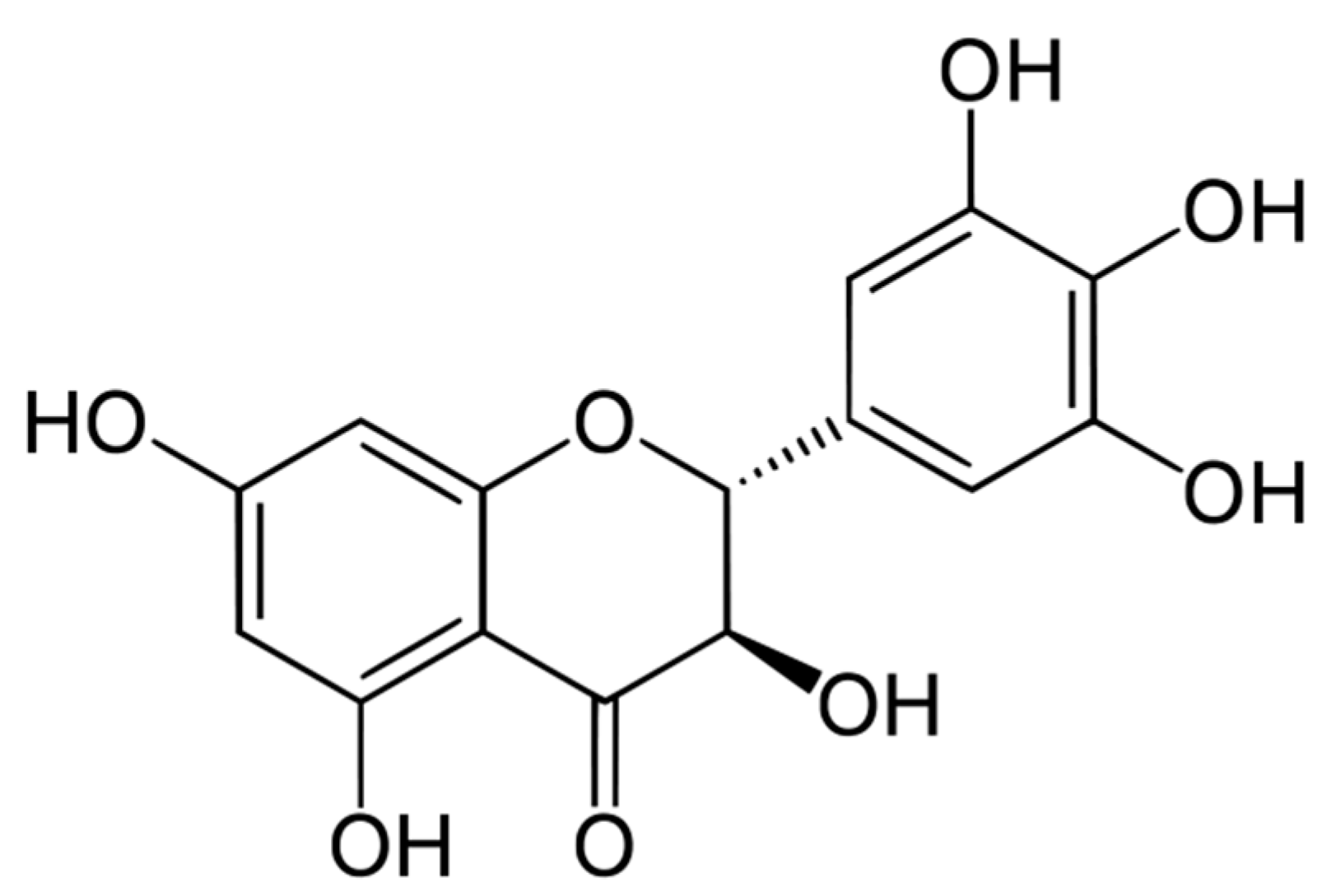
Optimizing the Maximum Recovery of Dihydromyricetin from Chinese Vine Tea, Ampelopsis grossedentata, Using Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
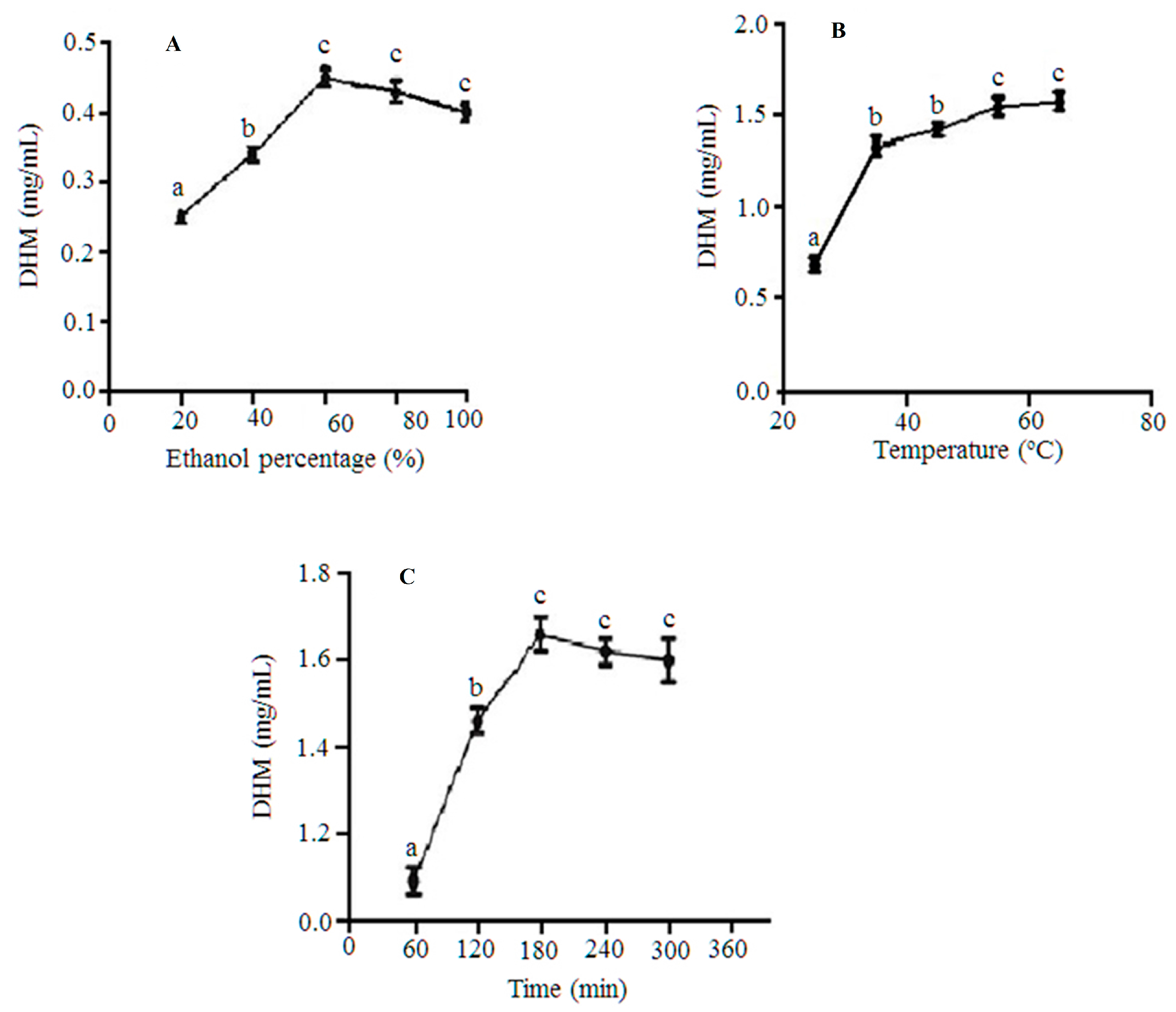
2.1. Solvent Composition/Ethanol Percentage Affected DHM Extractability and Overall Yields
2.2. Increasing the Temperature of Extraction Mixtures Enhanced DHM Yields
2.3. Extending Extraction Times Positively Influenced DHM Overall Yields
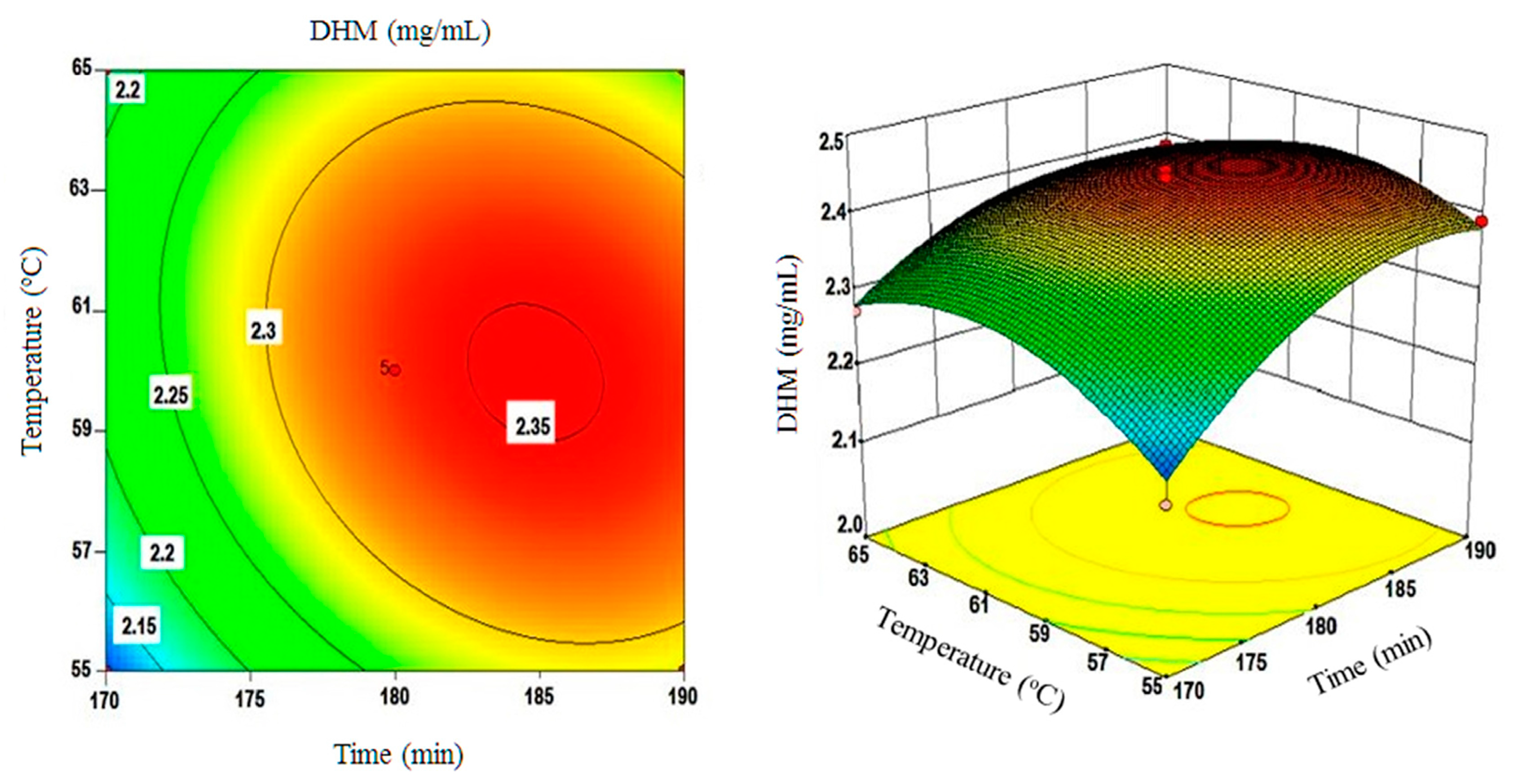
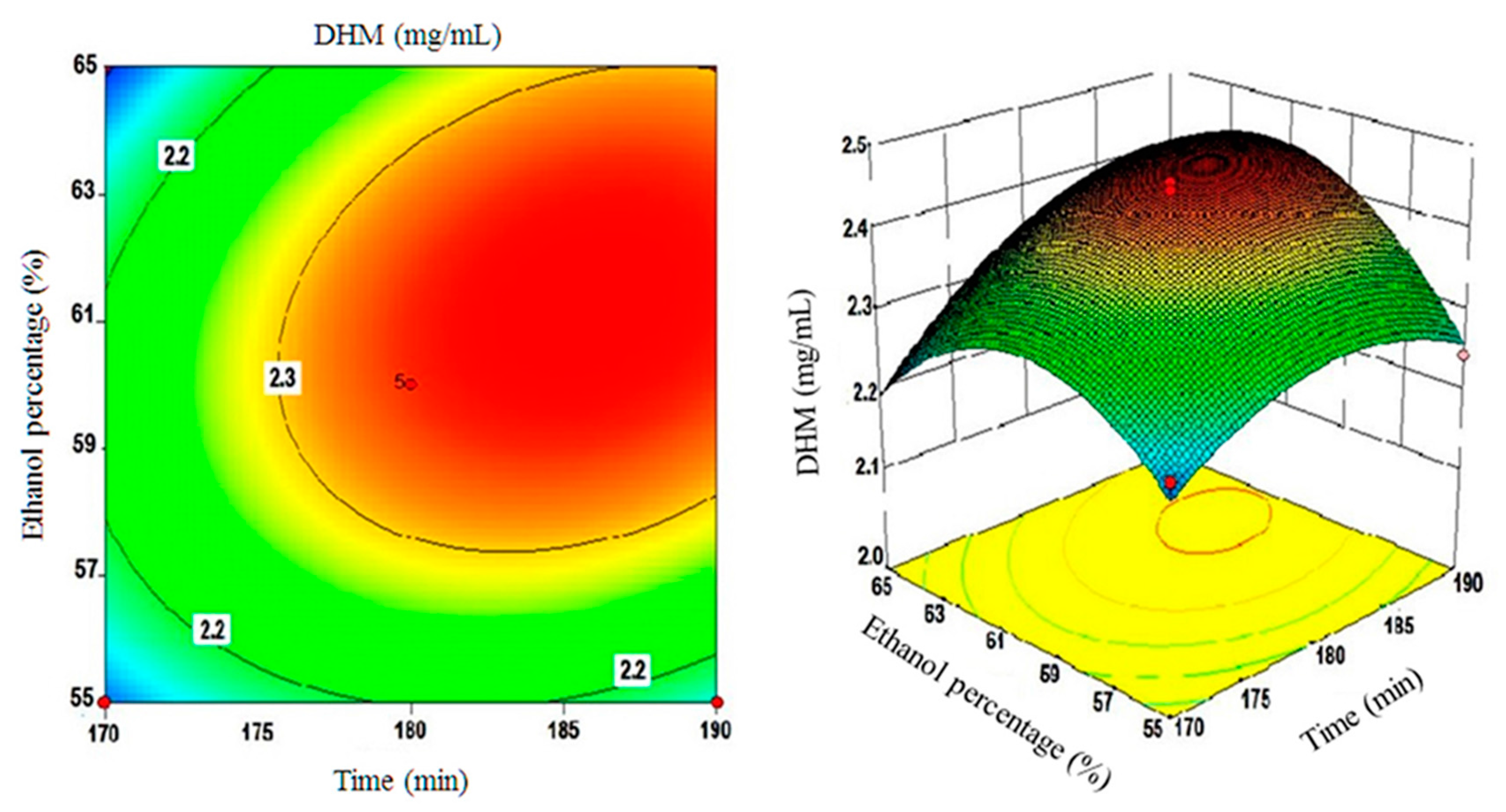
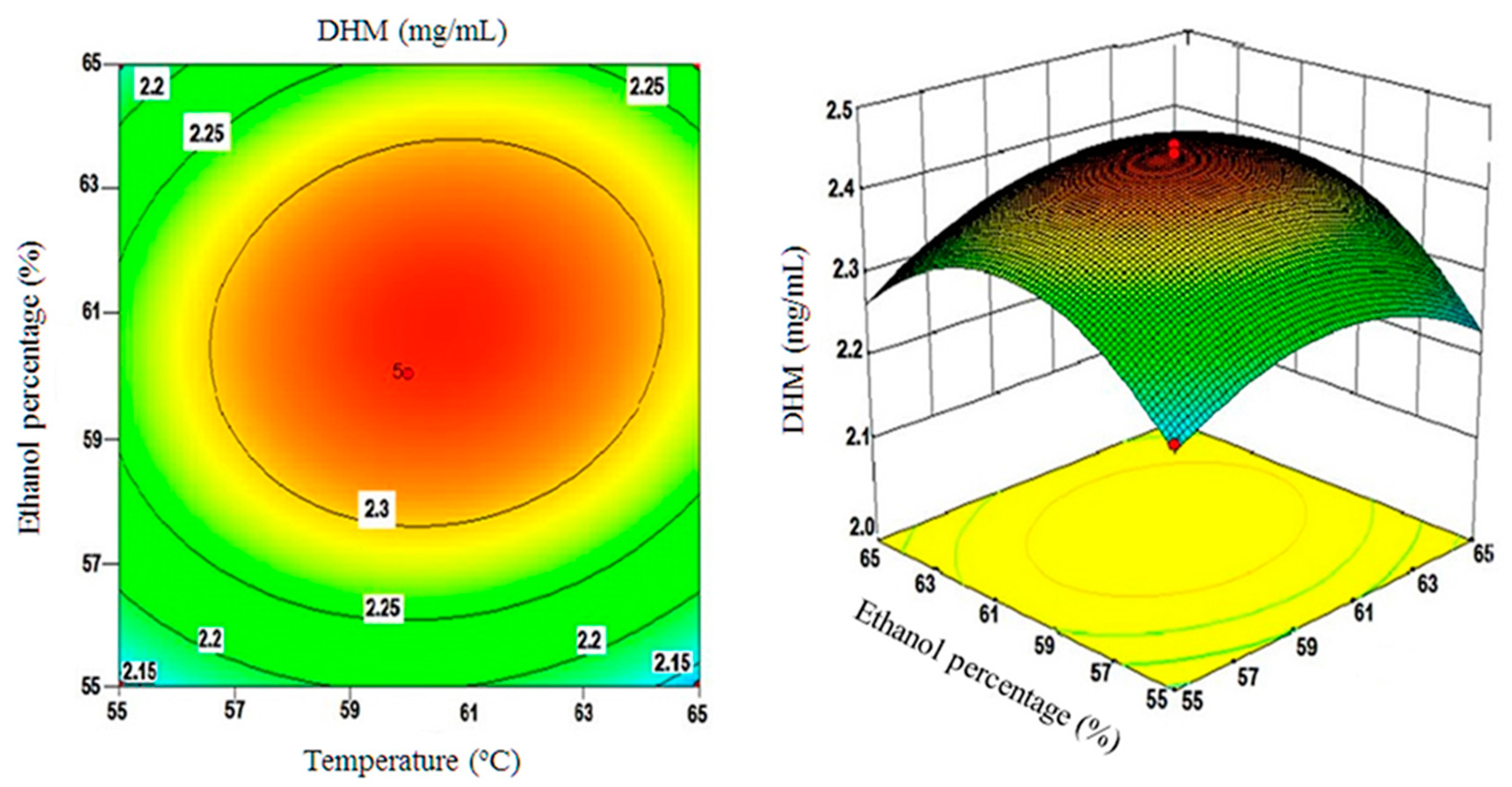
2.4. Using the Box-Behnken Design (BBD) to Optimize DHM Extractions and Testing Model Compliance with the Quadratic Fit
2.5. The Experimental Validation of Optimal Extraction Conditions/Parameters
3. Materials and Methods
3.1. Plant Materials and Chemical Reagents
3.2. Sample Preparation
3.3. DHM Analysis and Quantification by High Performance Liquid Chromatography (HPLC)
3.4. Confirmatory Analysis of DHM Using Matrix Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) Mass Spectrometry
3.5. Delineating the Influence of Single Factors on DHM Extraction/Recovery Rates and Establishing Variability Ranges
3.5.1. The Influence of Solvent Composition/Ethanol (%) on DHM Recovery
3.5.2. Extraction Times
3.5.3. Temperature of Extraction
3.6. Optimizing DHM Extraction Parameters through Response Surface Methodology (RSM) and Model Fitting
3.7. Empirical Validation of the Established Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Lopez, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallosbc, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; de la Rosa, A.P.B. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Bang, W.Y.; Nair, V.; Angulo-Escalante, M.A.; Cisneros-Zevallos, L.; Heredia, J.B. Protective Role of Flavonoids and Lipophilic Compounds from Jatropha platyphylla on the Suppression of Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2016, 64, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Lekhak, S.; Sharma, A. Antibacterial property of different medicinal plants: Ocimum sanctum, Cinnamomum zeylanicum, Xanthoxylum armatum and Origanum majorana. Kathmandu Univ. J. Sci. Eng. Technol. 2009, 5, 143–150. [Google Scholar] [CrossRef]
- Ahmad, I.; Mehmood, Z.; Mohammad, F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998, 62, 183–193. [Google Scholar] [CrossRef]
- Kamboj, V.P. Herbal Medicine. Curr. Sci. 2000, 78, 35–39. [Google Scholar]
- Arora, D.S.; Kaur, J. Antimicrobial activity of spices. Int. J. Antimicrob. Agents 1999, 12, 257–262. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective Role of Ternatin Anthocyanins and Quercetin Glycosides from Butterfly Pea (Clitoria ternatea Leguminosae) Blue Flower Petals against Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef] [PubMed]
- Job, K.M.; Kiang, T.K.L.; Constance, J.E.; Sherwin, C.M.T.; Enioutina, E.Y. Herbal medicines: Challenges in the modern world. Part. 4. Canada and United States. Expert Rev. Clin. Pharmacol. 2016, 9, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Pecorari, R.; Appendino, G.; Miraldi, E.; Magnano, R.A.; Governa, P.; Cettolin, G.; Giachetti, D. Herbal Products in Italy: The Thin Line between Phytotherapy, Nutrition and Parapharmaceuticals; A Normative Overview of the Fastest Growing Market. in Europe. Pharmaceutics (Basel) 2016, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, A.; Liu, B.; Li, G.; Zhang, Z.; Chen, S. Antioxidant and cytotoxic activity of dihydromyricetin from Ampelopsis Grossedentata leaves. Agro Food Ind. Hi-Tech 2009, 20, 14–17. [Google Scholar]
- Xiong, H.P.; Ji, H.W.; Yang, W.L.; Zhang, Y.S. Antimicrobial activity of extracts from Ampelopsis grossedentata on common respiratory tract pathogens. J. Guangxi Agric. Biol. Sci. 2007, 26, 150–153. [Google Scholar]
- Zhou, F.Z.; Huang, M.; Zhang, X.Y.; Guo, Y. Synergy and Attenuation Effects of Dihydromyricetin on Tumor-Bearing Mice Affected by Breast Cancer Treated with Chemotherapy. JSCUT (Nat. Sci. Ed.) 2011, 39, 147–151. [Google Scholar]
- Shen, Y.; Lindemeyer, A.K.; Claudia, G.; Shao, X.M.; Igor, S.; Richard, W.O.; Jing, L. Dihydromyricetin as a novel anti-alcohol intoxication medication. J. Neurosci. 2012, 32, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Cai, W.; Xia, M.; Ito, Y. Purification of (+)-dihydromyricetin from leaves extract of Ampelopsis grossedentata using high-speed countercurrent chromatograph with scale-up triple columns. J. Chromatogr. A 2002, 973, 217–220. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, G.; Tian, M.; Pu, Q.; Qin, M. Optimization of the Ultrasonic-Assisted Extraction of Bioactive Flavonoids from Ampelopsis grossedentata and Subsequent Separation and Purification of Two Flavonoid Aglycones by High.-Speed Counter-Current Chromatography. Molecules 2016, 21, 1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-S.; Li, D.; Wang, L.-J.; Ozkan, N.; Chen, X.D.; Mao, Z.-H.; Yang, H.Z. Optimization of ethanol–water extraction of lignans from flaxseed. Sep. Purif. Technol. 2007, 57, 17–24. [Google Scholar] [CrossRef]
- Zhang, P.; Cai, S.; Song, L.; Zhang, L.; Fan, H.; Zhou, L.; Lin, R.; Yang, G.; Bian, X.; Wang, W.; Zhang, J. Solubility of dihydromyricetin in ethanol and water mixtures from 288.15 to 323.15 K. J. Mol. Liq. 2015, 211, 197–202. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Vongsangnak, W.; Jian, G.; Somchai, C.; Zhong, J.-J. Towards efficient extraction of notoginseng saponins from cultured cells of Panax notoginseng. Biochem. Eng. J. 2004, 18, 115–120. [Google Scholar] [CrossRef]
- Kim, S.-J.; Murthy, H.N.; Hahn, E.-J.; Lee, H.L.; Paek, K.-Y. Parameters affecting the extraction of ginsenosides from the adventitious roots of ginseng (Panax ginseng C.A. Meyer). Sep. Purif. Technol. 2007, 56, 401–406. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Bauer, F.; Siebenhandl, S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int. J. Food Sci. Technol. 2006, 41, 121–133. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Boziaris, I.S.; Nychas, G.-J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.; Yang, J.; Zhang, G.; Chen, Y.; Luo, Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Ramić, M.; Vidovic, S.; Zekovic, Z.; Vladic, J.; Cvejin, A.; Pavlic, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Jia, C.; Zhang, X.; Xia, W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Eloff, J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- De Boer, H.J.; Kool, A.; Broberg, A.; Mziray, W.R.; Hedberg, I.; Levenfors, J.J. Anti-fungal and anti-bacterial activity of some herbal remedies from Tanzania. J. Ethnopharmacol. 2005, 96, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lightfoot, D.A.; Kinsel, M.; Watson, D.G. Employing response surface methodology for the optimization of ultrasound assisted extraction of lutein and beta-carotene from spinach. Molecules 2015, 20, 6611–6625. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Factors | −1 | 0 | 1 |
|---|---|---|---|
| X1: extraction time (min) | 170 | 180 | 190 |
| X2: extraction temperature (°C) | 55 | 60 | 65 |
| X3: solvent composition/ethanol (%) | 55 | 60 | 65 |
| Run | X1 (Time/min) | X2 (Temp./°C) | X3 (Ethanol/%) | Response Values (Y) as DHM (mg/mL) | Predicted Value | Residual |
|---|---|---|---|---|---|---|
| 1 | 0 (180) | −1 (55) | 1 (65) | 2.305 ± 0.29 | 2.230 | 0.08 |
| 2 | −1 (170) | 1 (65) | 0 (60) | 2.172 ± 0.32 | 2.146 | 0.03 |
| 3 | 1 (190) | −1 (55) | 0 (60) | 2.142 ± 0.16 | 2.270 | −0.13 |
| 4 | 1 (190) | 0 (60) | 1 (65) | 2.355 ± 0.22 | 2.297 | 0.06 |
| 5 | 0 (180) | 0 (60) | 0 (60) | 2.352 ± 0.32 | 2.340 | 0.01 |
| 6 | −1 (170) | 0 (60) | 1 (65) | 2.210 ± 0.11 | 2.091 | 0.12 |
| 7 | −1 (170) | 0 (60) | −1 (55) | 2.290 ± 0.28 | 2.117 | 0.17 |
| 8 | 0 (180) | 1 (65) | −1 (55) | 2.117 ± 0.21 | 2.134 | −0.02 |
| 9 | 0 (180) | −1 (55) | −1 (55) | 2.345 ± 0.13 | 2.234 | 0.11 |
| 10 | 0 (180) | 0 (60) | 0 (60) | 2.110 ± 0.23 | 2.340 | −0.23 |
| 11 | 0 (180) | 0 (60) | 0 (60) | 2.277 ± 0.19 | 2.340 | −0.06 |
| 12 | 0 (180) | 1 (65) | 1 (65) | 2.335 ± 0.25 | 2.240 | 0.09 |
| 13 | 0 (180) | 0 (60) | 0 (60) | 2.285 ± 0.22 | 2.340 | −0.05 |
| 14 | −1 (170) | −1 (55) | 0 (60) | 2.080 ± 0.14 | 2.146 | −0.07 |
| 15 | 0 (180) | 0 (60) | 0 (60) | 2.147 ± 0.18 | 2.340 | −0.19 |
| 16 | 1 (190) | 0 (60) | −1 (55) | 2.140 ± 0.22 | 2.159 | −0.02 |
| 17 | 1 (190) | 1 (65) | 0 (60) | 2.177 ± 0.32 | 2.270 | −0.09 |
| Parameters | Response | |||
|---|---|---|---|---|
| Estimated Coefficients | F-Value | Prob > F | Status | |
| Intercept | ||||
| Model | 20.13 | 0.0003 | Significant | |
| Linear effect | ||||
| A (time) | 0.062 | 38.63 | 0.0004 | Significant |
| B (temperature) | 0.011 | 1.29 | 0.2934 | NS** |
| C (solvent) | 0.028 | 8.24 | 0.0240 | Significant |
| Interactive effect | ||||
| AB | −0.024 | 3.03 | 0.1254 | NS |
| AC | 0.041 | 8.67 | 0.0216 | Significant |
| BC | 0.016 | 1.24 | 0.3015 | NS |
| Quadratic effect | ||||
| A2 | −0.064 | 21.72 | 0.0023 | Significant |
| B2 | −0.068 | 24.81 | 0.0016 | Significant |
| C2 | −0.11 | 61.86 | 0.0001 | Significant |
| The statistical parameters for fitting analysis | ||||
| Lack of Fit | - | 3.12 | 0.1503 | NS |
| R2 | - | - | 0.9600 | - |
| Adj. R2 | - | - | 0.9100 | - |
| C.V. % | - | - | 1.2600 | - |
| Adequate precision | - | - | 11.420 | - |
| Extraction Times (min) | Extraction Temp. (°C) | Ethanol (%) | DHM (mg/mL) | |
|---|---|---|---|---|
| Predicated | 180 | 60 | 60 | 2.33 |
| Experimental | 180 | 60 | 60 | 2.31 |
| RE (%) | - | - | - | 0.87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, U.; Lu, H.; Wang, J.; Han, J.; Zhu, X.; Lu, Z.; Tayyaba, S.; Hassan, Y.I. Optimizing the Maximum Recovery of Dihydromyricetin from Chinese Vine Tea, Ampelopsis grossedentata, Using Response Surface Methodology. Molecules 2017, 22, 2250. https://doi.org/10.3390/molecules22122250
Muhammad U, Lu H, Wang J, Han J, Zhu X, Lu Z, Tayyaba S, Hassan YI. Optimizing the Maximum Recovery of Dihydromyricetin from Chinese Vine Tea, Ampelopsis grossedentata, Using Response Surface Methodology. Molecules. 2017; 22(12):2250. https://doi.org/10.3390/molecules22122250
Chicago/Turabian StyleMuhammad, Umair, Hedong Lu, Juan Wang, Jinzhi Han, Xiaoyu Zhu, Zhaoxin Lu, Sultana Tayyaba, and Yousef I. Hassan. 2017. "Optimizing the Maximum Recovery of Dihydromyricetin from Chinese Vine Tea, Ampelopsis grossedentata, Using Response Surface Methodology" Molecules 22, no. 12: 2250. https://doi.org/10.3390/molecules22122250
APA StyleMuhammad, U., Lu, H., Wang, J., Han, J., Zhu, X., Lu, Z., Tayyaba, S., & Hassan, Y. I. (2017). Optimizing the Maximum Recovery of Dihydromyricetin from Chinese Vine Tea, Ampelopsis grossedentata, Using Response Surface Methodology. Molecules, 22(12), 2250. https://doi.org/10.3390/molecules22122250







