Studies on the Anti-Oxidative Function of trans-Cinnamaldehyde-Included β-Cyclodextrin Complex
Abstract
:1. Introduction
2. Results and Discussion
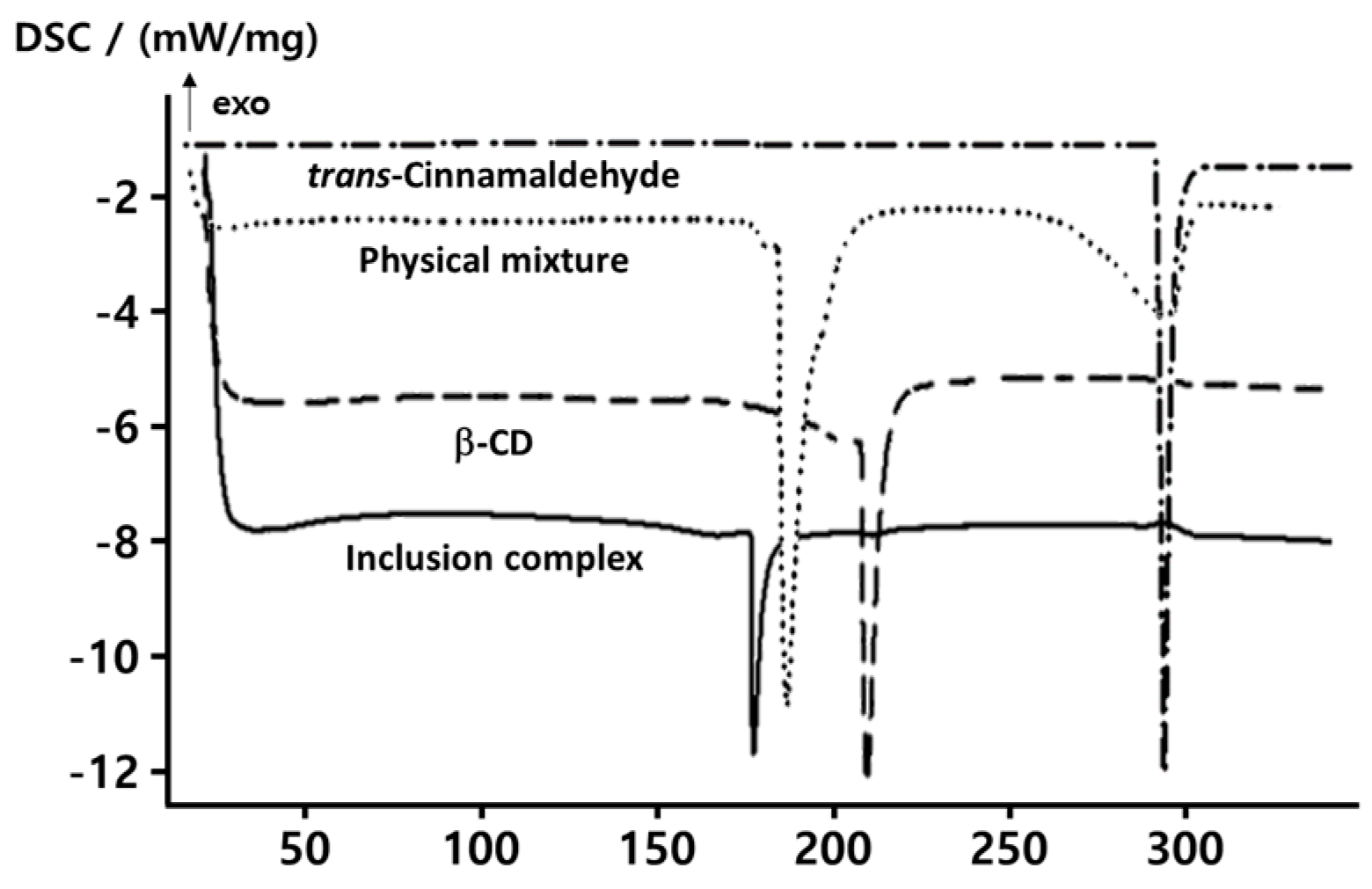
2.1. Thermal Properties
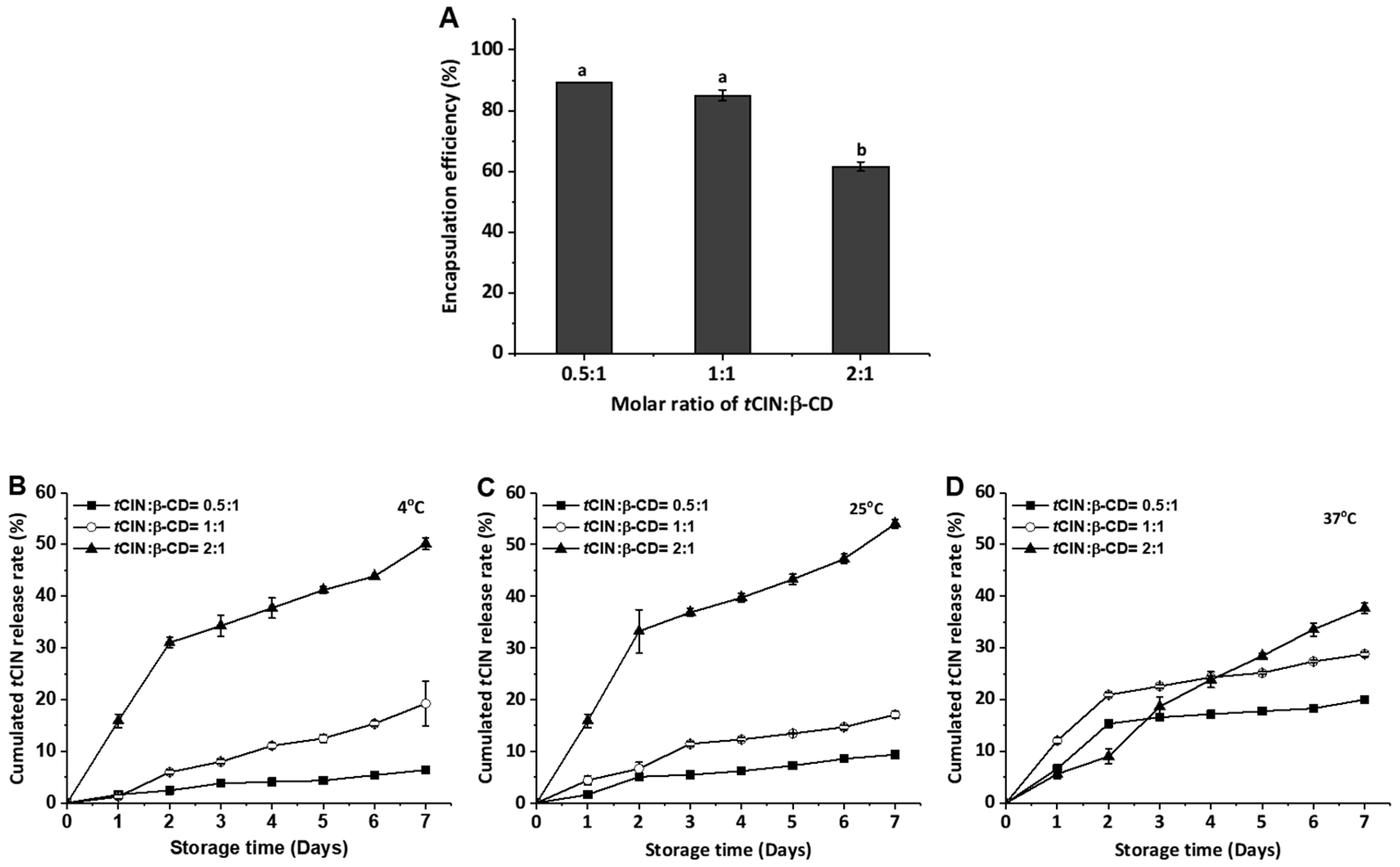
2.2. Encapsulation Efficiency and Release Characteristics of the CIs
2.3. Anti-Oxidant Activities of the CIs
2.4. Cell Viability
2.5. Inhibition of NO Production
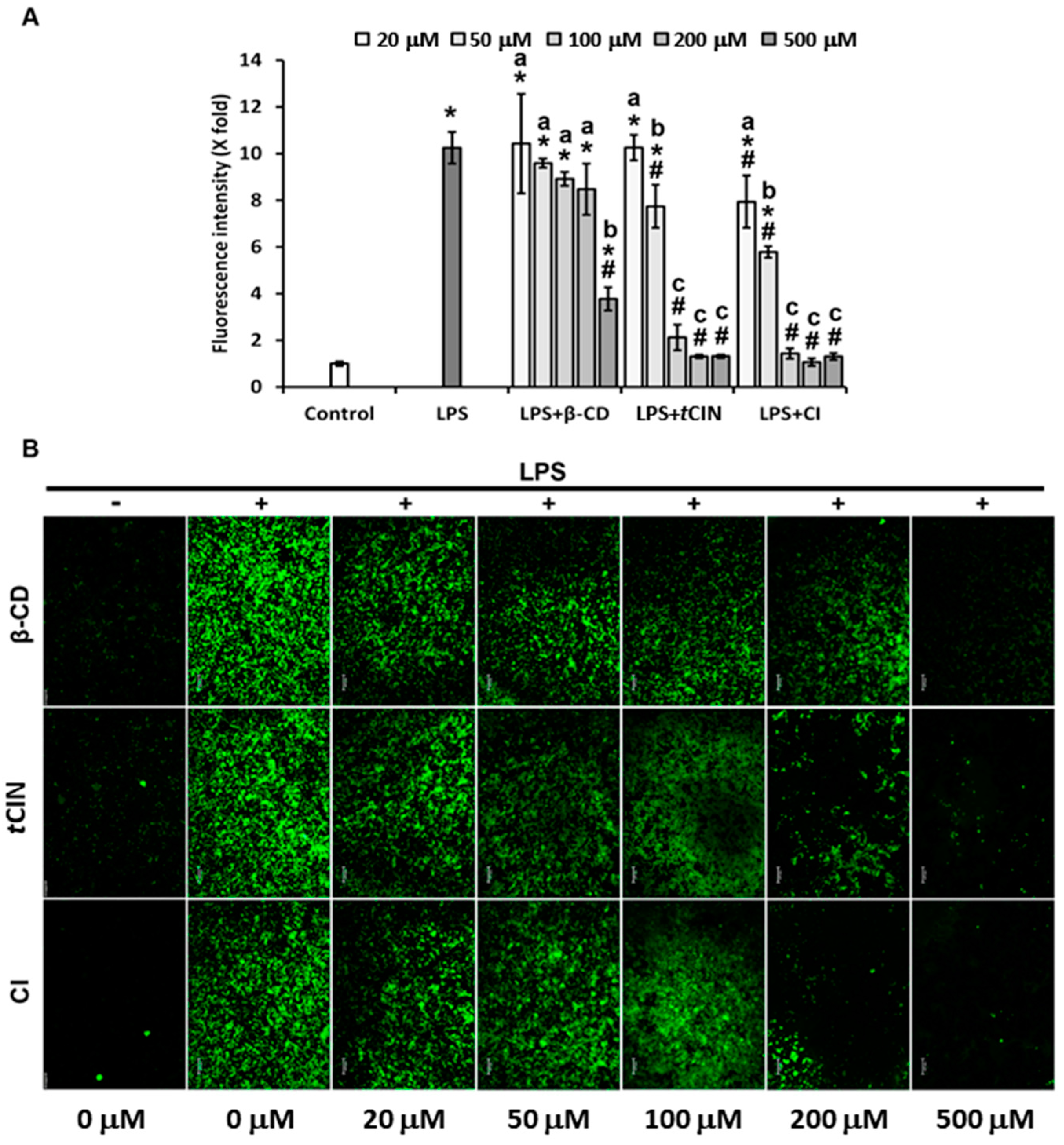
2.6. ROS Suppression
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. DSC Measurement
3.4. Encapsulation Efficiency and Release Study
3.5. Anti-Oxidative Activity
3.6. Cell Culture
3.7. Cell Viability
3.8. NO Production
3.9. ROS Determination
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, R.; Balick, M.J. Sweet wood-cinnamon and its importance as a spice and medicine. J. Sci. Heal. 2005, 1, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Chen, P.F.; Chang, S.C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Sartorius, T.; Peter, A.; Schulz, N.; Drescher, A.; Bergheim, I.; Machann, J.; Schick, F.; Siegel-Axel, D.; Schürmann, A.; Weigert, C.; et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS ONE 2014, 9, e92358. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Hwang, J.S.; So, J.S.; Lee, C.G.; Sahoo, A.; Ryu, J.H.; Jeon, W.K.; Ko, B.S.; Im, C.R.; Lee, S.J.; et al. Cinnamon extract induces tumor cell death through inhibition of NFκB and AP1. BMC Cancer 2010, 10, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Chang, K.S.; Chang, P.W. Inhibition of neuroinflammation by cinnamon and its main components. Food Chem. 2013, 138, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Dong, X.; Jiang, G.; Zhang, H.; Xie, H.; Jiang, Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov. Food Sci. Emerg. Technol. 2009, 10, 627–632. [Google Scholar] [CrossRef]
- Cabello, C.M.; Bair, W.B.; Lamore, S.D.; Ley, S.; Bause, A.S.; Azimian, S.; Wondrak, G.T. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic. Biol. Med. 2009, 46, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Liu, L.Q.; He, Y.L.; Kong, W.J.; Huang, S.A. Cytotoxic effect of trans-cinnamaldehyde on human leukemia K562 cells. Acta Pharmacol. Sin. 2010, 31, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.K.; Hua, K.F.; Hsu, H.Y.; Cheng, S.S.; Lin, I.F.; Chen, C.J.; Chen, S.T.; Chang, S.T. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem. Toxicol. 2008, 46, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.Y.; Son, D.J.; Lee, H.; Yoo, H.S.; Song, S.; Oh, K.W.; Han, D.C.; Kwon, B.M.; Hong, J.T. Inhibitory effect of 2′-hydroxycinnamaldehyde on nitric oxide production through inhibition of NF-κB activation in RAW 264.7 cells. Biochem. Pharmacol. 2005, 69, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.Y.; Guh, J.Y.; Chao, L.K.; Lu, Y.C.; Hwang, J.Y.; Yang, Y.L.; Cheng, T.H.; Yang, W.Y.; Chien, Y.J.; Huang, J.S. Anti-proliferative effects of cinnamaldehyde on human hepatoma cell lines. Food Chem. 2012, 133, 1603–1610. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Fahmy, A.; Badawy, D. Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem. Toxicol. 2011, 49, 3007–3012. [Google Scholar] [CrossRef] [PubMed]
- Hooth, M.J.; Sills, R.C.; Burka, L.T.; Haseman, J.K.; Witt, K.L.; Orzech, D.P.; Fuciarelli, A.F.; Graves, S.W.; Johnson, J.D.; Bucher, J.R. Toxicology and carcinogenesis studies of microencapsulated trans-cinnamaldehyde in rats and mice. Food Chem. Toxicol. 2004, 42, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Smith, R.L.; Tagami, H. A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem. Toxicol. 2005, 43, 799–836. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.E.; Eissa, S. Electrochemistry of cyclodextrin inclusion complexes of pharmaceutical compounds. Open Chem. Biomed. Methods J. 2010, 3, 74–85. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.; Al-Sadeh, K.S.; Al-Bitar, M.B. Inclusion complexes of sunscreen agents with β-cyclodextrin: Spectroscopic and molecular modeling studies. J. Spectrosc. 2013, 2013, 841409–841420. [Google Scholar] [CrossRef]
- Marangoci, N.; Mares, M.; Silion, M.; Fifere, A.; Varganici, C.; Nicolescu, A.; Deleanu, C.; Coroaba, A.; Pinteala, M.; Simionescu, B.C. Inclusion complex of a new propiconazole derivative with β-cyclodextrin: NMR, ESI–MS and preliminary pharmacological studies. Results Pharm. Sci. 2011, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Auda, S.H. Nimesulide/Methyl β-cyclodextrin inclusion complexes: Physicochemical characterization, solubility, dissolution, and biological studies. Drug Dev. Res. 2014, 75, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, C.; Arama, C.; Monciu, C.M. Preparation and characterization of inclusion complexes between repaglinide and β-cyclodextrin, 2-hydroxypropyl-β-cyclodextrin and randomly methylated β-cyclodextrin. Farmacia 2010, 58, 78–88. [Google Scholar]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Cravotto, G.; Binello, A.; Baranelli, E.; Carraro, P.; Trotta, F. Cyclodextrins as food additives and in food processing. Curr. Nutr. Food Sci. 2006, 2, 343–350. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Leonardi, D.; Salazar, M.O.; Lamas, M.C. Modified β-cyclodextrin inclusion complex to improve the physicochemical properties of albendazole. Complete in vitro evaluation and characterization. PLoS ONE 2014, 9, e88234. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Min, S.G.; Choi, M.J. Release characteristics of freeze-dried eugenol encapsulated with β-cyclodextrin by molecular inclusion method. J. Microencapsul. 2010, 27, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Lila, M.A.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Choi, M.J.; Ruktanonchai, U.; Soottitantawat, A.; Min, S.G. Morphological characterization of encapsulated fish oil with β-cyclodextrin and polycaprolactone. Food Res. Int. 2009, 42, 989–997. [Google Scholar] [CrossRef]
- Lee, K.H.; Choi, E.M. Stimulatory effects of extract prepared from the bark of Cinnamomum cassia blume on the function of osteoblastic MC3T3-E1 cells. Phytother. Res. 2006, 20, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Nedovic, V.A. Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: London, UK, 2010; pp. 127–1160. ISBN 9781441910073. [Google Scholar]
- Lakkis, J.M. Encapsulation and Controlled Release Technologies in Food Systems; Blackwell Publishing: Oxford, UK, 2007; pp. 13–40. ISBN 9780470277881. [Google Scholar]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Yang, R.; Chen, J.B.; Xiao, C.F.; Liu, Z.C.; Gao, Z.Y.; Yan, S.J.; Zhang, J.H.; Zhang, H.B.; Lin, J. Inclusion complex of GA-13316 with β-cyclodextrin: Preparation, characterization, molecular modeling, and in vitro evaluation. Carbohydr. Polym. 2014, 111, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.J.; Davaatseren, M.; Kim, W.; Park, S.K.; Kim, S.H.; Hur, H.J.; Kim, M.S.; Kim, Y.S.; Kwon, D.Y. Vitisin A suppresses LPS-induced NO production by inhibiting ERK, p38, and NF-κB activation in RAW 264.7 cells. Int. Immunopharmacol. 2009, 9, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.Y.; Jo, Y.J.; Bjrapha, P.; Choi, M.J.; Min, S.G. Antimicrobial effect of α- or β-cyclodextrin complexes with trans-cinnamaldehyde against staphylococcus aureus and Escherichia coli. Dry. Technol. 2015, 33, 377–383. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davaatseren, M.; Jo, Y.-J.; Hong, G.-P.; Hur, H.J.; Park, S.; Choi, M.-J. Studies on the Anti-Oxidative Function of trans-Cinnamaldehyde-Included β-Cyclodextrin Complex. Molecules 2017, 22, 1868. https://doi.org/10.3390/molecules22121868
Davaatseren M, Jo Y-J, Hong G-P, Hur HJ, Park S, Choi M-J. Studies on the Anti-Oxidative Function of trans-Cinnamaldehyde-Included β-Cyclodextrin Complex. Molecules. 2017; 22(12):1868. https://doi.org/10.3390/molecules22121868
Chicago/Turabian StyleDavaatseren, Munkhtugs, Yeon-Ji Jo, Geun-Pyo Hong, Haeng Jeon Hur, Sujin Park, and Mi-Jung Choi. 2017. "Studies on the Anti-Oxidative Function of trans-Cinnamaldehyde-Included β-Cyclodextrin Complex" Molecules 22, no. 12: 1868. https://doi.org/10.3390/molecules22121868
APA StyleDavaatseren, M., Jo, Y.-J., Hong, G.-P., Hur, H. J., Park, S., & Choi, M.-J. (2017). Studies on the Anti-Oxidative Function of trans-Cinnamaldehyde-Included β-Cyclodextrin Complex. Molecules, 22(12), 1868. https://doi.org/10.3390/molecules22121868




