Optimization of Dissolution Compartments in a Biorelevant Dissolution Apparatus Golem v2, Supported by Multivariate Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Design
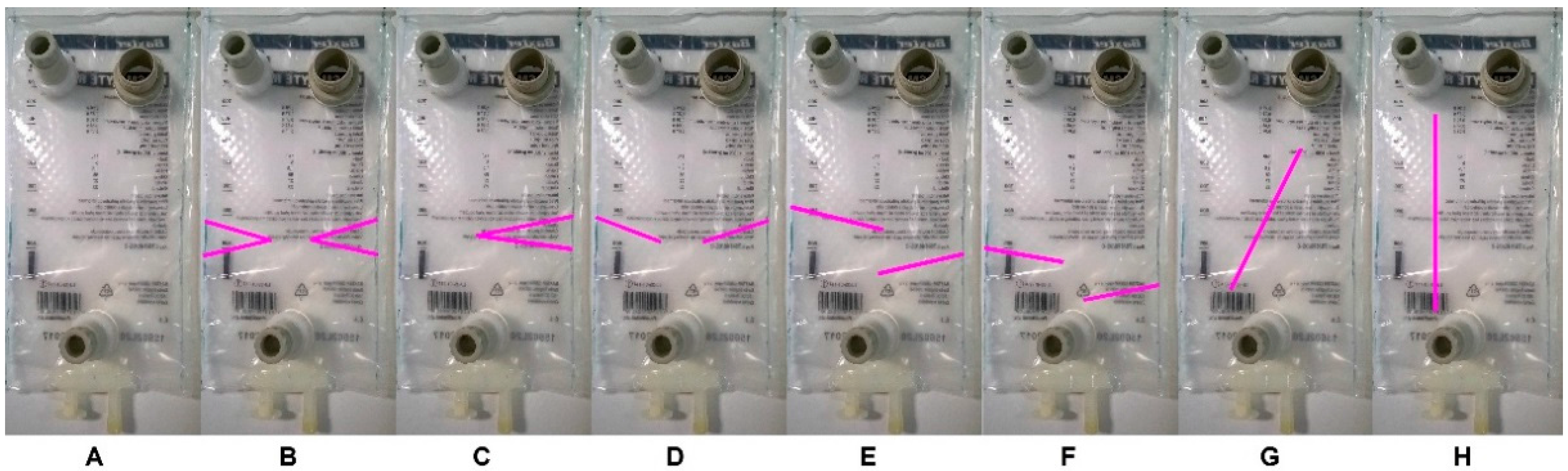
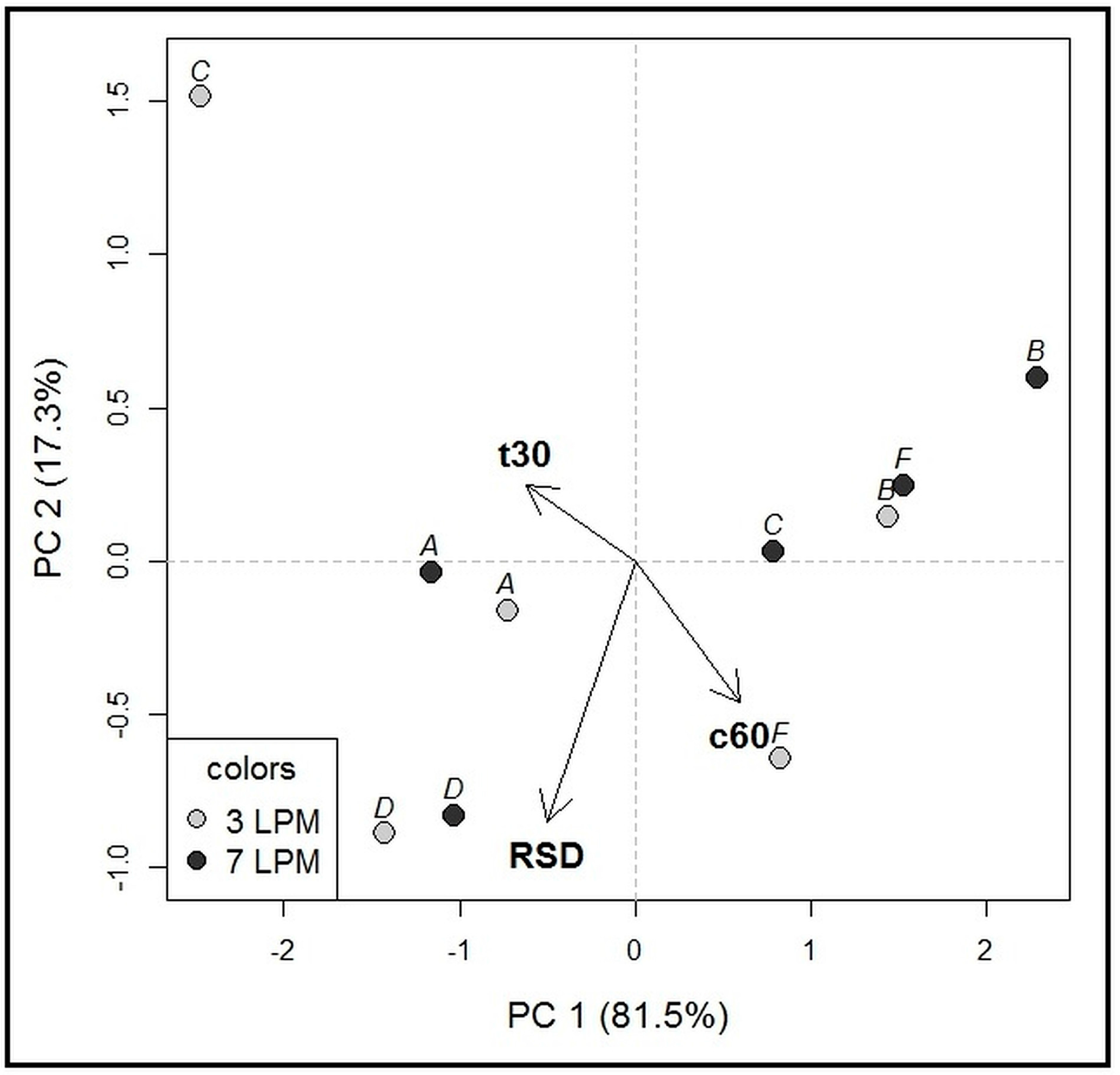
2.1.1. Compartment Type Analysis—Phase 1
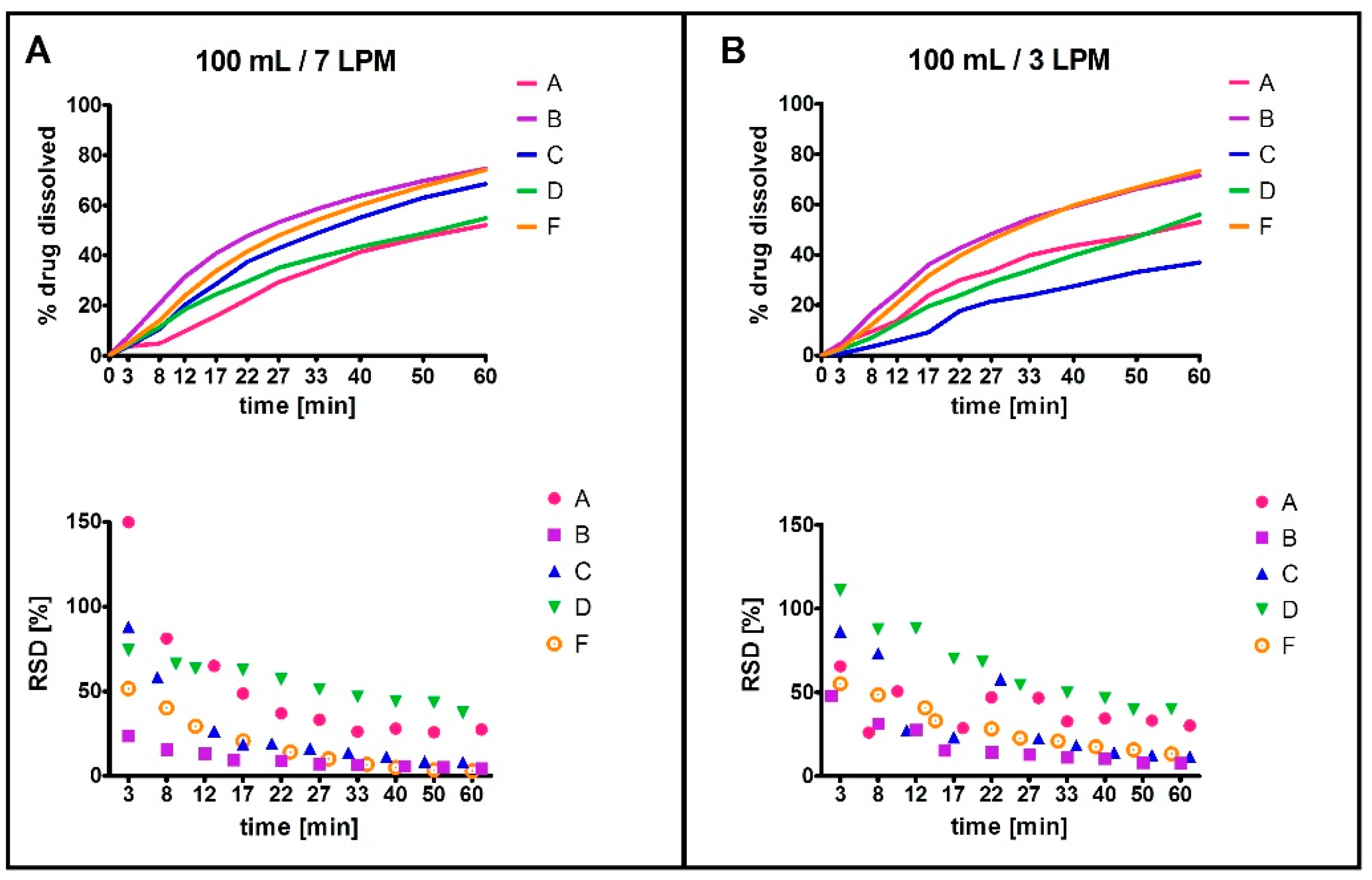
2.1.2. Compartment Type Analysis—Phase 2
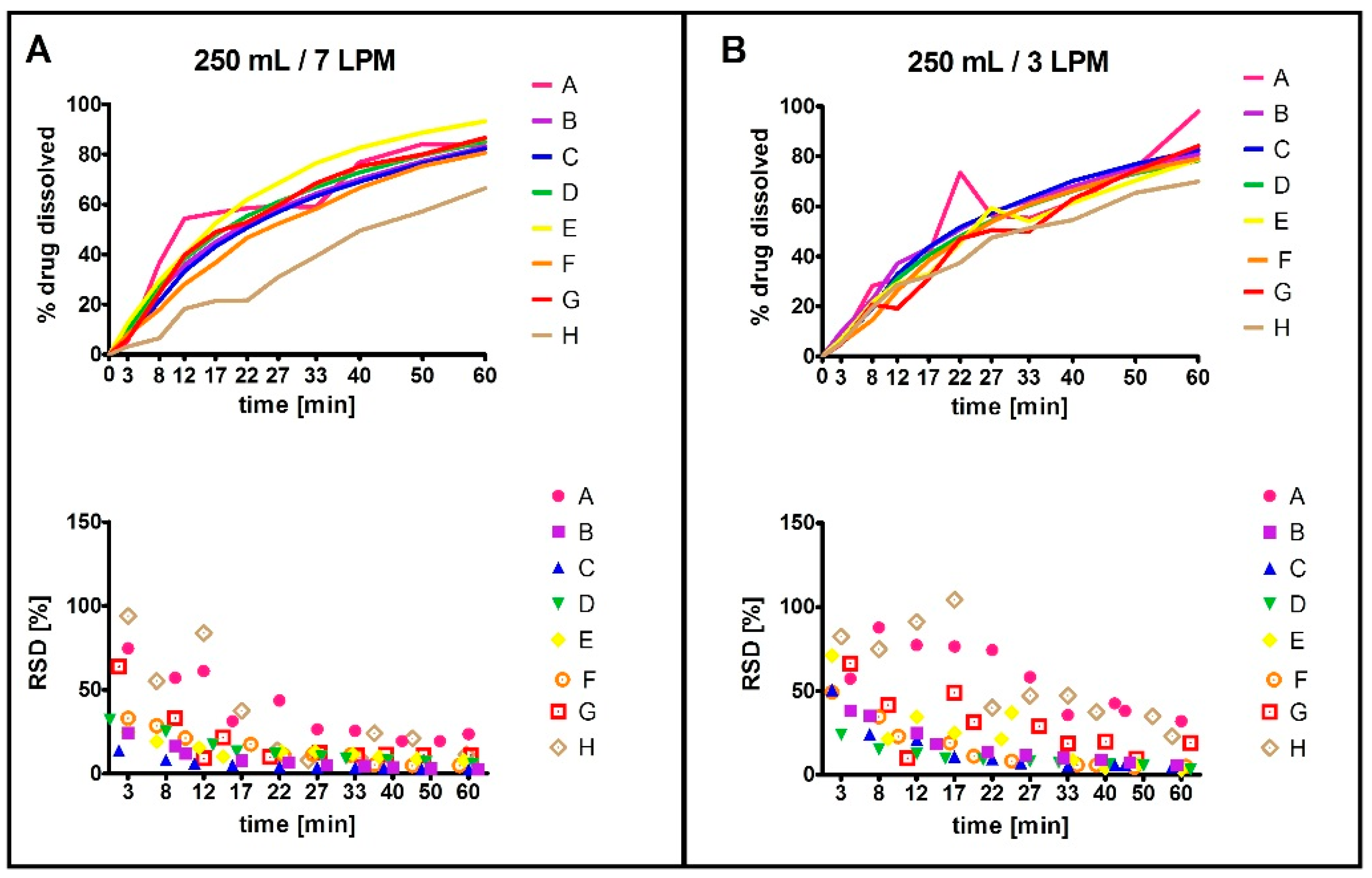
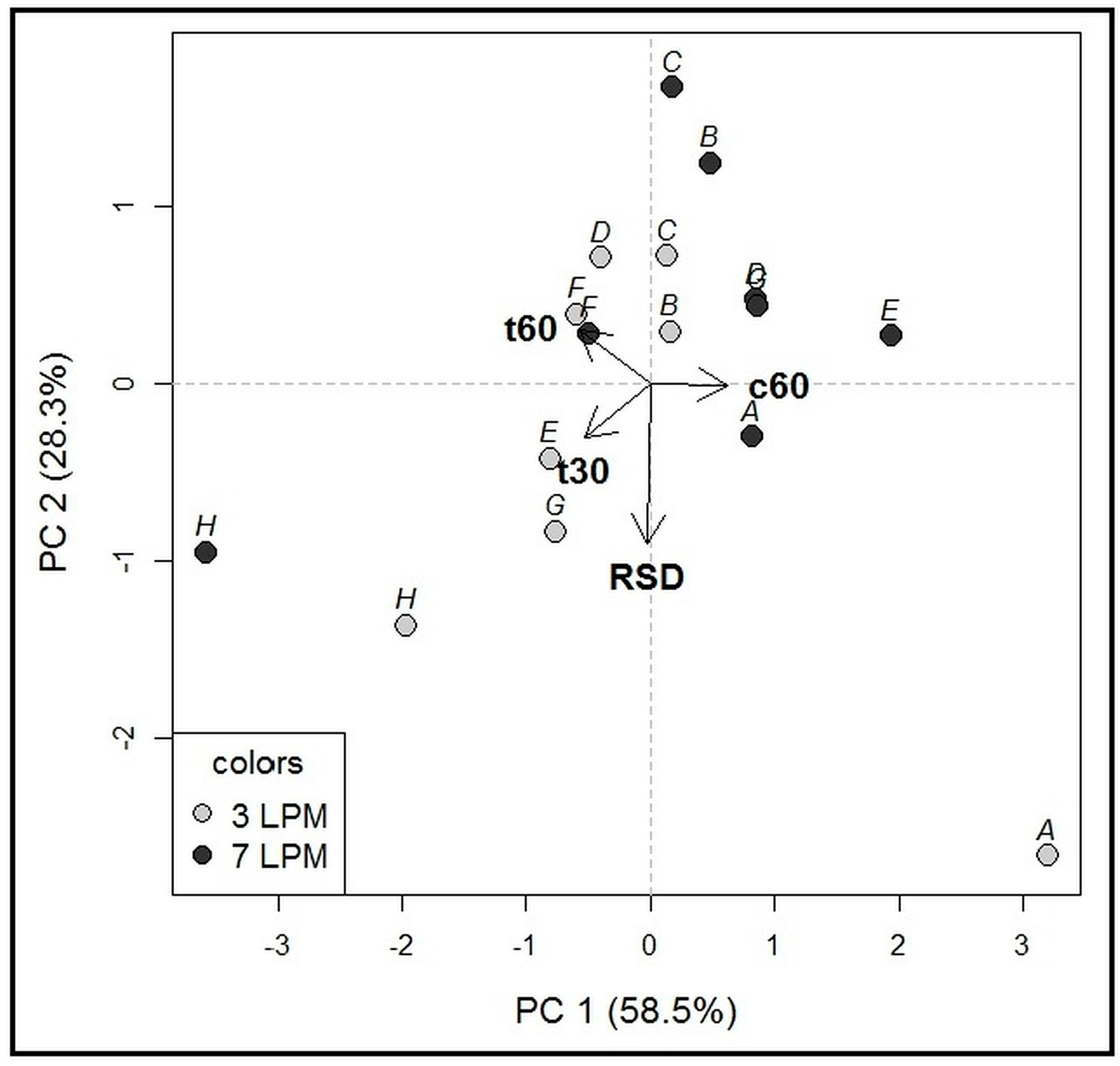
2.1.3. Influence of Agitation Rate and Dissolution Volumes
2.1.4. USP 2 Comparison
3. Materials and Methods
3.1. Tablets
3.2. Golem v2 Dissolution Compartments
3.3. Dissolution Testing in Golem v2 Apparatus
3.4. HPLC Analysis
3.5. USP Dissolution Apparatus 2 Test
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z. The role of bcs (biopharmaceutics classification system) and bddcs (biopharmaceutics drug disposition classification system) in drug development. J. Pharm. Sci. 2013, 102, 34–42. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration/Center for Drug Evaluation and Research. Guidance for Industry: Extended Release oral Dosage Forms: Development, Evaluation, and Application of in Vitro/In Vivo Correlations; Food and Drug Administration: Rockville, MD, USA, 1997.
- Kaur, P.; Jiang, X.; Duan, J.; Stier, E. Applications of in vitro-in vivo correlations in generic drug development: Case studies. AAPS J. 2015, 17, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Davit, B.M.; Kanfer, I.; Tsang, Y.C.; Cardot, J.M. BCS biowaivers: Similarities and differences among EMA, FDA, and WHO requirements. AAPS J. 2016, 18, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Culen, M.; Rezacova, A.; Jampilek, J.; Dohnal, J. Designing a dynamic dissolution method: A review of instrumental options and corresponding physiology of stomach and small intestine. J. Pharm. Sci. 2013, 102, 2995–3017. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M. Dynamic dissolution: A step closer to predictive dissolution testing? Mol. Pharm. 2010, 7, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Kostewicz, E.S.; Abrahamsson, B.; Brewster, M.; Brouwers, J.; Butler, J.; Carlert, S.; Dickinson, P.A.; Dressman, J.; Holm, R.; Klein, S.; et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur. J. Pharm. Sci. 2014, 57, 342–366. [Google Scholar] [CrossRef] [PubMed]
- Vardakou, M.; Mercuri, A.; Naylor, T.A.; Rizzo, D.; Butler, J.M.; Connolly, P.C.; Wickham, M.S.; Faulks, R.M. Predicting the human in vivo performance of different oral capsule shell types using a novel in vitro dynamic gastric model. Int. J. Pharm. 2011, 419, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Wickham, M.J.S.; Faulks, R.M.; Mann, J.; Mandalari, G. The design, operation, and application of a dynamic gastric model. Dissolut. Technol. 2012, 19, 15–22. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. A human gastric simulator (HGS) to study food digestion in human stomach. J. Food Sci. 2010, 75, E627–E635. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, S.; Lelieveld, J.; Gorissen, T.; Minekus, M.; Havenaar, R. Development of an advanced in vitro model of the stomach and its evaluation versus human gastric physiology. Food Res. Int. 2016, 88, 191–198. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R. Multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern. Lab. Anim. ATLA 1995, 23, 197–209. [Google Scholar]
- Minekus, M.; Smeets-Peeters, M.; Bernalier, A.; Marol-Bonnin, S.; Havenaar, R.; Marteau, P.; Alric, M.; Fonty, G. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl. Microbiol. Biotechnol. 1999, 53, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Denis, S.; le Goff, O.; Sicardi, V.; Francois, O.; Yao, A.F.; Garrait, G.; Manzi, A.P.; Beyssac, E.; Alric, M.; et al. Development and validation of a new dynamic computer-controlled model of the human stomach and small intestine. Biotechnol. Bioeng. 2016, 113, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Hribar, M.; Trontelj, J.; Klancar, U.; Markun, B.; Celigoj Dujc, T.; Legen, I. A novel intestine model apparatus for drug dissolution capable of simulating the peristaltic action. AAPS PharmSciTech 2017, 18, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Culen, M.; Tuszynski, P.K.; Polak, S.; Jachowicz, R.; Mendyk, A.; Dohnal, J. Development of in vitro-in vivo correlation/relationship modeling approaches for immediate release formulations using compartmental dynamic dissolution data from “golem”: A novel apparatus. Biomed. Res. Int. 2015, 2015, 328628. [Google Scholar] [CrossRef] [PubMed]
- Reimann, C.; Filzmoser, P.; Garrett, R.G.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R, 1st ed.; John Wiley & Sons: Chichester, UK, 2008; pp. 218–219. ISBN 978-0-470-98581-6. [Google Scholar]
- Franek, F.; Holm, P.; Larsen, F.; Steffansen, B. Interaction between fed gastric media (ensure plus®) and different hypromellose based caffeine controlled release tablets: Comparison and mechanistic study of caffeine release in fed and fasted media versus water using the usp dissolution apparatus 3. Int. J. Pharm. 2014, 461, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mudie, D.M.; Murray, K.; Hoad, C.L.; Pritchard, S.E.; Garnett, M.C.; Amidon, G.L.; Gowland, P.A.; Spiller, R.C.; Amidon, G.E.; Marciani, L. Quantification of gastrointestinal liquid volumes and distribution following a 240 ml dose of water in the fasted state. Mol. Pharm. 2014, 11, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2013; Volume 1, Chapter 4.1.3.; p. 542. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds are available from the authors. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stupák, I.; Pavloková, S.; Vysloužil, J.; Dohnal, J.; Čulen, M. Optimization of Dissolution Compartments in a Biorelevant Dissolution Apparatus Golem v2, Supported by Multivariate Analysis. Molecules 2017, 22, 2042. https://doi.org/10.3390/molecules22122042
Stupák I, Pavloková S, Vysloužil J, Dohnal J, Čulen M. Optimization of Dissolution Compartments in a Biorelevant Dissolution Apparatus Golem v2, Supported by Multivariate Analysis. Molecules. 2017; 22(12):2042. https://doi.org/10.3390/molecules22122042
Chicago/Turabian StyleStupák, Ivan, Sylvie Pavloková, Jakub Vysloužil, Jiří Dohnal, and Martin Čulen. 2017. "Optimization of Dissolution Compartments in a Biorelevant Dissolution Apparatus Golem v2, Supported by Multivariate Analysis" Molecules 22, no. 12: 2042. https://doi.org/10.3390/molecules22122042
APA StyleStupák, I., Pavloková, S., Vysloužil, J., Dohnal, J., & Čulen, M. (2017). Optimization of Dissolution Compartments in a Biorelevant Dissolution Apparatus Golem v2, Supported by Multivariate Analysis. Molecules, 22(12), 2042. https://doi.org/10.3390/molecules22122042




