Enological Tannin Effect on Red Wine Color and Pigment Composition and Relevance of the Yeast Fermentation Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Model Systems
2.1.1. Evolution of Pigment Composition
2.1.2. Color Evolution
2.2. Wines
2.2.1. Evolution of Pigment Composition
2.2.2. Color Evolution
3. Material and Methods
3.1. Chemicals
3.2. Model Systems
3.3. Wines
3.4. HPLC-DAD-MS Analysis
3.5. Colorimetric Measurements
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments

Author Contributions
Conflicts of Interest
References
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [PubMed]
- Salas, E.; Le Guerneve, C.; Fulcrand, H.; Poncet-Legrand, C.; Cheynier, W. Structure determination and colour properties of a new directly linked flavanol-anthocyanin dimer. Tetrahedron Lett. 2004, 45, 8725–8729. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Álvarez-García, M.; Rivas-Gonzalo, J.C.; Heredia, F.J.; Santos-Buelga, C. Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin 3-O-glucoside and (+)-catechin. J. Agric. Food Chem. 2001, 49, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef]
- Fulcrand, H.; dosSantos, P.J.C.; SarniManchado, P.; Cheynier, V.; FavreBonvin, J. Structure of new anthocyanin-derived wine pigments. J. Chem. Soc. Perkin Trans. 1996, 7, 735–739. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Benabdeljalil, C.; Cheynier, V.; Fulcrand, H.; Hakiki, A.; Mosaddak, M.; Moutounet, M. Evidence of new pigments resulting from reaction between anthocyanins and yeast metabolites. Sci. Aliment. 2000, 20, 203–219. [Google Scholar] [CrossRef]
- Quijada-Morín, N.; Dangles, O.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Physico-chemical and chromatic characterization of malvidin 3-glucoside-vinylcatechol and malvidin 3-glucoside-vinylguaiacol wine pigments. J. Agric. Food Chem. 2010, 58, 9744–9752. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, Y.; Asenstorfer, R.E. Screening for potential pigments derived from anthocyanins in red wine using nanoelectrospray tandem mass spectrometry. J. Agric. Food Chem. 2002, 50, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, A.E.; Pardon, K.; Hayasaka, Y.; de Sa, M.; Herderich, M. Structures and colour properties of new red wine pigments. Tetrahedron Lett. 2003, 44, 4887–4891. [Google Scholar] [CrossRef]
- Schwarz, M.; Wabnitz, T.C.; Winterhalter, P. Pathway leading to the formation of anthocyanin-vinylphenol adducts and related pigments in red wines. J. Agric. Food Chem. 2003, 51, 3682–3687. [Google Scholar] [CrossRef] [PubMed]
- Francia-Aricha, E.M.; Guerra, M.T.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. New anthocyanin pigments formed after condensation with flavanols. J. Agric. Food Chem. 1997, 45, 2262–2266. [Google Scholar] [CrossRef]
- Rivas-Gonzalo, J.C.; Bravo-Haro, S.; Santos-Buelga, C. Detection of compounds formed through the reaction of malvidin 3-monoglucoside and catechin in the presence of acetaldehyde. J. Agric. Food Chem. 1995, 43, 1444–1449. [Google Scholar] [CrossRef]
- Blanco-Vega, D.; López-Bellido, F.J.; Alia-Robledo, J.M.; Hermosin-Gutierrez, I. HPLC-DAD-ESI-MS/MS Characterization of Pyranoanthocyanins Pigments Formed in Model Wine. J. Agric. Food Chem. 2011, 59, 9523–9531. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- García-Estevéz, I.; Alcalde-Eon, C.; Escribano-Bailón, M.T. Flavanol Quantification of Grapes via Multiple Reaction Monitoring Mass Spectrometry: Application to Differentiation among Clones of Vitis vinifera L. cv. Rufete Grapes. J. Agric. Food Chem. 2017, 65, 6359–6368. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Vivas, N.; Glories, Y. Role of oak wood ellagitannins in the oxidation process of red wines during aging. Am. J. Enol. Vitic. 1996, 47, 103–107. [Google Scholar]
- Timberlake, C.F.; Bridle, P. Interactions between anthocyanins, phenolic compounds, and acetalheyde and their significance in red wines. Am. J. Enol. Vitic. 1976, 27, 97–105. [Google Scholar]
- García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Validation of a Mass Spectrometry Method to Quantify Oak Ellagitannins in Wine Samples. J. Agric. Food Chem. 2012, 60, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Cadahía, E.; Varea, S.; Muñoz, L.; Fernández de Simón, B.; García-Vallejo, M.C. Evolution of ellagitannins in Spanish, French, and American oak woods during natural seasoning and toasting. J. Agric. Food Chem. 2001, 49, 3677–3684. [Google Scholar] [CrossRef]
- Versari, A.; du Toit, W.; Parpinello, G.P. Oenological tannins: A review. Aust. J. Grape Wine Res. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, A.; López-Solis, R.; Ramírez-Escudero, C.; Zamora-Marín, F. Phenolic characterization of commercial enological tannins. Eur. Food Res. Technol. 2009, 229, 859–866. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. The effects of enological practices in anthocyanins, phenolic compounds and wine colour and their dependence on grape characteristics. J. Food Comp. Anal. 2007, 20, 546–552. [Google Scholar] [CrossRef]
- Manfroi, V.; Rizzon, L.A.; Guerra, C.C.; Fialho, F.B.; Dall’Agnol, I.; Carlos Ferri, V.; Valmor Rombaldi, C. Influence of different doses and distinct times of application of Enological tannins on the physicochemical characteristics of the Cabernet Sauvignon wine. Ciencia Tecnol. Aliment. 2010, 30, 127–135. [Google Scholar] [CrossRef]
- Neves, A.C.; Spranger, M.I.; Zhao, Y.; Leandro, M.C.; Sun, B. Effect of Addition of Commercial Grape Seed Tannins on Phenolic Composition, Chromatic Characteristics, and Antioxidant Activity of Red Wine. J. Agric. Food Chem. 2010, 58, 11775–11782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Liang, N.-N.; Wang, J.; Pan, Q.-H.; Duan, C.-Q. Effect of the Prefermentative Addition of Five Enological Tannins on Anthocyanins and Color in Red Wines. J. Food Sci. 2013, 78, C25–C30. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Smith, P.A.; Birse, M.; Farancis, I.L.; Kwiatkowski, M.J.; Lattey, K.A.; Liebich, B.; Herderich, M.J. The effect of pre- and post-ferment additions of grape derived tannin on Shiraz wine sensory properties and phenolic composition. Aust. J. Grape Wine Res. 2007, 13, 30–37. [Google Scholar] [CrossRef]
- Main, G.L.; Morris, J.R. Effect of macerating enzymes and postfermentation grape-seed tannin on the color of cynthiana wines. Am. J. Enol. Vitic. 2007, 58, 365–372. [Google Scholar]
- Alcalde-Eon, C.; García-Estévez, I.; Ferreras-Charro, R.; Rivas-Gonzalo, J.C.; Ferrer-Gallego, R.; Escribano-Bailón, M.T. Adding oenological tannin vs. overripe grapes: Effect on the phenolic composition of red wines. J. Food Comp. Anal. 2014, 34, 99–113. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Puente, V.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Color stabilization of red wines. A chemical and colloidal approach. J. Agric. Food Chem. 2014, 62, 6984–6994. [Google Scholar] [CrossRef] [PubMed]
- Asenstorfer, R.E.; Markides, A.J.; Iland, P.G.; Jones, G.P. Formation of vitisin A during red wine fermentation and maturation. Aust. J. Grape Wine Res. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- García-Estévez, I.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Alcalde-Eon, C. Evaluation of the influence of oak ellagitannins in the synthesis of pyranoanthocyanins in model systems. In Proceedings of the 8th International Workshop on Anthocyanins (IWA), Montpellier, France, 16–18 September 2015; p. 125. [Google Scholar]
- García-Puente Rivas, E.; Alcalde-Eon, C.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Behaviour and characterisation of the colour during red wine making and maturation. Anal. Chim. Acta 2006, 563, 215–222. [Google Scholar] [CrossRef]
- González-Manzano, S.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Santos-Buelga, C. Studies on the copigmentation between anthocyanins and flavan-3-ols and their influence in the colour expression of red wine. Food Chem. 2009, 114, 649–656. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Darias-Martín, J.; Carrillo, M.; Díaz, E.; Boulton, R.B. Enhancement of red wine colour by pre-fermentation addition of copigments. Food Chem. 2001, 73, 217–220. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing—A comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- García-Estévez, I.; Jacquet, R.; Alcalde-Eon, C.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Quideau, S. Hemisynthesis and Structural and Chromatic Characterization of Delphinidin 3-O-Glucoside-Vescalagin Hybrid Pigments. J. Agric. Food Chem. 2013, 61, 11560–11568. [Google Scholar] [CrossRef] [PubMed]
- García-Marino, M.; Rivas-Gonzalo, J.C.; Ibáñez, E.; García-Moreno, C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal. Chim. Acta 2006, 563, 44–50. [Google Scholar] [CrossRef]
- Ough, C.S.; Davenport, M.; Joseph, K. Effects of certain vitamins on growth and fermentation rate of several commercial active dry wine yeasts. Am. J. Enol. Vitic. 1989, 40, 208–213. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
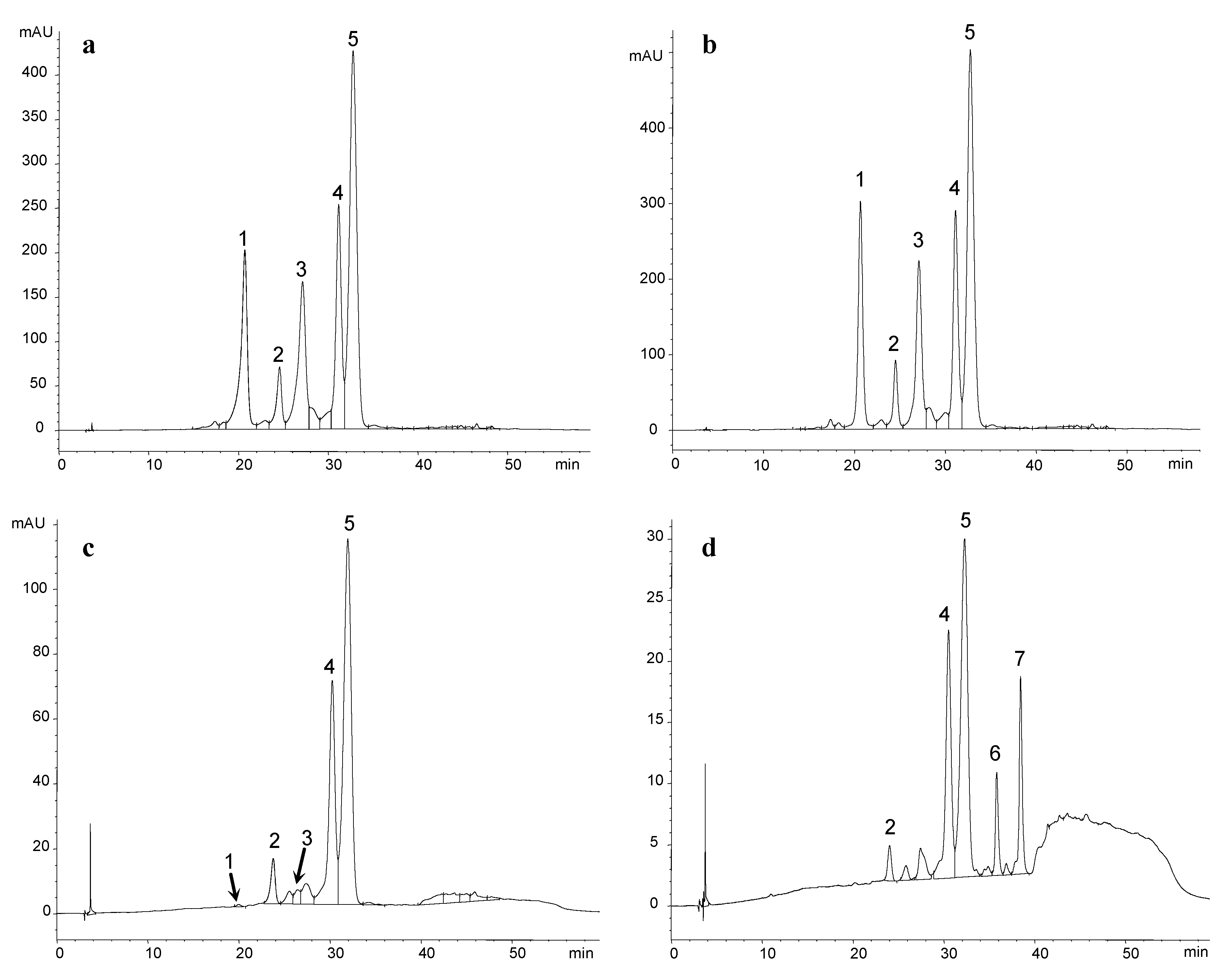

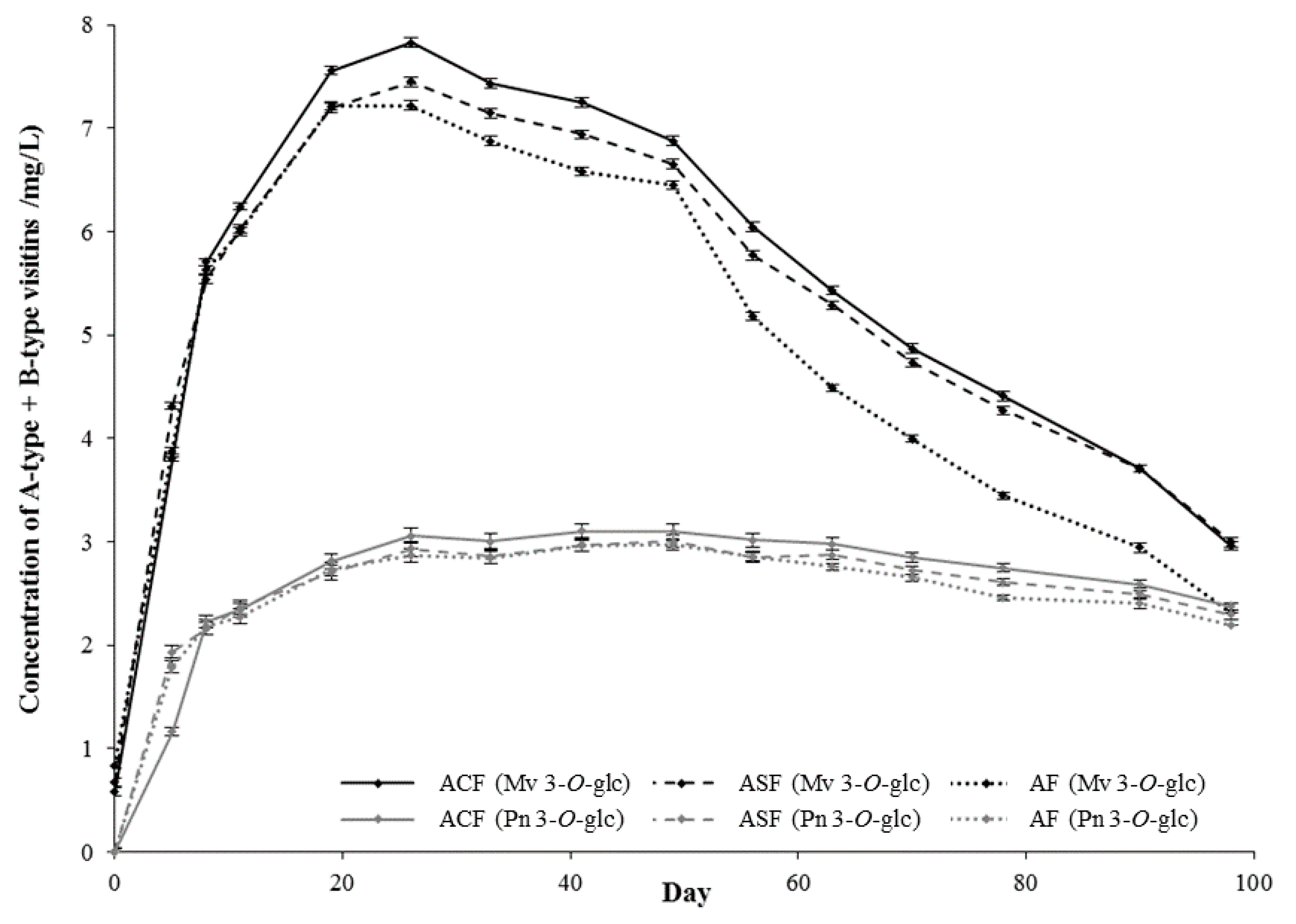
| Day | Standard Wine 1 | Fermentative Medium 1 | ||||
|---|---|---|---|---|---|---|
| AC | AS | A | ACF | ASF | AF | |
| 0 | 86.97 ± 0.08 a | 86.64 ± 0.09 a | 87.18 ± 0.08 a | 77.97 ± 0.08 a | 78.62 ± 0.06 a | 78.35 ± 0.09 a |
| 5 | 86.75 ± 0.03 a | 86.48 ± 0.05 a | 87.05 ± 0.04 b | 79.25 ± 0.08 a | 79.79 ± 0.08 b | 79.99 ± 0.02 c |
| 19 | 88.33 ± 0.06 a | 87.85 ± 0.01 b | 88.68 ± 0.03 c | 83.35 ± 0.04 a | 83.71 ± 0.02 b | 84.31 ± 0.02 c |
| 41 | 89.74 ± 0.05 a | 89.24 ± 0.02 b | 90.24 ± 0.05 c | 85.91 ± 0.05 a | 86.25 ± 0.02 b | 87.26 ± 0.06 c |
| 70 | 88.50 ± 0.04 a | 88.18 ± 0.01 b | 90.08 ± 0.02 c | 89.51 ± 0.06 a | 89.54 ± 0.04 a | 90.58 ± 0.07 b |
| 90 | 88.95 ± 0.03 a | 88.86 ± 0.02 b | 90.87 ± 0.03 c | 89.97 ± 0.01 a | 89.99 ± 0.04 a | 90.99 ± 0.02 c |
| 98 | 89.02 ± 0.01 a | 89.26 ± 0.07 b | 91.10 ± 0.03 c | 90.32 ± 0.02 a | 90.44 ± 0.01 b | 91.41 ± 0.05 c |
| Day | Standard Wine 1 | Fermentative Medium 1 | ||||
|---|---|---|---|---|---|---|
| AC | AS | A | ACF | ASF | AF | |
| 0 | 22.65 ± 0.06 a | 22.90 ± 0.08 a | 21.42 ± 0.08 a | 40.71 ± 0.09 a | 39.54 ± 0.06 a | 40.40 ± 0.07 b |
| 5 | 21.30 ± 0.04 a | 21.52 ± 0.03 a | 21.09 ± 0.02 b | 36.35 ± 0.04 a | 35.24 ± 0.05 b | 35.60 ± 0.05 c |
| 19 | 17.02 ± 0.02 a | 17.44 ± 0.01 a | 16.93 ± 0.03 b | 24.26 ± 0.06 a | 23.53 ± 0.05 b | 22.98 ± 0.03 c |
| 41 | 13.56 ± 0.05 a | 13.92 ± 0.05 b | 13.02 ± 0.01 c | 17.21 ± 0.03 a | 16.37 ± 0.03 b | 15.39 ± 0.08 c |
| 70 | 13.23 ± 0.06 a | 12.90 ± 0.02 b | 11.01 ± 0.02 c | 12.75 ± 0.04 a | 12.11 ± 0.03 a | 10.88 ± 0.05 b |
| 90 | 12.36 ± 0.02 a | 11.91 ± 0.03 b | 9.69 ± 0.04 c | 11.98 ± 0.03 a | 11.37 ± 0.02 a | 9.96 ± 0.02 b |
| 98 | 12.24 ± 0.02 a | 11.41 ± 0.04 b | 9.35 ± 0.05 c | 11.68 ± 0.01 a | 10.93 ± 0.04 b | 9.55 ± 0.01 c |
| Day | Standard Wine 1 | Fermentative Medium 1 | ||||
|---|---|---|---|---|---|---|
| AC | AS | A | ACF | ASF | AF | |
| 0 | −3.69 ± 0.09 a | −3.20 ± 0.08 b | −4.22 ± 0.01 c | −1.36 ± 0.08 a | −1.35 ± 0.06 a | −1.25 ± 0.03 b |
| 5 | 2.10 ± 0.03 a | 2.63 ± 0.04 b | 1.32 ± 0.04 c | 0.51 ± 0.08 a | 0.59 ± 0.01 a | 0.16 ± 0.01 b |
| 19 | 14.16 ± 0.03 a | 13.46 ± 0.03 b | 10.32 ± 0.05 c | 7.80 ± 0.04 a | 6.74 ± 0.02 b | 6.42 ± 0.07 c |
| 41 | 26.73 ± 0.02 a | 24.05 ± 0.01 b | 18.57 ± 0.08 c | 21.58 ± 0.03 a | 18.36 ± 0.02 b | 15.84 ± 0.06 c |
| 70 | 33.83 ± 0.05 a | 30.21 ± 0.08 b | 28.12 ± 0.02 c | 37.02 ± 0.07 a | 34.44 ± 0.07 a | 28.80 ± 0.07 b |
| 90 | 41.34 ± 0.05 a | 37.49 ± 0.03 b | 36.23 ± 0.01 c | 43.17 ± 0.04 a | 39.16 ± 0.04 a | 34.57 ± 0.02 c |
| 98 | 43.23 ± 0.01 a | 39.28 ± 0.02 b | 38.50 ± 0.03 c | 44.72 ± 0.02 a | 40.76 ± 0.04 b | 36.16 ± 0.01 c |
| Sampling Point 1 | ||||||
|---|---|---|---|---|---|---|
| 17 Days | 20 Days | 2 Months | 5 Months | 8 Months | ||
| Total anthocyanins | Wine C | 380 ± 4 a | 319 ± 8 a | 281 ± 3 a | 264 ± 7 a | 202 ± 3 a |
| Wine R | 397 ± 7 b | 396 ± 5 b | 325 ± 5 b | 314 ± 6 b | 257 ± 5 b | |
| F-A+ products 2 | Wine C | 0.97 ± 0.08 a | 1.01 ± 0.09 a | 1.31 ± 0.01 a | 0.28 ± 0.01 a | 0.17 ± 0.01 a |
| Wine R | 0.98 ± 0.09 a | 1.15 ± 0.08 b | 1.39 ± 0.02 b | 0.32 ± 0.01 b | 0.22 ± 0.03 b | |
| F-ethyl-A+ products 3 | Wine C | 0.27 ± 0.02 a | 0.21 ± 0.01 a | 0.12 ± 0.01 a | n.q. | n.q. |
| Wine R | 0.38 ± 0.01 b | 0.33 ± 0.02 b | 0.2 ± 0.01 b | n.q. | n.q. | |
| A-type + B-type vitisins of malvidin 3-O-glucoside | Wine C | 3.43 ± 0.05 a | 2.85 ± 0.17 a | 1.72 ± 0.08 a | 1.45 ± 0.09 a | 1.59 ± 0.01 a |
| Wine R | 3.51 ± 0.07 a | 3.04 ± 0.12 b | 1.73 ± 0.06 a | 1.47 ± 0.06 a | 1.74 ± 0.03 b | |
| Vinylphenol piranoanthocyanin of malvidin 3-O-glucoside | Wine C | 0.06 ± 0.01 a | 0.09 ± 0.02 a | 0.1 ± 0.03 a | 0.06 ± 0.02 a | 0.14 ± 0.05 a |
| Wine R | 0.05 ± 0.02 a | 0.09 ± 0.02 a | 0.1 ± 0.02 a | 0.07 ± 0.02 a | 0.18 ± 0.02 a | |
| Sampling Point 1 | ||||||
|---|---|---|---|---|---|---|
| 17 Days | 20 Days | 2 Months | 5 Months | 8 Months | ||
| L* | Wine C | 63.76 ± 0.04 a | 66.7 ± 0.09 a | 79.8 ± 0.07 a | 85.95 ± 0.04 a | 87.68 ± 0.03 a |
| Wine R | 61.44 ± 0.04 b | 61.66 ± 0.02b | 76.21 ± 0.01 b | 84.12 ± 0.03 b | 82.98 ± 0.01 b | |
| C*ab | Wine C | 45.93 ± 0.05 a | 40.76 ± 0.09 a | 23.33 ± 0.06 a | 14.43 ± 0.07 a | 11.76 ± 0.01 a |
| Wine R | 47.58 ± 0.07 b | 46.05 ± 0.03 b | 26.79 ± 0.02 b | 15.39 ± 0.06 b | 16.53 ± 0.01 b | |
| hab | Wine C | −7.81 ± 0.03 a | −7.26 ± 0.04 a | −5.89 ± 0.05 a | 2.58 ± 0.04 a | 11.32 ± 0.14 a |
| Wine R | −7.13 ± 0.03 b | −6.49 ± 0.02 b | −5.91 ± 0.05 b | 6.79 ± 0.06 b | 7.97 ± 0.02 b | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Estévez, I.; Alcalde-Eon, C.; Puente, V.; Escribano-Bailón, M.T. Enological Tannin Effect on Red Wine Color and Pigment Composition and Relevance of the Yeast Fermentation Products. Molecules 2017, 22, 2046. https://doi.org/10.3390/molecules22122046
García-Estévez I, Alcalde-Eon C, Puente V, Escribano-Bailón MT. Enological Tannin Effect on Red Wine Color and Pigment Composition and Relevance of the Yeast Fermentation Products. Molecules. 2017; 22(12):2046. https://doi.org/10.3390/molecules22122046
Chicago/Turabian StyleGarcía-Estévez, Ignacio, Cristina Alcalde-Eon, Víctor Puente, and M. Teresa Escribano-Bailón. 2017. "Enological Tannin Effect on Red Wine Color and Pigment Composition and Relevance of the Yeast Fermentation Products" Molecules 22, no. 12: 2046. https://doi.org/10.3390/molecules22122046
APA StyleGarcía-Estévez, I., Alcalde-Eon, C., Puente, V., & Escribano-Bailón, M. T. (2017). Enological Tannin Effect on Red Wine Color and Pigment Composition and Relevance of the Yeast Fermentation Products. Molecules, 22(12), 2046. https://doi.org/10.3390/molecules22122046







