Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study
Abstract
:1. Introduction
2. Results and Discussion
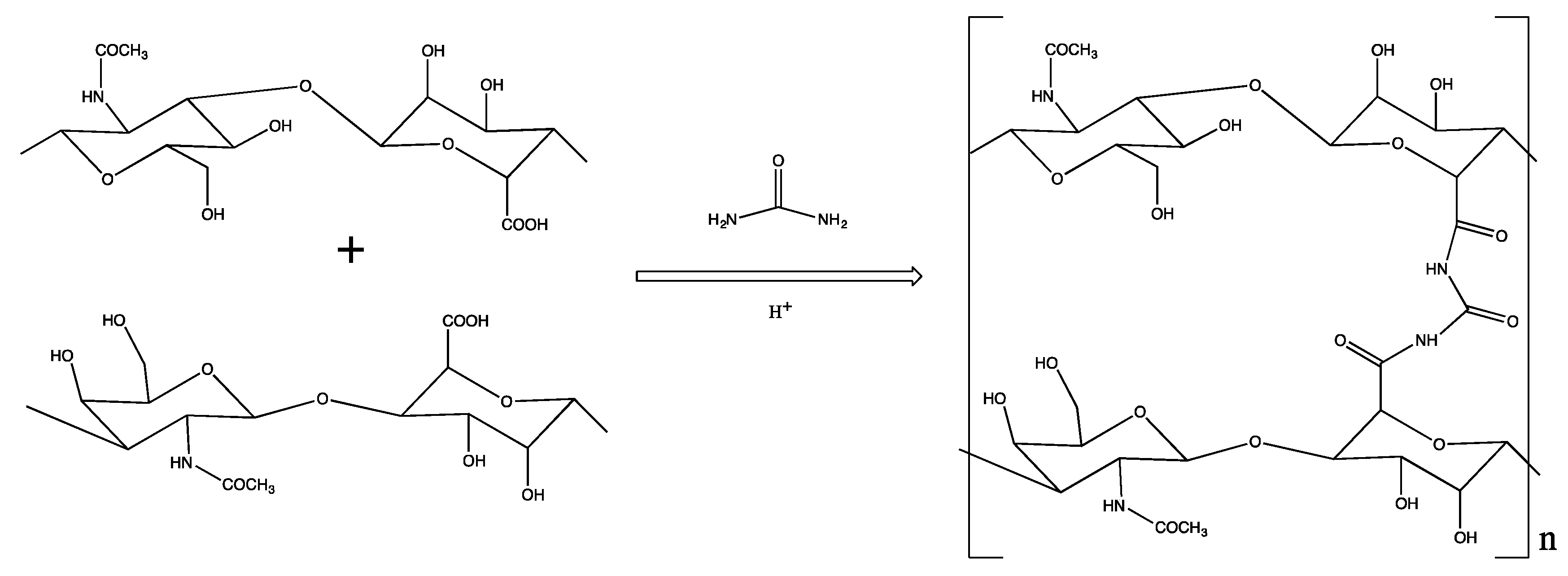
2.1. Synthesis of HA-CL
2.2. Physical-Chemical Characterization and Stability of HA-CL Solutions
2.3. Cell Viability
2.4. Effect of HA-CL on IL-8 Levels
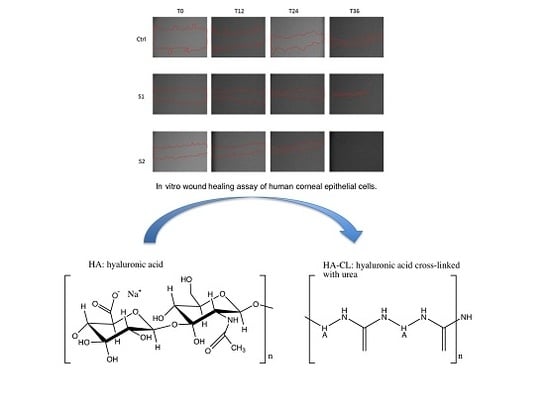
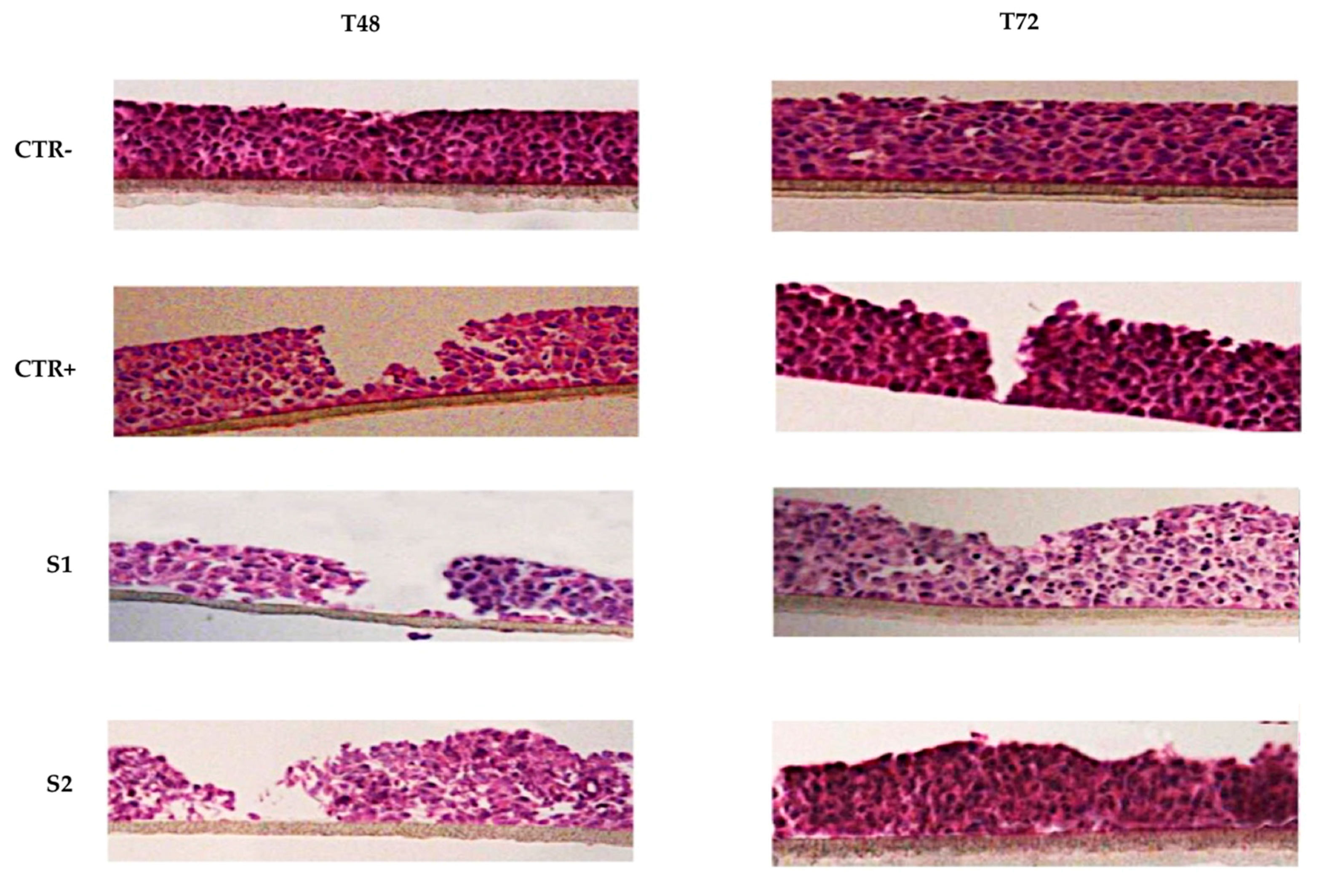
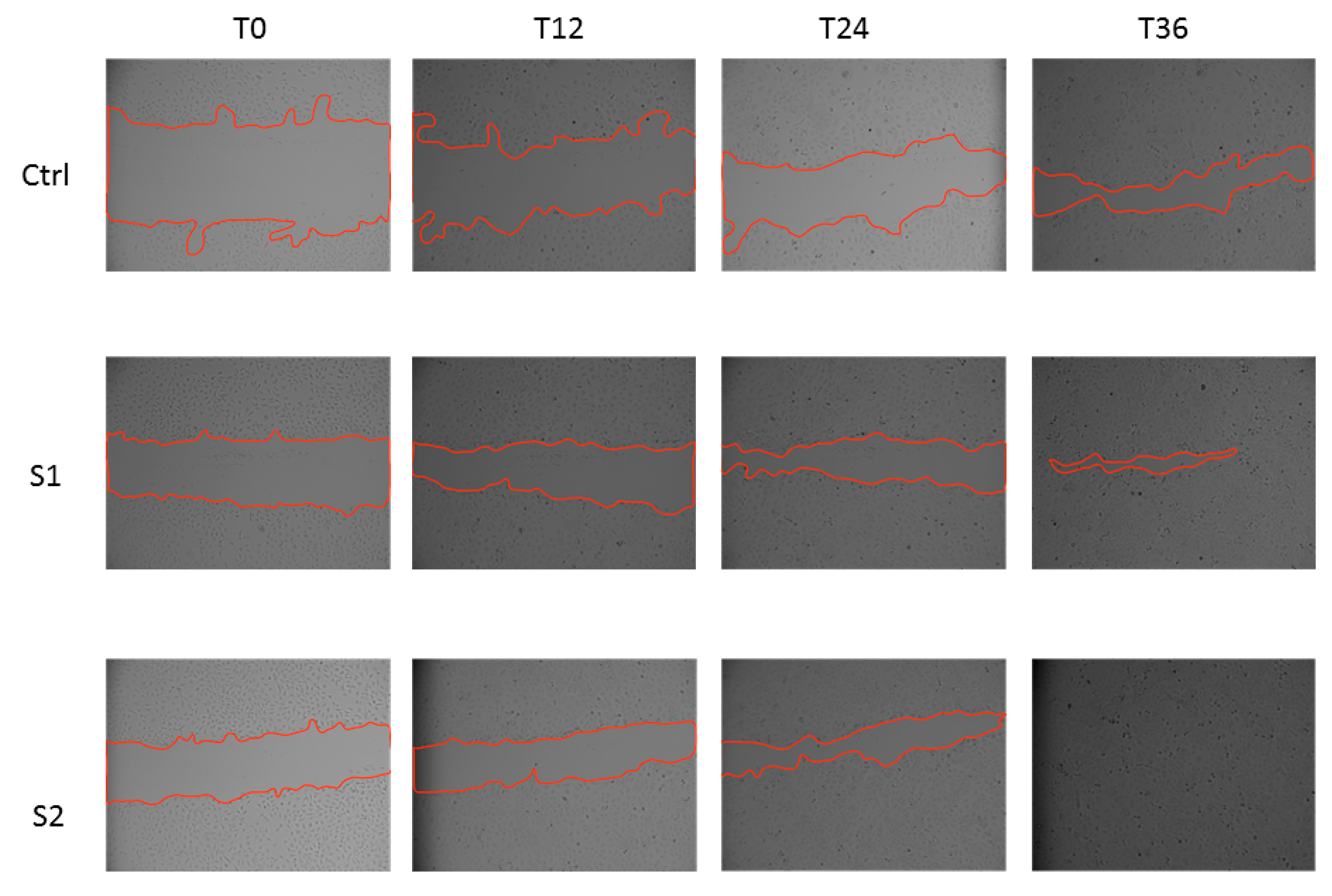
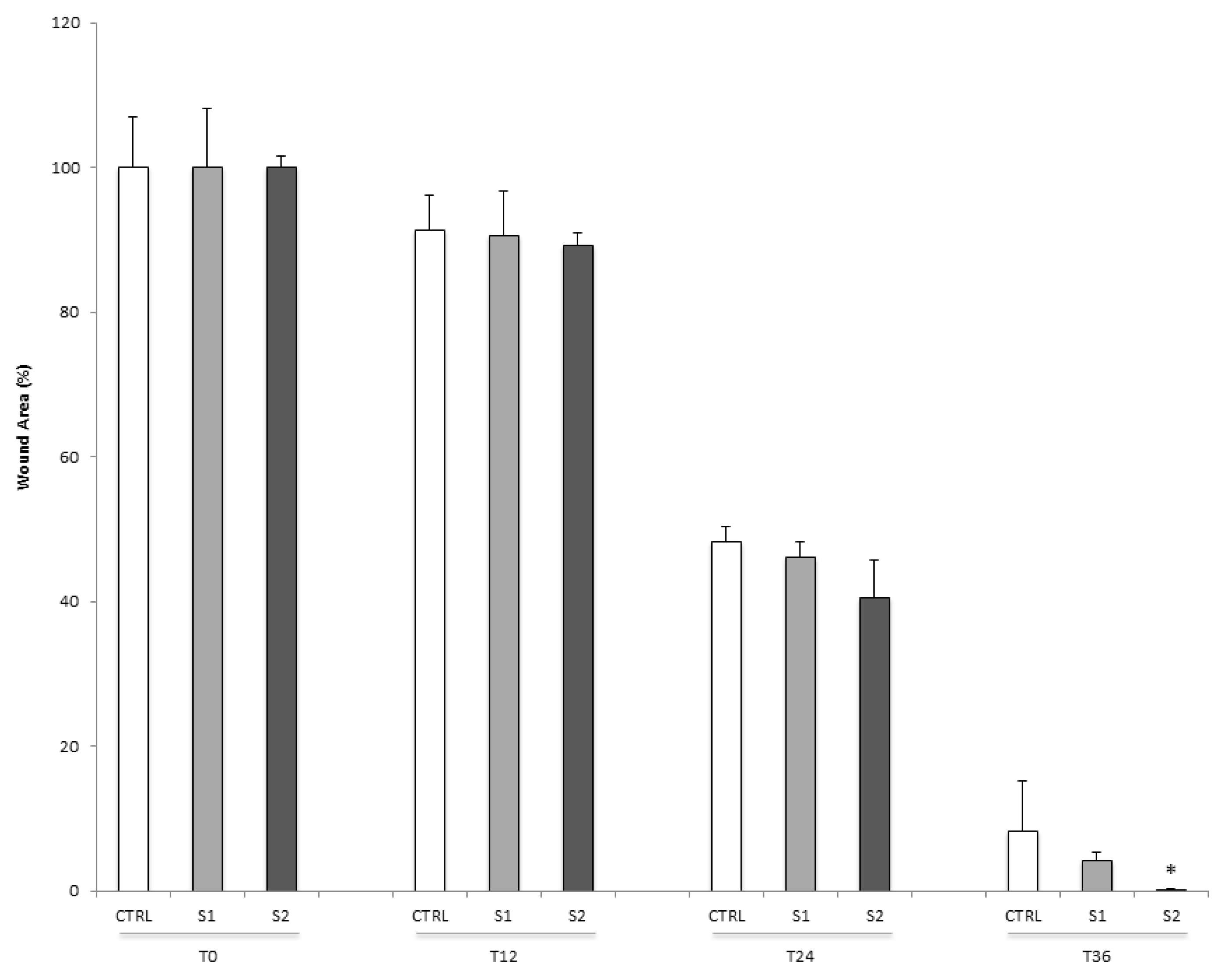
2.5. Epithelial Corneal Wound Closure
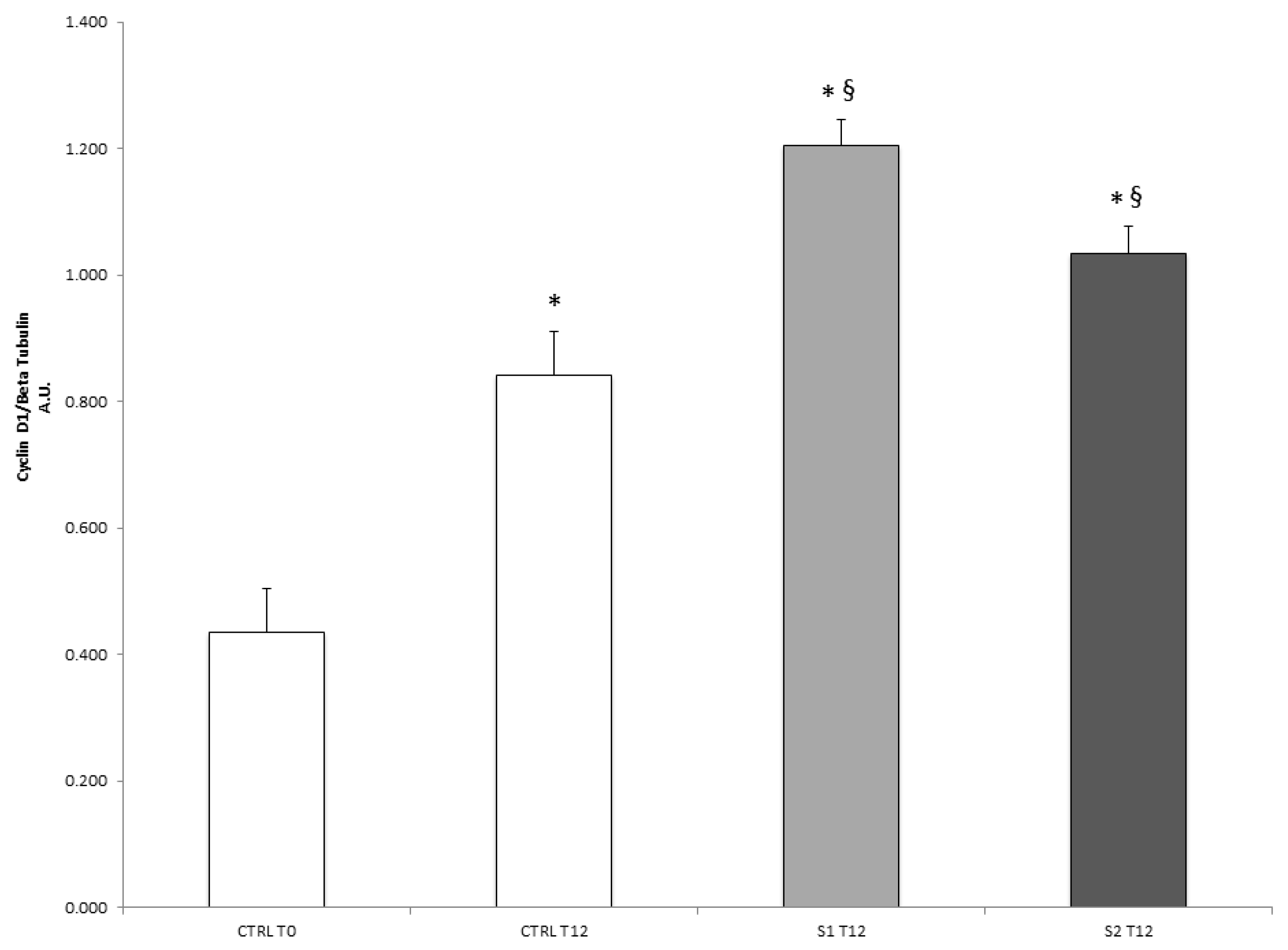
2.6. Cyclin D1 Protein Levels
3. Experimental Section
3.1. Materials
3.2. Formulation of HA-CL Solutions
3.3. Physical-Chemical Characterization and Stability of HA-CL Solutions
3.4. In Vitro Efficacy Study
3.4.1. Experimental Scheme
- -
- negative control condition: tissues not wounded and not treated (CTR−);
- -
- positive control condition: tissues wounded but not treated (CTR+);
- -
- treated condition: tissues wounded and treated with the samples (S1 and S2).
3.4.2. Cell Cultures
3.4.3. Cell Viability
3.4.4. IL-8 ELISA Test
3.4.5. Epithelial Corneal Wound Closure
3.4.6. Western Blot Analysis
3.4.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CTR− | negative control condition |
| CTR+ | positive control condition |
| ELISA | enzyme-linked immunosorbent assay |
| HA-CL | cross-linked hyaluronic acid |
| HA | hyaluronic acid |
| HCE | 3D reconstructed tissues of human corneal epithelium |
| HCEpiC | 2D human corneal epithelial cells |
| HE | hematoxylin and eosin |
| IL-8 | interleukin-8 |
| KCS | keratoconjunctivitis sicca |
| MTT | 3(4,5-dimethylthiazol-2)2,5 difeniltetrazolium bromide |
| S1 | solution 1, 0.02% (w/v) HA-CL |
| S2 | solution 2, 0.4% (w/v) HA-CL |
References
- Lemp, M.A.; Baudouin, C.; Bau, J.; Dogru, M.; Foulks, G.N.; Kinoshita, S.; Laibson, P.; Mc Culley, J.; Murube, J.; Pflugfelder, S.C.; et al. DEWS definition and classification subcommittee of the international dry eye workshop. The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the international dry eye workshop. Ocular Surf. 2007, 5, 75–92. [Google Scholar]
- Berriaud, N.; Milas, M.; Rinaudo, M. Characterization and properties of hyaluronic acid (hyaluronan). In Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Dumitriu, S., Dekker, M., Eds.; CRC Press: New York, NY, USA, 2005; pp. 535–549. [Google Scholar]
- Lapcik, L., Jr.; Lapcik, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, structure, properties, and applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Stuart, J.C.; Linn, J.G. Dilute sodium hyaluronate (Healon) in the treatment of ocular surface disorders. Ann. Ophthalmol. 1985, 17, 190–192. [Google Scholar] [PubMed]
- Yoshida, K.; Nitatori, Y.; Uchiyama, Y. Localization of glycosaminoglycans and CD44 in the human lacrimal gland. Arch. Histol. Cytol. 1996, 59, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Pastis, K.W.; Ellingham, R.B.; Frost, L.; Corfield, A.P.; Easty, D.L. Hyaluronan in dry eye and contact lens wearers. In Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2; Sullivan, D.A., Dartt, D.A., Meneray, M.A., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 785–790. [Google Scholar]
- Frescura, M.; Berry, M.; Corfield, A.; Carrington, S.; Easty, D.L. Evidence of hyaluronan in human tears and secretions of conjunctival cultures. Biochem. Soc. Trans. 1994, 22, 228s. [Google Scholar] [CrossRef]
- Fukuda, M.; Miyamoto, Y.; Miyara, Y.; Mishima, H.; Otori, T. Hyaluronic acid concentrations in human tear fluids. Investig. Ophthalmol. Vis. Sci. 1996, 37, 3916. [Google Scholar]
- Nakamura, M.; Hikida, M.; Nakano, T.; Ito, S.; Hamano, T.; Kinoshita, S. Characterization of water retentive properties of hyaluronan. Cornea 1993, 12, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Amankwah, R.; Powell–Richards, A.; Dua, H.S. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br. J. Ophthalmol. 2004, 88, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Katakami, C. The effect of hyaluronic acid on corneal epithelial cell proliferation. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2313–2315. [Google Scholar]
- Nishida, T.; Nakamura, M.; Mishima, H.; Otori, T. Hyaluronan stimulates corneal epithelial migration. Exp. Eye Res. 1991, 53, 753–758. [Google Scholar] [CrossRef]
- Presti, D.; Scott, J.E. Hyaluronan–mediated protective effect against cell damage caused by enzymatically produced hydroxyl (OH.) radicals is dependent on hyaluronan molecular mass. Cell Biochem. Funct. 1994, 12, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. Extracellular matrix, supramolecular organization and shape. J. Anat. 1995, 187, 259–269. [Google Scholar] [PubMed]
- Aragona, P.; Papa, V.; Micali, A.; Santocono, M.; Milazzo, G. Long term treatment with sodium hyaluronate–containing artificial tears reduces ocular surface damage in patients with dry eye. Br. J. Ophthalmol. 2002, 86, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, K.; Woods, C.; Fonn, D. An investigation of the efficacy of a novel ocular lubricant. Eye Contact Lens 2009, 35, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hamano, T.; Horimoto, K.; Lee, M.; Komemushi, S. Sodium hyaluronate eye drops enhance tear film stability. Jpn. J. Ophthalmol. 1996, 40, 62–65. [Google Scholar] [PubMed]
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Effectiveness of sodium hyaluronate eye drops in the treatment of dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Prabhasawat, P.; Tesavibul, N.; Kasetsuwan, N. Performance profile of sodium hyaluronate in patients with lipid tear deficiency: Randomised, double–blind, controlled, exploratory study. Br. J. Ophthalmol. 2007, 91, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Sand, B.B.; Marner, K.; Norn, M.S. Sodium hyaluronate in the treatment of keratoconjunctivitis sicca. A double masked clinical trial. Acta Ophthalmol. 1989, 67, 181–183. [Google Scholar] [CrossRef]
- Fallacara, A.; Manfredini, S.; Durini, E.; Vertuani, S. Hyaluronic acid fillers in soft tissue regeneration. Facial Plast. Surg. 2017, 33, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Mann, B.K. Efficacy of a crosslinked hyaluronic acid–based hydrogel as a tear film supplement: A masked controlled study. PLoS ONE 2014, 9, e99766. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Mann, B.K. A crosslinked HA–based hydrogel ameliorates dry eye symptoms in dogs. Int. J. Biomater. 2013, 2013, 460437. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Espandar, L.; Mamalis, N.; Prestwich, G.D. A cross–linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet. Ophthalmol. 2010, 13, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Wirostko, B.; Mann, K.B.; Williams, D.L.; Prestwich, G.D. Ophthalmic uses of a thiol–modified hyaluronan–based hydrogel. Adv. Wound Care (New Rochelle) 2014, 3, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Wirostko, B.M.; Gum, G.; Mann, B.K. Topical cross–linked HA–based hydrogel accelerates closure of corneal epithelial defects and repair of stromal ulceration in companion animals. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Calles, J.A.; Tàrtara, L.I.; Lopez–Garcìa, A.; Diebold, Y.; Palma, S.D.; Vallés, E.M. Novel bioadhesive hyaluronan–itaconic acid crosslinked films for ocular therapy. Int. J. Pharm. 2013, 455, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Calles, J.A.; Lopez–Garcìa, A.; Vallés, E.M.; Palma, S.D.; Diebold, Y. Preliminary characterization of dexamethasone–loaded cross–linked hyaluronic acid films for topical ocular therapy. Int. J. Pharm. 2016, 509, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Postorino, E.I.; Rania, L.; Aragona, E.; Mannucci, C.; Alibrandi, A.; Calapai, G.; Puzzolo, D.; Aragona, P. Efficacy of eye drops containing cross–linked hyaluronic acid and coenzyme Q10 in treating patients with mild to moderate dye eye. Eur. J. Ophthalmol. 2017. [Google Scholar] [CrossRef]
- Cagini, C.; Torroni, G.; Fiore, T.; Cerquaglia, A.; Lupidi, M.; Aragona, P.; Iaccheri, B. Tear film stability in Sjogren syndrome patients treated with hyaluronic acid versus crosslinked hyaluronic acid–based eye drops. J. Ocul. Pharmacol. Ther. 2017, 33, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Citernesi, U.R.; Beretta, L.; Citernesi, L. Cross–Linked Hyaluronic Acid, Process for the Preparation Thereof and Use Thereof. Patent WO/2015/007773 A1, 22 January 2015. [Google Scholar]
- Charlton, J.F.; Schwab, I.R.; Stuchell, R. Topical urea as a treatment for non-infectious keratopathy. Acta Ophthalmol. Scand. 1996, 74, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, F.; Adriaens, E.; Alepee, N.; Straube, F.; De Wever, B.; Cappadoro, M.; Catoire, S.; Hansen, E.; Wolf, A.; Vanparys, P. Prevalidation of a new in vitro reconstituted human cornea model to assess the eye irritating potential of chemicals. Toxicol. In Vitro 2006, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pecorelli, A.; Mencarelli, M.; Carbotti, P.; Fortino, V.; Muscettola, M.; Maioli, E. Rottlerin: A multifaced regulator of keratinocyte cell cycle. Exp. Dermatol. 2009, 18, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Condon, P.I.; McEwen, C.G.; Wright, M.; Mackintosh, G.; Prescott, R.J.; McDonald, C. Double blind, randomized, placebo controlled, crossover, multicenter study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br. J. Ophthalmol. 1999, 83, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Papa, V.; Aragona, P.; Russo, S.; Di Bella, A.; Russo, P.; Milazzo, G. Comparison of hypotonic and isotonic solutions containing sodium hyaluronate on the symptomatic treatment of dry eye patients. Ophthalmology 2001, 215, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Middleton, S.; Fattahian, H.; Moridpour, R. Comparison of hyaluronic acid–containing topical eye drops with carbomer-based topical ocular gel as a tear replacement in canine keratoconjunctivitis sicca: a prospective study in twenty-five dogs. Vet. Res. Forum 2012, 3, 229–232. [Google Scholar] [PubMed]
Sample Availability: Samples of the compound HA-CL with urea are not available from the authors. |
| Time | T (°C) | S1 | S2 | ||
|---|---|---|---|---|---|
| pH | η (mPa∙s) | pH | η (mPa∙s) | ||
| Day 7 | 23 ± 2 | 7.0 ± 0.0 | 1.6 ± 0.0 | 7.1 ± 0.0 | 85.9 ± 0.0 |
| 40 ± 2 | 7.0 ± 0.0 | 1.6 ± 0.0 | 7.1 ± 0.0 | 85.8 ± 0.0 | |
| Month 1 | 23 ± 2 | 7.0 ± 0.1 | 1.6 ± 0.0 | 7.0 ± 0.0 | 85.9 ± 0.0 |
| 40 ± 2 | 7.0 ± 0.0 | 1.6 ± 0.0 | 7.0 ± 0.0 | 85.8 ± 0.2 | |
| Month 2 | 23 ± 2 | 7.0 ± 0.0 | 1.6 ± 0.1 | 7.0 ± 0.1 | 85.9 ± 0.0 |
| 40 ± 2 | 7.0 ± 0.0 | 1.6 ± 0.2 | 7.0 ± 0.2 | 85.8 ± 0.1 | |
| Month 3 | 23 ± 2 | 7.0 ± 0.0 | 1.6 ± 0.0 | 7.0 ± 0.0 | 85.9 ± 0.0 |
| 40 ± 2 | 7.1 ± 0.1 | 1.5 ± 0.0 | 7.0 ± 0.0 | 85.6 ± 0.3 | |
| Month 6 | 23 ± 2 | 7.0 ± 0.1 | 1.6 ± 0.0 | 7.0 ± 0.0 | 86.0 ± 0.0 |
| 40 ± 2 | 7.1 ± 0.0 | 1.5 ± 0.2 | 7.0 ± 0.0 | 85.7 ± 0.0 | |
| Condition | Cell Viability | % Variation vs. CTR− | % Variation vs. CTR+ | |||
|---|---|---|---|---|---|---|
| 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | |
| CTR− | 100.0 ± 0.5 | 100.0 ± 8.4 | - | - | - | - |
| CTR+ | 72.4 ± 2.2 | 81.8 ± 4.4 | −27.6 | −18.2 | - | - |
| S1 | 76.4 ± 2.1 | 107.2 ± 0.5 | −23.6 | +7.2 | +4.0 | +25.4 |
| S2 | 74.4 ± 0.7 | 102.3 ± 2.0 | −25.6 | +2.3 | +1.9 | +20.5 |
| Condition | vs. CTR− | vs. CTR+ | vs. S1 | vs. S2 | ||||
|---|---|---|---|---|---|---|---|---|
| 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | |
| CTR− | - | - | 0.00000 | 0.00983 | 0.00000 | 0.41310 | 0.00000 | 0.97145 |
| CTR+ | 0.00000 | 0.00983 | - | - | 0.07789 | 0.00116 | 0.58301 | 0.00474 |
| S1 | 0.00000 | 0.41310 | 0.07789 | 0.00116 | - | - | 0.52885 | 0.72684 |
| S2 | 0.00000 | 0.97145 | 0.58301 | 0.00474 | 0.52885 | 0.72684 | - | - |
| Condition | IL-8 pg/mL | % Variation vs. CTR− | % Variation vs. CTR+ | |||
|---|---|---|---|---|---|---|
| 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | |
| CTR− | 616.5 ± 37.7 | 595.7 ± 35.9 | - | - | - | - |
| CTR+ | 762.2 ± 40.3 | 720.7 ± 49.5 | +23.6 | +21.0 | - | - |
| S1 | 699.5 ± 32.4 | 701.5 ± 36.4 | +13.5 | +13.8 | −8.2 | −2.7 |
| S2 | 664.3 ± 52.3 | 714.5 ± 58.2 | +7.8 | +15.9 | −12.8 | −0.9 |
| Condition | 48 h | |||
|---|---|---|---|---|
| vs. CTR− | vs. CTR+ | vs. S1 | vs. S2 | |
| CTR− | - | 0.00625 | 0.15548 | 0.61723 |
| CTR+ | 0.00625 | - | 0.37554 | 0.07523 |
| S1 | 0.15548 | 0.37554 | - | 0.82461 |
| S2 | 0.61723 | 0.07523 | 0.82461 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallacara, A.; Vertuani, S.; Panozzo, G.; Pecorelli, A.; Valacchi, G.; Manfredini, S. Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules 2017, 22, 2104. https://doi.org/10.3390/molecules22122104
Fallacara A, Vertuani S, Panozzo G, Pecorelli A, Valacchi G, Manfredini S. Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules. 2017; 22(12):2104. https://doi.org/10.3390/molecules22122104
Chicago/Turabian StyleFallacara, Arianna, Silvia Vertuani, Giacomo Panozzo, Alessandra Pecorelli, Giuseppe Valacchi, and Stefano Manfredini. 2017. "Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study" Molecules 22, no. 12: 2104. https://doi.org/10.3390/molecules22122104
APA StyleFallacara, A., Vertuani, S., Panozzo, G., Pecorelli, A., Valacchi, G., & Manfredini, S. (2017). Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules, 22(12), 2104. https://doi.org/10.3390/molecules22122104