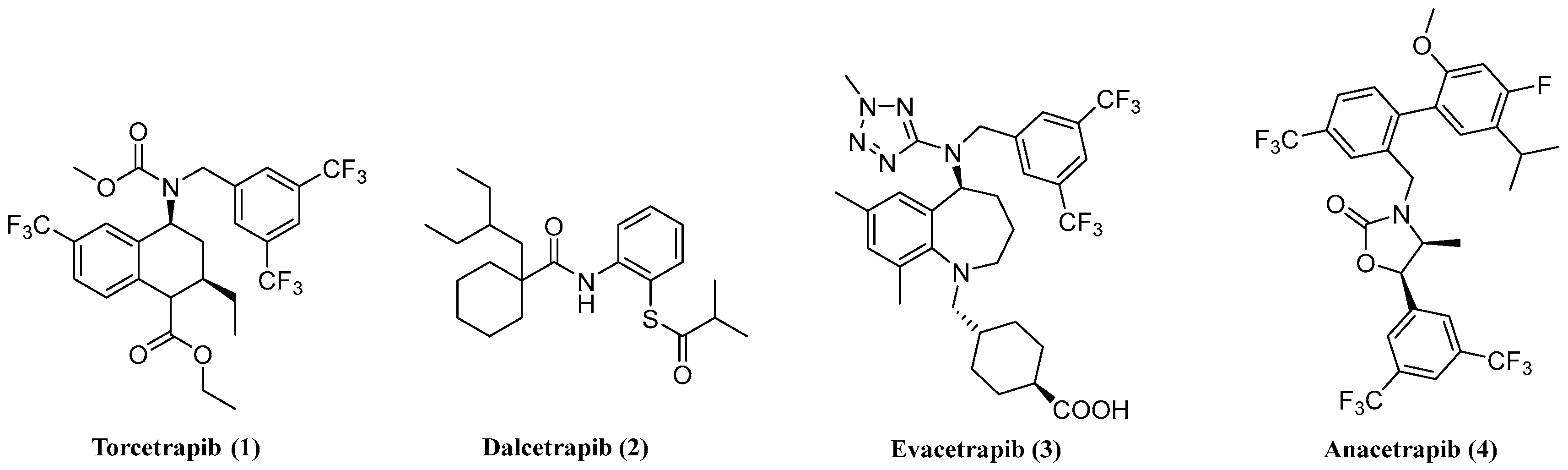
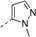3.2. Synthesis
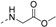
2-Chloro-N-(2-oxo-2-phenylethyl)acetamide (7). 2-amino-1-phenylethan-1-one hydrochloride 6 (0.50 g, 2.9 mmol) was dissolved in dichloromethane (10 mL), and triethylamine (13 mL, 8.9 mmol) was added. The mixture was cooled, and 2-chloroacetyl chloride (0.45 g, 4.0 mmol) was added in drops at 0 °C. After 2 h, the solution was recovered to room temperature for 16 h. Then the mixture was poured into water (20 mL) and extracted with dichloromethane (10 mL × 3), and the combined organic layers were washed with water (10 mL × 3) and brine (10 mL × 3), dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 4:1) to give 7 (0.39 g, 62.9%) as a white solid.
2-(Chloromethyl)-5-phenyloxazole (13a). Intermediate 7 (0.20 g, 1 mmol) was dissolved in acetonitrile (10 mL), and phosphorus oxychloride (0.17 mL, 1.9 mmol) was added in a slow stream. The solution was heated at reflux for 4 h and then cooled to room temperature and concentrated. Ethyl acetate (20 mL) was added, washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 20:1) to give 13a (0.13 g, 66.2%) as a white solid.
2-(Chloromethyl)-5-phenylthiazole (13b). Intermediate 7 (0.30 g, 1.4 mmol) was dissolved in tetrahydrofuran (5 mL), and Lawesson’s reagent (0.34 g, 0.80 mmol) was added. The solution was heated at reflux for 4 h and then cooled to room temperature. The solvent was removed under reduced pressure, and the resulting residue was dissolved in ethyl acetate. The solution was washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 10:1) to give 13b (0.10 g, 59.6%) as a white solid.
Ethyl 5-Methyl-1-phenyl-1H-pyrazole-3-carboxylate (9). Phenylhydrazine (0.50 g, 5.0 mmol) and ethyl acetopyruvate (1.1 g, 7.0 mmol) were dissolved in ethanol (10 mL). After being heated at reflux for 2 h, the reaction mixture was cooled to room temperature and concentrated in vacuo. The residue was dissolved in ethyl acetate, washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 10:1) to give 9 (0.50 g, 43.6%) as a white solid.
3-(Chloromethyl)-5-methyl-1-phenyl-1H-pyrazole (13c). In a solution of intermediate 9 (92.1 mg, 0.40 mmol) dissolved in ethanol (5 mL), sodium borohydride (19.0 mg, 0.50 mmol) was added. The mixture was stirred at room temperature for 30 min, and then water was added. The solution was extracted with ethyl acetate, and the combined organic layers were washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was dissolved in DMF (2 mL); then thionyl chloride (0.10 mL, 1.4 mmol) was added. After reflux for 1 h, water was added and extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 10:1) to give 13c (66.6 mg, 80.5%) as a white solid.
Ethyl 4-Phenylthiazole-2-carboxylate (11). A mixture of 2-bromo-1-phenylethan-1-one (0.50 g, 2.5 mmol) and ethyl 2-amino-2-thioxoacetate (0.50 g, 3.8 mmol) was dissolved in ethanol (10 mL). The solution was heated at reflux for 6 h and then cooled to room temperature. After being concentrated, the residue was dissolved in ethyl acetate (20 mL); then the solution was washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 20:1) to give 11 (0.43 g, 73.6%) as a white solid.
2-(Chloromethyl)-4-phenylthiazole (13d). The ester function in 11 was reduced with NaBH4 and reacted subsequently with SOCl2 to afford 13d, and this operation was the same as the step in the synthetic intermediate 13c. Compound 13d was obtained as a white solid. Yield: 75.9%.
2-(Chloromethyl)-5-phenyl-1,3,4-oxadiazole (13e). A mixture of benzohydrazide (0.50 g, 3.7 mmol), 2-chloroacetic acid (0.35 g, 3.7 mmol), and phosphorus oxychloride (1.0 mL, 11.0 mmol) was added to a three-necked round bottom flask. The solution was heated at reflux for 6 h and then cooled to 0 °C and neutralized to pH 9 with a saturated sodium carbonate aqueous solution. The precipitate was filtered, washed with water, and dried under an infrared lamp. Compound 13e (0.58 g, 81.1%) was obtained as a white solid.
2-(Chloromethyl)-1H-benzo[d]imidazole (13f). A mixture of benzene-1,2-diamine (2.0 g, 18.0 mmol) and ethyl 2-chloroacetate, (2.6 mL, 24.0 mmol) was dissolved in dilute hydrochloric acid solution (4 mol/L, 16 mL). The solution was heated at 110 °C for 4 h and then cooled to room temperature. The reaction solution was poured into ice water and then neutralized to pH 9 with ammonium hydroxide. The precipitate was filtered, washed with water, and dried under an infrared lamp to obtain compound 13f (2.7 g, 91.3%) was obtained as a white solid.
2-(Chloromethyl)benzo[d]oxazole (13g). In a solution of 2-aminophenol (0.50 g, 4.6 mmol) dissolved in chlorobenzene (5 mL), 2-chloroacetyl chloride (0.52 g, 4.6 mmol) and pyridine (0.02 mL) were added. The mixture was stirred at room temperature for 2 h; then p-toluene sulfonic acid (0.08 g, 0.46 mmol) was added. The mixture was heated at reflux for 8 h and then cooled to room temperature. The solvent was removed under reduced pressure, and the resulting residue was dissolved in ethyl acetate (30 mL). The solution was washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 10:1) to give 13g (0.70 g, 90.2%) as a yellow oil.
3-Phenoxy-N-((5-phenyloxazol-2-yl)methyl)aniline (16a). A mixture of 3-phenoxyaniline (0.20 g, 18.0 mmol) and 13a (0.20 g, 18.0 mmol) was dissolved in DMF (10 mL), followed by the addition of potassium carbonate (0.90 g, 6.48 mmol) and KI. The solution was stirred at room temperature for 12 h and then poured into water and extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 4:1) to give 16a (0.26 g, 70.3%) as a colourless oil.
N-(4-Fluorobenzyl)-3-phenoxy-N-((5-phenyloxazol-2-yl)methyl)aniline (17a). Compound 16a (0.20 g, 0.60 mmol) was added to a solution of (Bromomethyl)-4-fluorobenzene (0.08 mL, 0.60 mmol) in DMF (5 mL), followed by the addition of potassium carbonate (0.50 g, 3.6 mmol) and KI. The solution was stirred at room temperature for 24 h and then poured into water and extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether:ethyl acetate = 4:1) to give 17a (0.18 g, 66.9%) as a yellow solid. m.p. 81.0–82.4 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 7.65~7.60 (m, 3H), 7.46 (t, J = 7.6 Hz, 2H), 7.38~7.27 (m, 5H), 7.18~7.11 (m, 3H), 7.06 (t, J = 7.4 Hz, 1H), 6.90 (d, J = 7.7 Hz, 2H), 6.61 (dd, J = 8.3, 2.2 Hz, 1H), 6.46 (t, J = 2.2 Hz, 1H), 6.26 (dd, J = 7.9, 1.8 Hz, 1H), 4.84 (s, 2H), 4.72 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 161.65(d, J = 240 Hz), 161.50, 157.82, 157.03, 151.18, 149.93, 135.13, 135.11, 130.67(×2), 130.26(×2), 129.55, 129.02, 128.97, 127.87, 124.26(×2), 123.52, 122.98, 118.77(×2), 115.75, 115.61, 108.70, 107.51, 104.09, 54.71, 48.70. HRMS calcd. for C29H23FN2O2, [M + Na]+, 473.1641; found 473.1684. HPLC: tR = 11.84 min, 97.50%.
N-(4-Fluorobenzyl)-3-phenoxy-N-((5-phenylthiazol-2-yl)methyl)aniline (17b). Yellow solid, yield: 84.0%. m.p. 90.5–92.5 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 8.13 (s, 1H), 7.65~7.60 (m, 2H), 7.43 (t, J = 7.5 Hz, 2H), 7.37~7.26 (m, 5H), 7.19~7.11 (m, 3H), 7.07 (t, J = 7.4 Hz, 1H), 6.91~6.86 (m, 2H), 6.59 (dd, J = 8.4, 2.2 Hz, 1H), 6.41 (t, J = 2.2 Hz, 1H), 6.27 (dd, J = 7.9, 1.9 Hz, 1H), 4.96 (s, 2H), 4.74 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 169.45, 161.69 (d, J = 240 Hz), 157.88, 156.93, 149.50, 138.98, 138.90, 134.92, 131.32, 130.77, 130.25(×2), 129.73(×2), 129.18, 129.13, 128.76, 126.76(×2), 123.57, 118.84(×2), 115.82, 115.68, 108.94, 107.62, 104.32, 54.47, 53.54. HRMS calcd. for C29H23FN2OS, [M + Na]+, 489.1413; found 489.1462. HPLC: tR = 16.10 min, 95.77%.
N-(4-Fluorobenzyl)-N-((5-methyl-1-phenyl-1H-pyrazol-3-yl)methyl)-3-phenoxyaniline (17c). Yellow oil, yield: 64.9%. 1H-NMR (400 MHz, DMSO-d6) δ: 7.49~7.43 (m, 4H), 7.41~7.36 (m, 1H), 7.34~7.29 (m, 2H), 7.19~7.15 (m, 2H), 7.12~7.06 (m, 4H), 6.90~6.86 (m, 2H), 6.43~6.39 (m, 1H), 6.24~6.20 (m, 2H), 6.00 (s, 1H), 4.69 (s, 2H), 4.51 (s, 2H), 2.18 (s, 3H). 13C-NMR (150 MHz, DMSO-d6) δ: 160.96 (d, J = 240 Hz), 157.16, 156.37, 149.08, 147.95, 140.64, 139.21, 134.39, 130.02, 129.64(×2), 128.99(×2), 128.27, 128.22, 127.27, 124.17(×2), 122.90, 118.14(×2), 115.13, 114.99, 107.92, 106.47, 106.02, 103.20, 53.23, 47.01, 13.15. HRMS calcd. for C30H26FN3O, [M + H]+, 464.2138; found 464.2176. HPLC: tR = 10.74 min, 97.05%.
N-(4-Fluorobenzyl)-3-phenoxy-N-((4-phenylthiazol-2-yl)methyl)aniline (17d). Colourless oil, yield: 70.3%. 1H-NMR (400 MHz, DMSO-d6) δ: 8.02 (s, 1H), 7.95~7.91 (m, 2H), 7.44 (t, J = 7.5 Hz, 2H), 7.37~7.31 (m, 3H), 7.29~7.25 (m, 2H), 7.19~7.11 (m, 3H), 7.03 (t, J = 7.4 Hz, 1H), 6.90~6.86 (m, 2H), 6.58 (dd, J = 8.3, 2.2 Hz, 1H), 6.42 (t, J = 2.2 Hz, 1H), 6.26 (dd, J = 8.0, 2.0 Hz, 1H), 5.02 (s, 2H), 4.75 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 170.52, 161.68 (d, J = 241 Hz), 157.88, 156.91, 154.70, 149.51, 134.97, 134.95, 134.54, 130.75, 130.25(×2), 129.25(×2), 129.21, 129.16, 128.47, 126.42(×2), 123.55, 118.81(×2), 115.80, 115.65, 114.58, 108.90, 107.62, 104.27, 54.49, 53.63. HRMS calcd. for C29H23FN2OS, [M + Na]+, 489.1413; found 489.1467. HPLC: tR = 17.55 min, 98.71%.
N-(4-Fluorobenzyl)-3-phenoxy-N-((5-phenyl-1,3,4-oxadiazol-2-yl)methyl)aniline (17e). White solid, yield: 64.9%. m.p. 97.3–98.2 °C. 1H-NMR (400 MHz, CDCl3) δ: 7.99 (d, J = 7.0 Hz, 2H), 7.58~7.48 (m, 3H), 7.32~7.26 (m, 4H), 7.21 (t, J = 8.2 Hz, 1H), 7.11~6.97 (m, 5H), 6.71 (dd, J = 8.3, 2.0 Hz, 1H), 6.60 (s, 1H), 6.47 (d, J = 8.0 Hz, 1H), 4.78 (s, 2H), 4.67 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 164.72, 164.67, 161.68 (d, J = 241 Hz), 157.88, 157.03, 149.70, 134.90, 134.88, 132.53, 130.77, 130.27(×2), 129.93, 129.07, 129.01, 126.91(×2), 123.68, 123.56, 118.78(×2), 115.78, 115.64, 108.88, 107.87, 104.33, 54.49, 49.07. HRMS calcd. for C28H22FN3O2, [M + Na]+, 474.1594; found 474.1638. HPLC: tR = 8.75 min, 96.33%.
N-((1H-Benzo[d]imidazol-2-yl)methyl)-N-(4-fluorobenzyl)-3-phenoxyaniline (17f). Yellow oil, yield: 57.3%. 1H-NMR (600 MHz, DMSO-d6) δ: 7.65~7.60 (m, 1H), 7.42~7.39 (m, 1H), 7.31 (t, J = 7.9 Hz, 2H), 7.20~7.15 (m, 4H), 7.12~7.06 (m, 3H), 7.04 (t, J = 8.1 Hz, 1H), 6.93 (d, J = 7.8 Hz, 2H), 6.52~6.45 (m, 2H), 6.36 (t, J = 2.1 Hz, 1H), 6.16 (dd, J = 7.9, 2.0 Hz, 1H), 5.55 (s, 2H), 4.52 (d, J = 5.1 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 161.90 (d, J = 241 Hz), 157.88, 157.29, 152.73, 150.39, 142.43, 135.93, 133.48, 130.52, 130.26(×2), 129.34, 129.28, 123.44, 122.81, 122.16, 119.42, 118.83(×2), 115.97, 115.83, 110.90, 108.31, 106.99, 103.35, 46.16, 41.14. HRMS calcd. for C27H22FN3O, [M + H]+, 424.1825; found 424.1859. HPLC: tR = 5.72 min, 97.98%.
N-(Benzo[d]oxazol-2-ylmethyl)-N-(4-fluorobenzyl)-3-phenoxyaniline (17g). Colourless oil, yield: 71.0%. 1H-NMR (400 MHz, DMSO-d6) δ: 7.74~7.67 (m, 2H), 7.40~7.32 (m, 4H), 7.27~7.21 (m, 2H), 7.17~7.03 (m, 4H), 6.89~6.85 (m, 2H), 6.58 (dd, J = 8.3, 2.2 Hz, 1H), 6.41 (t, J = 2.2 Hz, 1H), 6.24 (dd, J = 8.0, 1.9 Hz, 1H), 5.01 (s, 2H), 4.76 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 164.45, 161.66 (d, J = 241 Hz), 157.89, 156.89, 150.76, 149.81, 141.04, 135.06, 130.72, 130.21(×2), 128.95, 128.90, 125.59, 124.96, 123.56, 120.10, 118.83(×2), 115.78, 115.64, 111.28, 108.50, 107.39, 103.89, 54.81, 48.96. HRMS calcd. for C27H21FN2O2, [M + H]+, 425.1665; found 425.1725. HPLC: tR = 18.55 min, 96.41%.
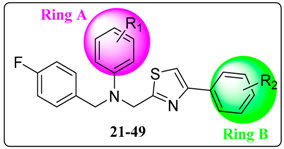
3.3. General Procedure for the Synthesis of Compounds 21–49
A mixture of substituted 2-bromo-1-phenylethan-1-one (2.5 mmol) and ethyl 2-amino-2-thioxoacetate (3.8 mmol) was dissolved in ethanol (10 mL). The solution was heated at reflux for 6 h and then cooled to room temperature. After being concentrated, the residue was dissolved in ethyl acetate (20 mL); then the solution was washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by chromatography on silica gel to give 18. The intermediate 19 was obtained by an operation similar to that described for the preparation of 13c. Subsequently, substituted arylamine reacted with 19 according to the synthesis condition of compound 16a to produce 20. Finally, target compounds 21 to 49 were prepared according to the procedure for 17a, except that different substrates were used.
N-(4-Fluorobenzyl)-3-methoxy-N-((4-phenylthiazol-2-yl)methyl)aniline (21). Colourless oil, yield: 64.1%. 1H-NMR (400 MHz, DMSO-d6) δ: 8.00 (s, 1H), 7.98~7.94 (m, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.39~7.32 (m, 3H), 7.17 (t, J = 12.3 Hz, 2H), 7.04 (t, J = 8.2 Hz, 1H), 6.39 (dd, J = 8.3, 2.2 Hz, 1H), 6.31 (t, J = 2.2 Hz, 1H), 6.27 (dd, J = 8.1, 2.1 Hz, 1H), 5.01 (s, 2H), 4.76 (s, 2H), 3.62 (s, 3H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.03, 162.47~160.87 (d, J = 240 Hz), 160.66, 154.60, 149.19, 135.30, 134.56, 130.30, 129.28(×3), 129.22, 128.47, 126.40(×2), 115.76, 115.62, 114.61, 106.54, 102.82, 100.12, 55.18, 54.44, 53.62. HRMS calcd. for C24H21FN2OS, [M + H]+, 405.1437; found 405.1480. HPLC: tR = 8.71 min, 95.81%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-phenylthiazol-2-yl)methyl)aniline (22). Colourless oil, yield: 65.6%. 1H-NMR (600 MHz, DMSO-d6) δ: 7.99 (s, 1H), 7.96~7.92 (m, 2H), 7.44 (t, J = 7.7 Hz, 2H), 7.40~7.36 (m, 2H), 7.34 (t, J = 7.4 Hz, 1H), 7.16 (t, J = 8.9 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.56 (d, J = 2.8 Hz, 1H), 6.27 (dd, J = 8.8, 2.8 Hz, 1H), 4.90 (s, 2H), 4.65 (s, 2H), 3.62 (d, J = 6.6 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.25, 161.67 (d, J = 240 Hz), 154.40, 149.86, 142.94, 142.04, 135.47, 134.59, 130.06, 129.69, 129.28(×2), 129.45, 126.37(×2), 115.67, 115.53, 114.67, 113.96, 106.28, 101.19, 56.54, 55.79, 55.27, 54.19. HRMS calcd. for C25H23FN2O2S, [M + Na]+, 457.1362; found 457.1409. HPLC: tR = 7.11 min, 98.24%.
N-(4-Fluorobenzyl)-N-((4-(4-nitrophenyl)thiazol-2-yl)methyl)-3-(trifluoromethoxy)aniline (23). Colourless oil, yield: 54.9%. 1H-NMR (600 MHz, DMSO-d6) δ: 8.39 (s, 1H), 8.31 (d, J = 9.0 Hz, 2H), 8.22 (d, J = 9.0 Hz, 2H), 7.40~7.35 (m, 2H), 7.24 (t, J = 8.3 Hz, 1H), 7.21~7.16 (m, 2H), 6.81 (dd, J = 8.5, 2.4 Hz, 1H), 6.72 (s, 1H), 6.61 (d, J = 8.1 Hz, 1H), 5.11 (s, 2H), 4.82 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.07, 161.76 (d, J = 241 Hz), 152.47, 149.92, 149.43, 147.14, 140.39, 134.54, 131.01, 129.30, 129.25, 127.31(×2), 124.75(×2), 119.15, 115.87, 115.73, 112.34, 109.27, 105.94, 62.92, 54.30, 53.32. HRMS calcd. for C24H17F4N3O3S, [M + H]+, 504.1005; found 504.1063. HPLC: tR = 12.63 min, 95.83%.
N-(4-Fluorobenzyl)-N-((4-(4-nitrophenyl)thiazol-2-yl)methyl)-3-(trifluoromethyl)aniline (24). Brown solid, yield: 65.9%. m.p. 107.0–108.4 °C. 1H-NMR (600 MHz, CDCl3) δ: 8.31~8.27 (m, 2H), 8.06~8.03 (m, 2H), 7.61 (s, 1H), 7.31 (t, J = 8.0 Hz, 1H), 7.26 (d, J = 7.2 Hz, 2H), 7.14 (s, 1H), 7.07~7.03 (m, 3H), 6.97 (dd, J = 8.4, 2.6 Hz, 1H), 4.92 (s, 2H), 4.73 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 170.91, 161.76 (d, J = 241 Hz), 152.48, 148.16, 147.15, 140.37, 134.49, 130.57, 129.27, 129.23, 127.30(×2), 125.71, 124.75(×2), 123.91, 119.19, 117.13, 115.91, 115.77, 113.85, 109.41, 54.26, 53.21. HRMS calcd. for C24H17F4N3O2S, [M + H]+, 488.1056; found 488.1104. HPLC: tR = 9.95 min, 96.05%.
N-(4-Fluorobenzyl)-N-((4-(4-nitrophenyl)thiazol-2-yl)methyl)-[1,1′-biphenyl]-4-amine (25). Light yellow solid, yield: 54.9%. m.p. 164.5–166.2 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 8.38 (s, 1H), 8.34~8.30 (m, 2H), 8.25~8.22 (m, 2H), 7.54 (dd, J = 8.3, 1.1 Hz, 2H), 7.48 (d, J = 8.9 Hz, 2H), 7.41~7.35 (m, 4H), 7.23 (dt, J = 8.5, 1.1 Hz, 1H), 7.21~7.16 (m, 2H), 6.88 (d, J = 9.0 Hz, 2H), 5.10 (s, 2H), 4.84 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 172.20, 161.72 (d, J = 240 Hz), 152.53, 147.20, 147.11, 140.48, 140.39, 135.08, 129.59, 129.29, 129.24(×3), 127.80(×2), 127.33(×2), 126.65, 126.12(×2), 124.77(×2), 119.07, 115.85, 115.70, 113.94(×2), 54.34, 54.35. HRMS calcd. for C29H22FN3O2S, [M + H]+, 496.1495; found 496.1552. HPLC: tR = 14.39 min, 97.20%.
N-(4-Fluorobenzyl)-N-((4-(4-nitrophenyl)thiazol-2-yl)methyl)-[1,1′-biphenyl]-3-amine (26). Light yellow solid, yield: 59.8%. m.p. 149.5–150.8 °C. 1H-NMR (600 MHz, CDCl3) δ: 8.27 (d, J = 8.8 Hz, 2H), 8.04 (d, J = 8.8 Hz, 2H), 7.59 (s, 1H), 7.48 (d, J = 7.3 Hz, 2H), 7.37 (t, J = 7.5 Hz, 2H), 7.32~7.27 (m, 4H), 7.10 (s, 1H), 7.06~7.02 (m, 3H), 6.83 (dd, J = 8.3, 2.3 Hz, 1H), 4.93 (s, 2H), 4.75 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 172.05, 161.70 (d, J = 240 Hz), 152.33, 148.25, 147.12, 141.53, 141.24, 140.46, 135.22, 130.16, 129.37, 129.31, 129.25(×2), 127.82, 127.32(×2), 127.12(×2), 124.76(×2), 119.21, 116.52, 115.83, 115.69, 112.88, 112.12, 55.47, 53.52. HRMS calcd. for C29H22FN3O2S, [M + H]+, 496.1495; found 496.1567. HPLC: tR = 13.88 min, 96.81%.
N-(4-Fluorobenzyl)-N-((4-(4-nitrophenyl)thiazol-2-yl)methyl)-[1,1′-biphenyl]-2-amine (27). Colourless oil, yield: 50.4%. 1H-NMR (600 MHz, DMSO-d6) δ: 8.31 (s, 1H), 8.28 (d, J = 8.9 Hz, 2H), 8.17 (d, J = 8.9 Hz, 2H), 7.61 (d, J = 7.2 Hz, 2H), 7.49 (t, J = 7.6 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H), 7.23~7.19 (m, 2H), 7.13~7.10 (m, 3H), 7.10~7.04 (m, 3H), 4.31 (s, 2H), 4.00 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.99, 161.71 (d, J = 240 Hz), 160.91, 152.34, 148.26, 147.09, 141.54, 141.24, 140.45, 135.17, 130.16, 129.35, 129.30, 129.24(×2), 127.80, 127.29(×2), 127.11(×2), 124.72(×2), 119.17, 116.54, 115.82, 115.68, 112.88, 112.13, 54.48, 53.51. HRMS calcd. for C29H22FN3O2S, [M + H]+, 496.1495; found 496.1563. HPLC: tR = 15.05 min, 95.79%.
N-(4-Fluorobenzyl)-N-((4-(4-nitrophenyl)thiazol-2-yl)methyl)-3-(thiophen-2-yl)aniline (28). Dark yellow solid, yield: 63.1%. m.p. 143.9–148.8 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 8.38 (s, 1H), 8.32 (d, J = 8.7 Hz, 2H), 8.24 (d, J = 8.7 Hz, 2H), 7.46 (d, J = 5.0 Hz, 1H), 7.41 (dd, J = 8.0, 5.8 Hz, 2H), 7.35 (d, J = 3.5 Hz, 1H), 7.18 (dd, J = 16.3, 8.2 Hz, 3H), 7.07 (t, J = 4.1 Hz, 2H), 6.96 (d, J = 7.5 Hz, 1H), 6.75 (dd, J = 8.3, 1.9 Hz, 1H), 5.12 (s, 2H), 4.84 (s, 2H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.84, 161.71 (d, J = 240 Hz), 152.38, 148.22, 148.12, 144.44, 140.46, 135.14, 134.90, 130.34, 129.36, 129.30, 128.75, 127.33(×2), 125.90, 124.76(×2), 123.91, 119.18, 115.85, 115.70, 115.31, 113.07, 110.66, 54.51, 53.55. HRMS calcd. for C27H20FN3O2S2, [M + H]+, 502.1059; found 502.1118. HPLC: tR = 14.17 min, 98.03%.
N-(4-Fluorobenzyl)-3-(1-methyl-1H-pyrazol-5-yl)-N-((4-(4-nitrophenyl)thiazol-2-yl)methyl)aniline (29) Brown solid, yield: 68.9%. m.p. 102.2–104.5 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 8.38 (s, 1H), 8.32 (d, J = 8.9 Hz, 2H), 8.22 (d, J = 8.9 Hz, 2H), 7.43~7.37 (m, 3H), 7.26 (t, J = 8.0 Hz, 1H), 7.19 (t, J = 8.8 Hz, 2H), 6.87 (dd, J = 8.4, 2.2 Hz, 1H), 6.84 (s, 1H), 6.80 (d, J = 7.5 Hz, 1H), 6.24 (d, J = 1.8 Hz, 1H), 5.14 (s, 2H), 4.85 (s, 2H), 3.59 (s, 3H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.92, 161.72 (d, J = 241 Hz), 152.46, 148.22, 147.11, 143.64, 140.41, 138.26, 134.99, 131.29, 130.05, 129.27, 129.22, 127.29(×2), 124.73(×2), 119.09, 117.99, 115.84, 115.70, 115.04, 113.58, 106.05, 54.48, 53.64, 49.08. HRMS calcd. for C27H22FN5O2S, [M + H]+, 500.1556; found 500.1626. HPLC: tR = 6.76 min, 95.89%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(4-nitrophenyl)thiazol-2-yl)methyl)aniline (30). Light yellow solid, yield: 63.8%. m.p. 111.0–112.8 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 8.37 (s, 1H), 8.32 (d, J = 9.0 Hz, 2H), 8.22 (d, J = 8.9 Hz, 2H), 7.39 (dd, J = 8.4, 5.7 Hz, 2H), 7.17 (t, J = 8.8 Hz, 2H), 6.75 (d, J = 8.8 Hz, 1H), 6.56 (d, J = 2.7 Hz, 1H), 6.28 (dd, J = 8.8, 2.7 Hz, 1H), 4.94 (s, 2H), 4.66 (s, 2H), 3.62 (d, J = 4.1 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 163.14, 162.02 (d, J = 241 Hz), 160.86, 153.36, 149.87, 142.92, 142.05, 135.47, 131.23, 129.64, 128.44, 128.38, 116.21, 116.07, 115.67(×2), 115.53, 114.48, 113.97, 106.27, 101.19, 56.55, 55.80, 55.26, 54.15. HRMS calcd. for C25H22FN3O4S, [M + Na]+, 502.1213; found 502.1217. HPLC: tR = 7.59 min, 98.98%.
N-(4-Fluorobenzyl)-N-((4-(4-fluorophenyl)thiazol-2-yl)methyl)-3,4-dimethoxyaniline (31). Light yellow oil, yield: 71.1%. 1H-NMR (400 MHz, DMSO-d6) δ: 8.05~7.94 (m, 3H), 7.38 (s, 2H), 7.27 (t, J = 7.6 Hz, 2H), 7.16 (t, J = 7.7 Hz, 2H), 6.74 (d, J = 8.1 Hz, 1H), 6.57 (s, 1H), 6.28 (d, J = 8.4 Hz, 1H), 4.90 (s, 2H), 4.65 (s, 2H), 3.63 (d, J = 2.7 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.42, 162.33 (d, J = 243 Hz), 162.47~160.86 (d, J = 241 Hz), 153.36, 149.87, 142.92, 142.05, 135.46, 131.24, 129.69, 129.64, 128.44, 128.38, 116.21, 116.07, 115.67, 115.53, 114.48, 113.97, 106.27, 101.19, 56.55, 55.80, 55.26, 54.15. HRMS calcd. for C25H22F2N2O2S, [M + Na]+, 475.1268; found 475.1324. HPLC: tR = 4.87 min, 99.31%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(4-(trifluoromethyl)phenyl)thiazol-2-yl)methyl)aniline (32). White solid, yield: 69.9%. m.p. 88.7–89.5 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 8.24 (s, 1H), 8.17 (d, J = 8.1 Hz, 2H), 7.81 (d, J = 8.3 Hz, 2H), 7.39 (dd, J = 8.5, 5.7 Hz, 2H), 7.17 (t, J = 8.9 Hz, 2H), 6.75 (d, J = 8.8 Hz, 1H), 6.56 (d, J = 2.8 Hz, 1H), 6.28 (dd, J = 8.8, 2.8 Hz, 1H), 4.93 (s, 2H), 4.66 (s, 2H), 3.62 (d, J = 3.5 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 172.03, 161.67 (d, J = 241 Hz), 152.76, 149.88, 142.87, 142.11, 138.26, 135.43, 135.42, 129.71, 129.66, 128.44, 126.94(×2), 126.28, 117.36, 115.67, 115.53, 113.94, 106.34, 101.24, 56.53, 55.80, 55.30, 54.16, 49.06. HRMS calcd. for C26H22F4N2O2S, [M + Na]+, 525.1236; found 525.1303. HPLC: tR = 9.44 min, 98.71%.
4-(2-(((3,4-Dimethoxyphenyl)(4-fluorobenzyl)amino)methyl)thiazol-4-yl)benzonitrile (33). Yellow solid, yield: 69.9%. m.p. 105.4–106.6 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 8.28 (s, 1H), 8.14 (d, J = 8.1 Hz, 2H), 7.91 (d, J = 8.3 Hz, 2H), 7.40~7.36 (m, 2H), 7.16 (t, J = 9.7 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.56 (d, J = 2.8 Hz, 1H), 6.28 (dd, J = 8.8, 2.9 Hz, 1H), 4.92 (s, 2H), 4.65 (s, 2H), 3.62 (d, J = 5.8 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 172.18, 161.67 (d, J = 241 Hz), 152.54, 149.88, 142.84, 142.12, 138.66, 135.42, 133.38(×2), 129.72, 129.67, 127.00, 119.35, 118.27, 115.68, 115.54, 113.95, 110.60, 106.34, 101.25, 56.53, 55.82, 55.29, 54.12, 49.07. HRMS calcd. for C26H22FN3O2S, [M + Na]+, 482.1314; found 482.1375. HPLC: tR = 5.87 min, 97.66%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(3-nitrophenyl)thiazol-2-yl)methyl)aniline (34). Light yellow solid, yield: 53.7%. m.p. 120.5–121.6 °C. 1H-NMR (600 MHz, CDCl3) δ: 8.74 (t, J = 1.9 Hz, 1H), 8.21~8.16 (m, 2H), 7.59 (t, J = 8.0 Hz, 1H), 7.56 (s, 1H), 7.32 (dd, J = 8.5, 5.4 Hz, 2H), 7.05~7.01 (m, 2H), 6.76~6.74 (m, 1H), 6.56 (d, J = 2.1 Hz, 1H), 6.43 (dd, J = 8.7, 2.5 Hz, 1H), 4.80 (s, 2H), 4.58 (s, 2H), 3.80 (s, 3H), 3.76 (s, 3H). 13C-NMR (150 MHz, DMSO-d6) δ: 172.21, 161.68 (d, J = 240 Hz), 151.94, 149.89, 148.86, 142.85, 142.14, 136.09, 135.44, 132.50, 130.98, 129.72, 129.69, 123.00, 120.71, 117.40, 115.68, 115.54, 113.95, 106.37, 101.28, 56.53, 55.81, 55.33, 54.16. HRMS calcd. for C25H22FN3O4S, [M + Na]+, 502.1213; found 502.1272. HPLC: tR = 6.87 min, 95.41%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(2-nitrophenyl)thiazol-2-yl)methyl)aniline (35). Light yellow oil, yield: 54.6%. 1H-NMR (600 MHz, DMSO-d6) δ: 7.95 (s, 1H), 7.90 (dd, J = 8.0, 1.0 Hz, 1H), 7.83~7.78 (m, 1H), 7.76~7.72 (m, 1H), 7.64~7.60 (m, 1H), 7.36 (dd, J = 8.5, 5.6 Hz, 2H), 7.18~7.12 (m, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.52 (d, J = 2.8 Hz, 1H), 6.27 (dd, J = 8.8, 2.8 Hz, 1H), 4.78 (s, 2H), 4.59 (s, 2H), 3.63 (d, J = 10.9 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.08, 161.68 (d, J = 241 Hz), 149.96, 149.86, 149.21, 142.87, 142.20, 135.35, 132.97, 131.17, 129.85, 129.85, 129.79, 128.17, 124.43, 118.86, 115.65, 115.51, 113.85, 106.64, 101.40, 56.51, 55.76, 55.03, 49.07. HRMS calcd. for C25H22FN3O4S, [M + Na]+, 502.1213; found 502.1277. HPLC: tR = 5.44 min, 98.06%.
N-((4-(4-Bromophenyl)thiazol-2-yl)methyl)-N-(4-fluorobenzyl)-3,4-dimethoxyaniline (36). Dark yellow oil, yield: 71.6%. 1H-NMR (400 MHz, DMSO-d6) δ: 8.06 (s, 1H), 7.91 (d, J = 8.6 Hz, 2H), 7.64 (d, J = 8.6 Hz, 2H), 7.38 (dd, J = 8.5, 5.7 Hz, 2H), 7.16 (t, J = 8.9 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.56 (d, J = 2.7 Hz, 1H), 6.27 (dd, J = 8.8, 2.8 Hz, 1H), 4.90 (s, 2H), 4.65 (s, 2H), 3.62 (d, J = 4.2 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.65, 161.67 (d, J = 240 Hz), 153.17, 149.88, 142.89, 142.07, 135.45, 133.79, 132.22(×2), 129.69, 129.64, 128.38(×2), 121.54, 115.67, 115.53(×2), 113.97, 106.28, 101.20, 56.55, 55.81, 55.26, 54.16. HRMS calcd. for C25H22BrFN2O2S, [M + Na]+, 535.0467; found 535.0539. HPLC: tR = 11.61 min, 97.19%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(4-(1-methyl-1H-pyrazol-4-yl)phenyl)thiazol-2-yl)methyl)aniline (37). Brown solid, yield: 42.1%. m.p. 126.1–130.3 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 8.18 (s, 1H), 7.96 (s, 1H), 7.94~7.90 (m, 3H), 7.63 (d, J = 8.4 Hz, 2H), 7.39 (dd, J = 8.5, 5.6 Hz, 2H), 7.16 (t, J = 8.8 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.57 (d, J = 2.8 Hz, 1H), 6.28 (dd, J = 8.8, 2.8 Hz, 1H), 4.91 (s, 2H), 4.65 (s, 2H), 3.87 (s, 3H), 3.62 (d, J = 9.6 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 170.07, 160.59 (d, J = 240 Hz), 153.23, 148.79, 141.88, 140.95, 135.47, 131.62, 131.44, 131.16, 130.91, 130.84, 128.56, 128.20, 128.12, 127.30, 125.77, 124.57, 120,89, 114.59, 114.45, 112.90, 105.18, 100.11, 55.48, 54.73, 54.17, 53.13, 47.99. HRMS calcd. for C29H27FN4O2S, [M + Na]+, 537.1736; found 537.1822. HPLC: tR = 5.19 min, 95.89%.
N-((4-(4-(3,5-Dimethylisoxazol-4-yl)phenyl)thiazol-2-yl)methyl)-N-(4-fluorobenzyl)-3,4-dimethoxyaniline (38). Yellow solid, yield: 51.6%. m.p. 69.0–69.8 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 8.08~8.02 (m, 3H), 7.46 (d, J = 8.4 Hz, 2H), 7.39 (dd, J = 8.5, 5.6 Hz, 2H), 7.16 (t, J = 8.9 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.58 (d, J = 2.8 Hz, 1H), 6.29 (dd, J = 8.8, 2.8 Hz, 1H), 4.92 (s, 2H), 4.66 (s, 2H), 3.63 (d, J = 9.1 Hz, 6H), 2.43 (s, 3H), 2.26 (s, 3H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.35, 165.67, 161.68 (d, J = 240 Hz), 158.59, 153.93, 149.89, 142.95, 142.07, 135.48, 133.68, 129.86, 129.71(×2), 129.65, 126.79(×2), 116.07, 115.67, 115.53, 115.07, 113.97, 106.29, 101.23, 56.55, 55.81, 55.28, 54.16, 49.07, 11.90, 11.02. HRMS calcd. for C30H28FN3O3S, [M + Na]+, 552.1733; found 552.1817. HPLC: tR = 7.26 min, 96.49%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(4-(1-methyl-1H-pyrazol-5-yl)phenyl)thiazol-2-yl)methyl)aniline (39). Dark yellow solid, yield: 49.0%. m.p. 102.4–103.3 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 8.11 (s, 1H), 8.06 (d, J = 8.3 Hz, 2H), 7.62 (d, J = 8.3 Hz, 2H), 7.49 (d, J = 1.8 Hz, 1H), 7.40 (dd, J = 8.4, 5.7 Hz, 2H), 7.17 (t, J = 8.8 Hz, 2H), 6.75 (d, J = 8.8 Hz, 1H), 6.57 (d, J = 2.8 Hz, 1H), 6.47 (d, J = 1.8 Hz, 1H), 6.29 (dd, J = 8.8, 2.8 Hz, 1H), 4.93 (s, 2H), 4.67 (s, 2H), 3.90 (s, 3H), 3.63 (d, J = 5.9 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.50, 161.67 (d, J = 241 Hz), 153.73, 149.88, 142.93, 142.78, 142.06, 138.44, 135.47, 134.38, 130.02, 129.71, 129.66, 129.27(×2), 126.67(×2), 115.68, 115.54, 115.48, 113.98, 106.34, 106.29, 101.22, 56.56, 55.82, 55.28, 54.17, 49.07. HRMS calcd. for C29H27FN4O2S, [M + Na]+, 537.1736; found 537.1815. HPLC: tR = 5.84 min, 99.44%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(4-(thiophen-2-yl)phenyl)thiazol-2-yl)methyl)aniline (40). Dark yellow solid, yield: 43.7%. m.p. 115.8–119.0 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 8.04 (s, 1H), 7.99 (d, J = 8.4 Hz, 2H), 7.74 (d, J = 8.4 Hz, 2H), 7.61~7.56 (m, 2H), 7.39 (dd, J = 8.4, 5.7 Hz, 2H), 7.20~7.14 (m, 3H), 6.75 (d, J = 8.8 Hz, 1H), 6.57 (d, J = 2.7 Hz, 1H), 6.28 (dd, J = 8.8, 2.7 Hz, 1H), 4.92 (s, 2H), 4.66 (s, 2H), 3.63 (d, J = 5.9 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.41, 161.68 (d, J = 241 Hz), 153.90, 149.89, 143.41, 142.95, 142.08, 135.45, 133.71, 133.67, 129.69, 129.64, 129.07, 127.05(×2), 126.31, 126.19(×2), 124.34, 115.67, 115.53, 114.84, 113.97, 106.31, 101.22, 56.54, 55.80, 55.28, 54.21. HRMS calcd. for C29H25FN2O2S2, [M + Na]+, 539.1239; found 539.1324. HPLC: tR = 13.67 min, 97.82%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(naphthalen-2-yl)thiazol-2-yl)methyl)aniline (41). Light yellow oil, yield: 76.1%. 1H-NMR (400 MHz, DMSO-d6) δ: 8.54 (s, 1H), 8.14~8.08 (m, 2H), 7.98 (t, J = 8.4 Hz, 2H), 7.92 (d, J = 7.3 Hz, 1H), 7.57~7.48 (m, 2H), 7.40 (dd, J = 8.3, 5.7 Hz, 2H), 7.17 (t, J = 8.8 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.60 (d, J = 2.6 Hz, 1H), 6.30 (dd, J = 8.8, 2.7 Hz, 1H), 4.95 (s, 2H), 4.67 (s, 2H), 3.63 (d, J = 12.8 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.60, 161.68 (d, J = 241 Hz), 154.35, 149.90, 142.96, 142.09, 135.50, 133.66, 133.07, 132.04, 129.71, 129.66, 128.84, 128.70, 128.09, 127.04, 126.71, 125.03, 124.61, 115.69, 115.54, 115.28, 113.99, 106.32, 101.25, 56.55, 55.82, 55.31, 49.07. HRMS calcd. for C29H25FN2O2S, [M + Na]+, 507.1518; found 507.1588. HPLC: tR = 11.73 min, 96.23%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(p-tolyl)thiazol-2-yl)methyl)aniline (42). Yellow solid, yield: 67.1%. m.p. 105.3–107.0 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 7.90 (s, 1H), 7.85 (d, J = 8.1 Hz, 2H), 7.38 (dd, J = 8.5, 5.7 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 7.16 (t, J = 8.9 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.57 (d, J = 2.8 Hz, 1H), 6.27 (dd, J = 8.8, 2.8 Hz, 1H), 4.90 (s, 2H), 4.65 (s, 2H), 3.62 (d, J = 5.9 Hz, 6H), 2.33 (s, 3H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.06, 161.67 (d, J = 240 Hz), 160.87, 154.50, 149.87, 142.97, 142.03, 137.76, 135.48, 131.97, 129.82(×2), 129.67, 129.62(×2), 126.31, 115.66, 115.52, 113.98, 113.75, 106.25, 101.17, 56.55, 55.78, 55.25, 54.21, 21.28. HRMS calcd. for C26H25FN2O2S, [M + Na]+, 471.1518; found 471.1578. HPLC: tR = 9.58 min, 97.85%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(4-methoxyphenyl)thiazol-2-yl)methyl)aniline (43). Dark yellow solid, yield: 83.7%. m.p. 76.3–79.0 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 7.88 (d, J = 8.8 Hz, 2H), 7.82 (s, 1H), 7.39 (dd, J = 8.5, 5.7 Hz, 2H), 7.16 (t, J = 8.8 Hz, 2H), 7.00 (d, J = 8.8 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.56 (d, J = 2.8 Hz, 1H), 6.27 (dd, J = 8.8, 2.8 Hz, 1H), 4.89 (s, 2H), 4.65 (s, 2H), 3.80 (s, 3H), 3.62 (d, J = 4.0 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 170.93, 161.67 (d, J = 241 Hz), 159.56, 154.34, 149.88, 142.99, 142.03, 135.48, 129.67, 129.61, 127.73(×2), 127.48, 115.66, 115.52, 114.60(×2), 113.98, 112.54, 106.25, 101.17, 56.54, 55.79, 55.60, 55.25, 54.20. HRMS calcd. for C26H25FN2O3S, [M + Na]+, 487.1468; found 487.1535. HPLC: tR = 7.05 min, 98.64%.
N-((4-(4-Aminophenyl)thiazol-2-yl)methyl)-N-(4-fluorobenzyl)-3,4-dimethoxyaniline (44). Light yellow solid, yield: 87.3%. m.p. 144.7–146.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 7.61 (d, J = 8.5 Hz, 2H), 7.55 (s, 1H), 7.40~7.36 (m, 2H), 7.16 (dd, J = 12.3, 5.4 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.60 (d, J = 8.6 Hz, 2H), 6.55 (d, J = 2.8 Hz, 1H), 6.25 (dd, J = 8.8, 2.8 Hz, 1H), 5.28 (s, 2H), 4.86 (s, 2H), 4.64 (s, 2H), 3.62 (d, J = 4.2 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 170.23, 161.65 (d, J = 241 Hz), 155.52, 149.86, 149.12, 143.04, 141.95, 135.54, 129.65, 129.60, 127.41(×2), 122.83, 115.65, 115.51, 114.27(×2), 114.00, 109.74, 106.17, 101.11, 56.57, 55.78, 55.21, 54.23. HRMS calcd. for C25H24FN3O2S, [M + Na]+, 472.1471; found 472.1538. HPLC: tR = 4.25 min, 95.78%.
N-(4-(2-(((3,4-Dimethoxyphenyl)(4-fluorobenzyl)amino)methyl)thiazol-4-yl)phenyl)-2,2,2-trifluoroacetamide (45). Light yellow solid, yield: 75.8%. m.p. 143.6–145.8 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 11.34 (s, 1H), 8.01~7.96 (m, 3H), 7.76 (d, J = 8.7 Hz, 2H), 7.38 (dd, J = 8.4, 5.7 Hz, 2H), 7.16 (t, J = 8.8 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.56 (d, J = 2.8 Hz, 1H), 6.28 (dd, J = 8.8, 2.8 Hz, 1H), 4.90 (s, 2H), 4.65 (s, 2H), 3.62 (d, J = 6.5 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.42, 161.67 (d, J = 240 Hz), 155.02, 154.78, 153.72, 149.88, 142.93, 142.06, 136.43, 135.47, 131.92, 129.69, 129.64, 126.95(×2), 121.69, 115.67, 115.53, 114.62, 113.98, 106.28, 101.20, 56.55, 55.80, 55.26, 54.19, 49.06. HRMS calcd. for C27H23F4N3O3S, [M + Na]+, 568.1294; found 568.1379. HPLC: tR = 5.34 min, 97.66%.
N-(4-(2-(((3,4-Dimethoxyphenyl)(4-fluorobenzyl)amino)methyl)thiazol-4-yl)phenyl)acetamide (46). Light yellow solid, yield: 86.1%. m.p. 134.6–137.0 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 10.03 (s, 1H), 7.88~7.83 (m, 3H), 7.64 (d, J = 8.6 Hz, 2H), 7.38 (dd, J = 8.4, 5.7 Hz, 2H), 7.16 (t, J = 8.8 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.55 (d, J = 2.8 Hz, 1H), 6.27 (dd, J = 8.8, 2.8 Hz, 1H), 4.89 (s, 2H), 4.64 (s, 2H), 3.62 (d, J = 6.2 Hz, 6H), 2.06 (s, 3H). 13C-NMR (150 MHz, DMSO-d6) δ: 171.06, 168.81, 161.66 (d, J = 241 Hz), 154.29, 149.87, 142.95, 142.03, 139.57, 135.48, 129.69, 129.63, 129.48, 126.81(×2), 119.49(×2), 115.66, 115.52, 113.98, 113.25, 106.26, 101.18, 56.55, 55.80, 55.24, 54.20, 49.07. HRMS calcd. for C27H26FN3O3S, [M + Na]+, 514.1577; found 514.1662. HPLC: tR = 3.84 min, 97.51%.
Methyl(4-(2-(((3,4-dimethoxyphenyl)(4-fluorobenzyl)amino)methyl)thiazol-4-yl)phenyl)glycinate (47). Light yellow solid, yield: 79.4%. m.p. 103.5–105.4 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 7.67 (d, J = 8.6 Hz, 2H), 7.59 (s, 1H), 7.37 (dd, J = 8.5, 5.6 Hz, 2H), 7.15 (t, J = 8.8 Hz, 2H), 6.73 (d, J = 8.8 Hz, 1H), 6.60 (d, J = 8.7 Hz, 2H), 6.54 (d, J = 2.8 Hz, 1H), 6.27~6.22 (m, 2H), 4.86 (s, 2H), 4.63 (s, 2H), 3.96 (d, J = 6.4 Hz, 2H), 3.66 (s, 3H), 3.61 (d, J = 5.2 Hz, 6H). 13C-NMR (150 MHz, DMSO-d6) δ: 172.19, 170.39, 161.65 (d, J = 240 Hz), 155.25, 149.85, 148.47, 143.02, 141.95, 135.53, 129.66, 129.61, 127.37(×2), 123.48, 115.65, 115.51, 114.00, 112.55, 110.26, 106.18, 101.12, 56.57, 55.79, 55.20, 54.22, 52.11, 49.07, 44.89. HRMS calcd. for C28H28FN3O4S, [M + Na]+, 544.1682; found 544.1757. HPLC: tR = 4.77 min, 95.90%.
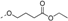
Ethyl-4-(4-(2-(((3,4-dimethoxyphenyl)(4-fluorobenzyl)amino)methyl)thiazol-4-yl)phenoxy)butanoate (48). yellow oil, yield: 32.9%. 1H-NMR (400 MHz, DMSO-d6) δ: 7.86 (d, J = 8.6 Hz, 2H), 7.81 (s, 1H), 7.38 (dd, J = 8.3, 5.8 Hz, 2H), 7.16 (t, J = 8.8 Hz, 2H), 6.98 (d, J = 8.8 Hz, 2H), 6.74 (d, J = 8.8 Hz, 1H), 6.55 (d, J = 2.6 Hz, 1H), 6.27 (dd, J = 8.8, 2.7 Hz, 1H), 4.89 (s, 2H), 4.64 (s, 2H), 4.08 (q, J = 7.1 Hz, 2H), 4.03 (t, J = 6.3 Hz, 2H), 3.62 (d, J = 3.7 Hz, 6H), 2.47 (t, J = 7.3 Hz, 2H), 2.03~1.95 (m, 2H), 1.19 (t, J = 7.1 Hz, 3H). 13C-NMR (150 MHz, DMSO-d6) δ: 173.04, 170.93, 161.67 (d, J = 240 Hz), 158.77, 154.31, 149.87, 142.98, 142.01, 135.50, 129.68, 129.62, 127.73(×2), 127.50, 115.67, 115.53, 115.09(×2), 113.99, 112.57, 106.23, 101.16, 67.00, 60.36, 56.56, 55.79, 55.24, 54.19, 30.61, 24.70, 14.58. HRMS calcd. for C31H33FN2O5S, [M + Na]+, 587.1992; found 587.2083. HPLC: tR = 8.39 min, 96.53%.
N-(4-Fluorobenzyl)-3,4-dimethoxy-N-((4-(4-morpholinophenyl)thiazol-2-yl)methyl)aniline (49). White solid, yield: 53.7%. m.p. 139.0–140.4 °C. 1H-NMR (600 MHz, DMSO-d6) δ: 7.81~7.78 (m, 2H), 7.74 (s, 1H), 7.38 (dd, J = 8.6, 5.6 Hz, 2H), 7.17~7.14 (m, 2H), 6.99 (d, J = 9.0 Hz, 2H), 6.73 (d, J = 8.8 Hz, 1H), 6.55 (d, J = 2.8 Hz, 1H), 6.26 (dd, J = 8.8, 2.9 Hz, 1H), 4.88 (s, 2H), 4.64 (s, 2H), 3.76~3.73 (m, 4H), 3.61 (d, J = 5.7 Hz, 6H), 3.17~3.14 (m, 4H). 13C-NMR (150 MHz, DMSO-d6) δ: 170.13, 161.08 (d, J = 241 Hz), 154.11, 150.51, 149.28, 142.42, 141.41, 134.93, 129.09, 129.04, 126.65(×2), 125.16, 115.08, 114.44, 114.20(×2), 113.42, 111.12, 105.63, 100.56, 65.93(×2), 55.99, 55.21, 54.65, 53.64, 48.49, 47.92. HRMS calcd. for C29H30FN3O3S, [M + Na]+, 542.1890; found 542.1975. HPLC: tR = 6.29 min, 98.33%.