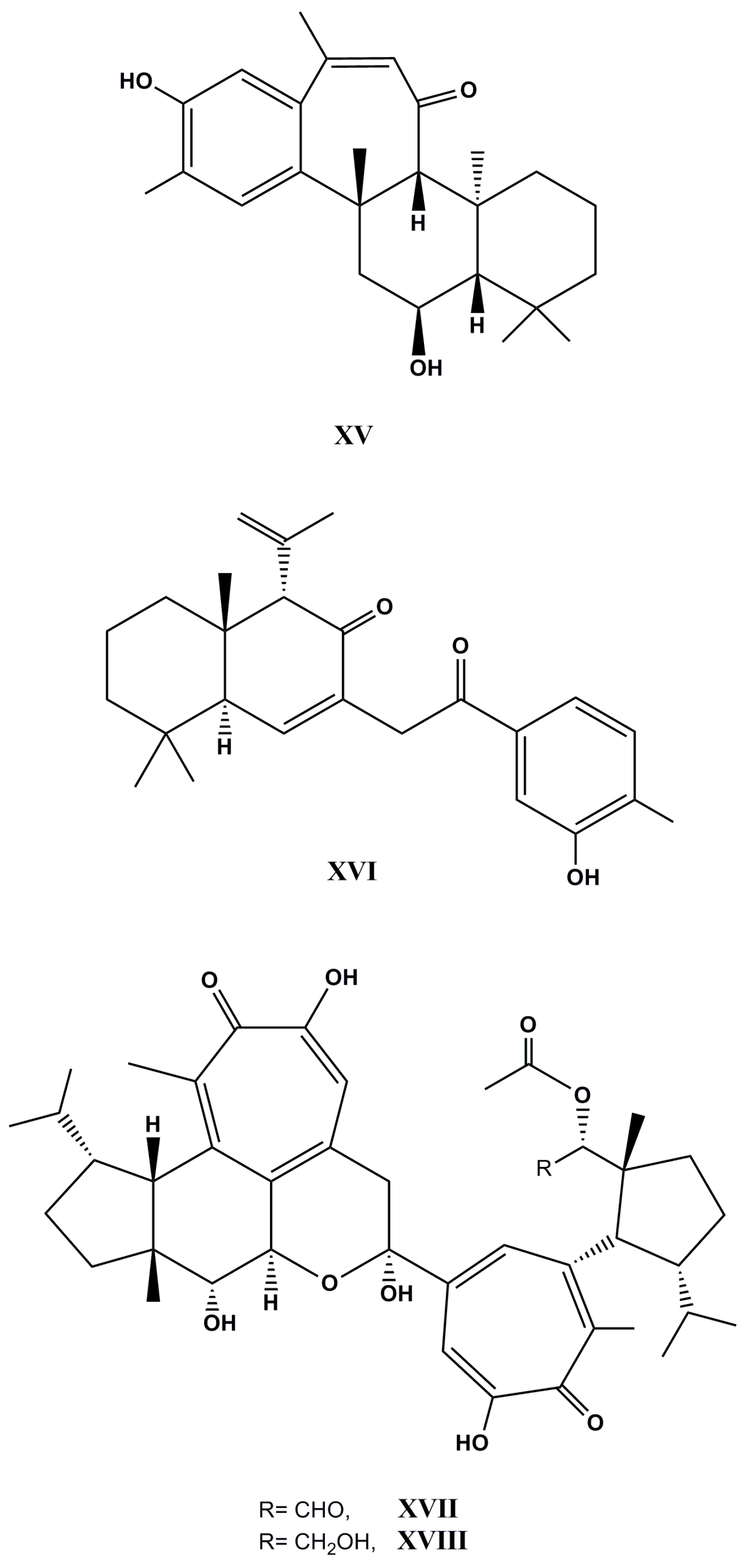
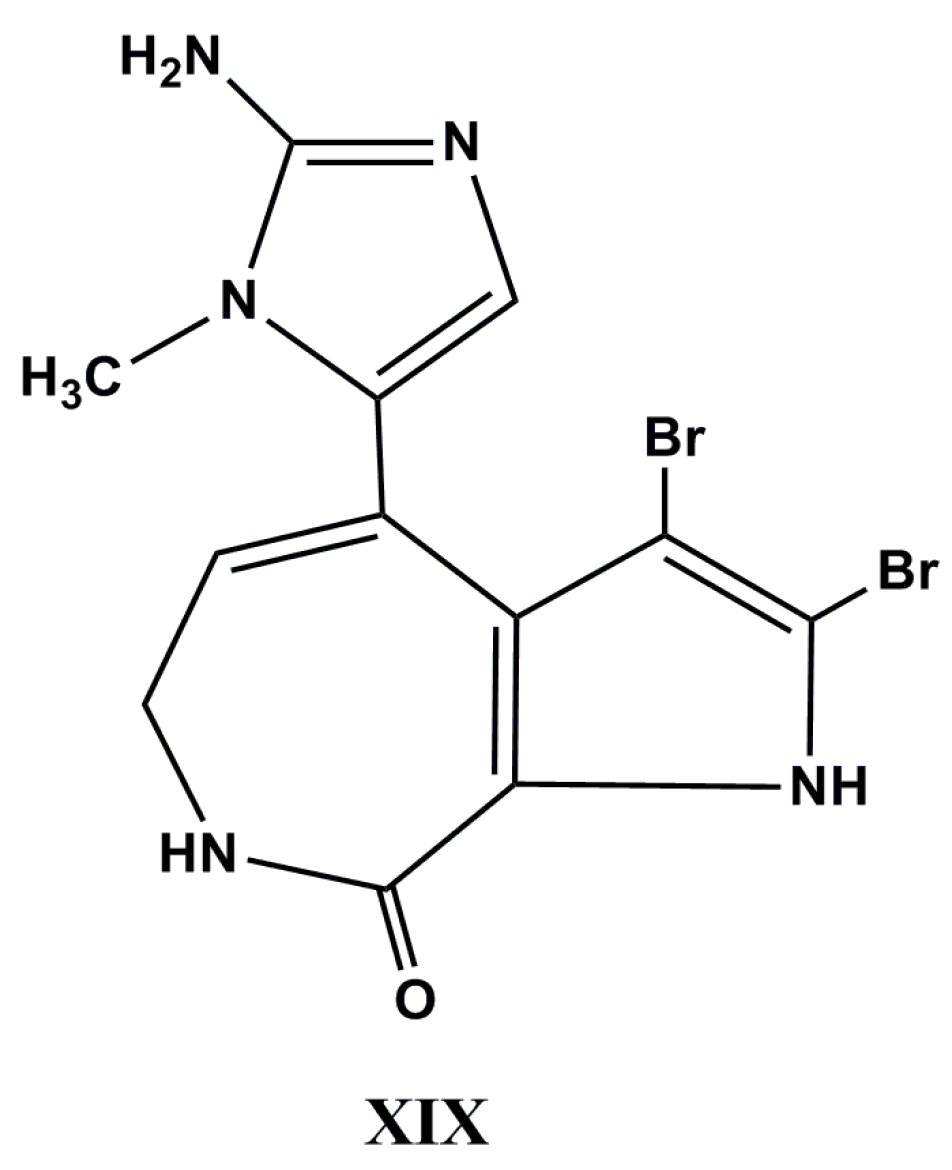
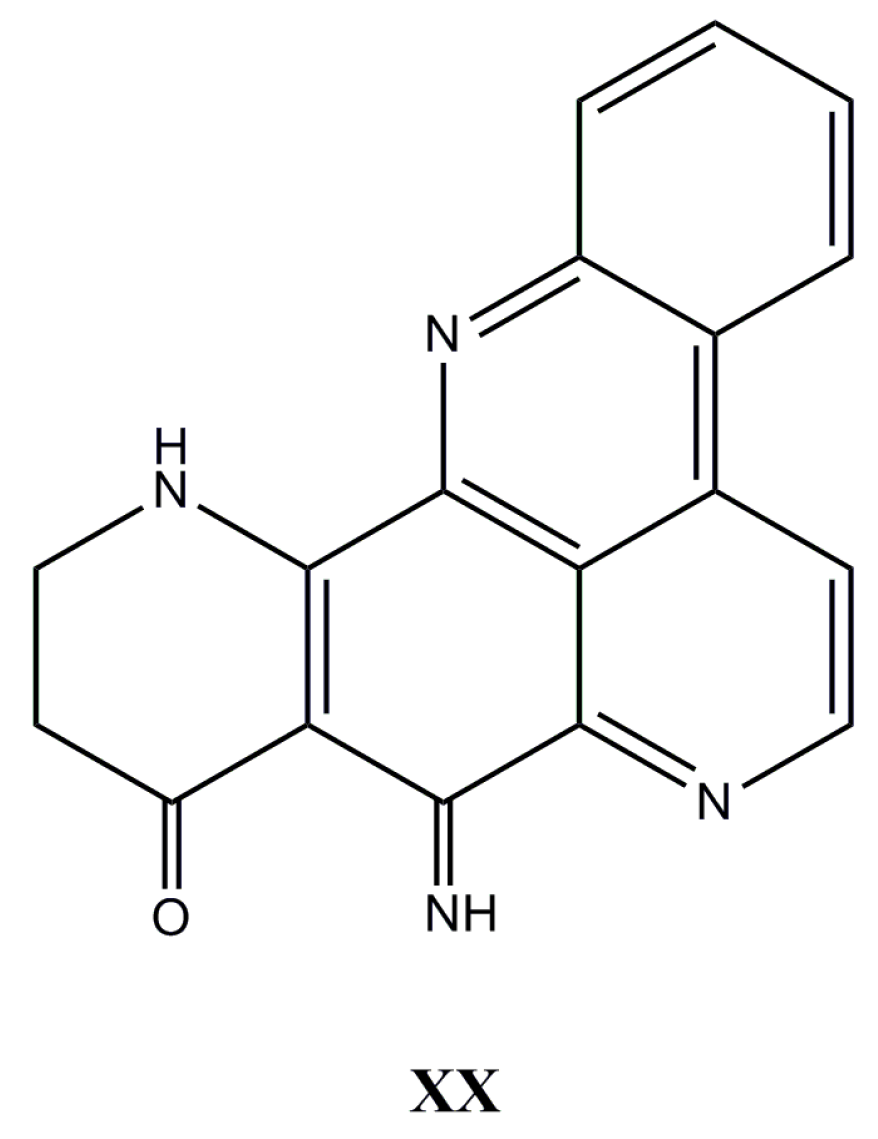
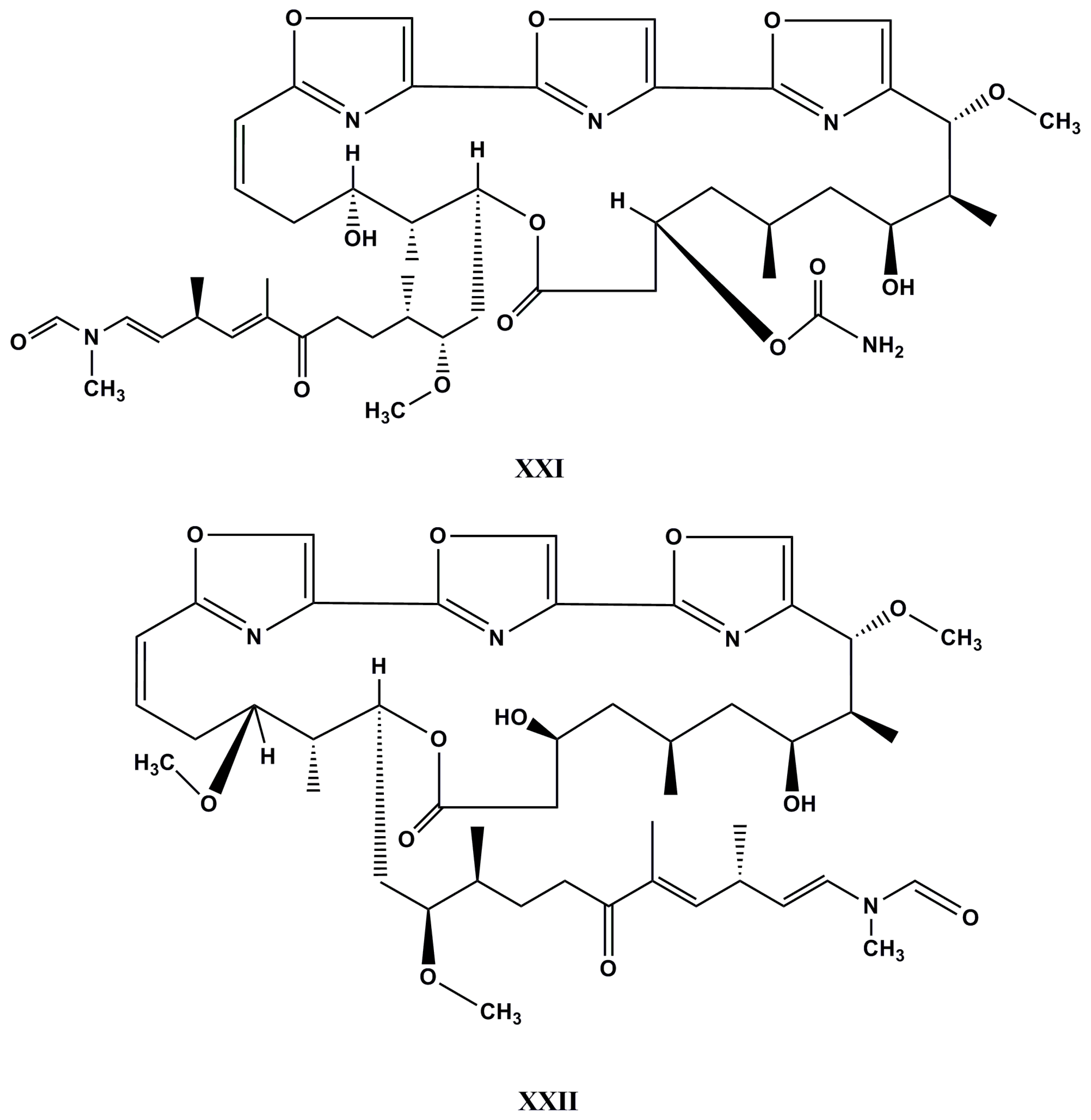
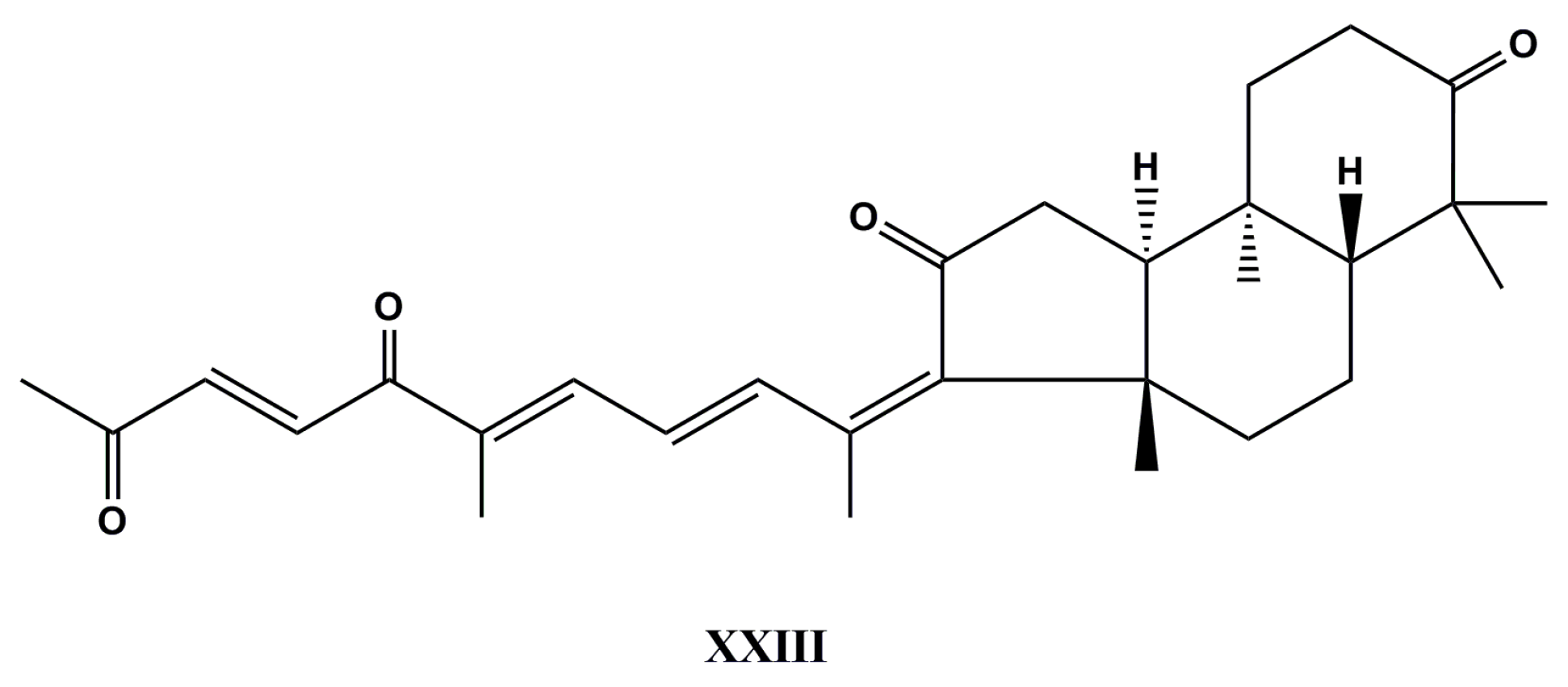
| Species (Order, Family) | Compounds | Cancer Cell Line (In Vitro Cytotoxicity) | Reference |
|---|---|---|---|
| Biemna sp. (Biemnida, Biemnidae) | Two pyridoacridines and the known isocystodamine ** | K562 (ED50 = 5 nM for each compound) | [155] |
| Cinachyrella enigmatica (Tetractinellida, Tetillidae) | Enigmazole-A * | NCL–H60 (mean GI50 of 1.7 µM) | [156] |
| Clathria sp. (Poecilosclerida, Microcionidae) | Mirabilins H–J and three known mirabilins | ECACC, AGS, HT29 and int-407 (LD50 values > 30 µM) | [157] |
| Haliclona sp. (Haplosclerida, Chalinidae) | Eight cyclic bis-1,3-dialkylpyridiniums and two known cyclostellettamines | A549 (LC50 = 14.7–28.9 µM) | [158] |
| Hyatella sp. (Dictyoceratida, Spongiidae) | Five new scalarane sesterterpenes and six known compounds | K562 (LC50 = 14.8–39.5 µM); one compound with LC50 > 100 µM | [159] |
| Myrmekioderma dendyi (Axinellida, Heteroxyidae) | Myrmekioside E, and peracetylated myrmekioside E (myrmekioside E-2) | NSCLC-N6 (IC50 = 7.3 µM); A549 (IC50 = 9.7 µM) | [160] |
| Spirastrella abata (Clionaida, Spirastrellidae) | Three phingosine 4-sulfates, and lysophosphatidylglycerol | K562 (LC50 = 4–8 µM) | [161] |
| Theonella swinhoei (Tetractinellida, Theonellidae) | Swinholide J, and the known swinholide A | KB (IC50 = 6.0 nM) | [162] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mioso, R.; Marante, F.J.T.; Bezerra, R.D.S.; Borges, F.V.P.; Santos, B.V.d.O.; Laguna, I.H.B.d. Cytotoxic Compounds Derived from Marine Sponges. A Review (2010–2012). Molecules 2017, 22, 208. https://doi.org/10.3390/molecules22020208
Mioso R, Marante FJT, Bezerra RDS, Borges FVP, Santos BVdO, Laguna IHBd. Cytotoxic Compounds Derived from Marine Sponges. A Review (2010–2012). Molecules. 2017; 22(2):208. https://doi.org/10.3390/molecules22020208
Chicago/Turabian StyleMioso, Roberto, Francisco J. Toledo Marante, Ranilson De Souza Bezerra, Flávio Valadares Pereira Borges, Bárbara V. de Oliveira Santos, and Irma Herrera Bravo de Laguna. 2017. "Cytotoxic Compounds Derived from Marine Sponges. A Review (2010–2012)" Molecules 22, no. 2: 208. https://doi.org/10.3390/molecules22020208
APA StyleMioso, R., Marante, F. J. T., Bezerra, R. D. S., Borges, F. V. P., Santos, B. V. d. O., & Laguna, I. H. B. d. (2017). Cytotoxic Compounds Derived from Marine Sponges. A Review (2010–2012). Molecules, 22(2), 208. https://doi.org/10.3390/molecules22020208






