Fluorescent “Turn-Off” Detection of Fluoride and Cyanide Ions Using Zwitterionic Spirocyclic Meisenheimer Compounds
Abstract
:1. Introduction
2. Results
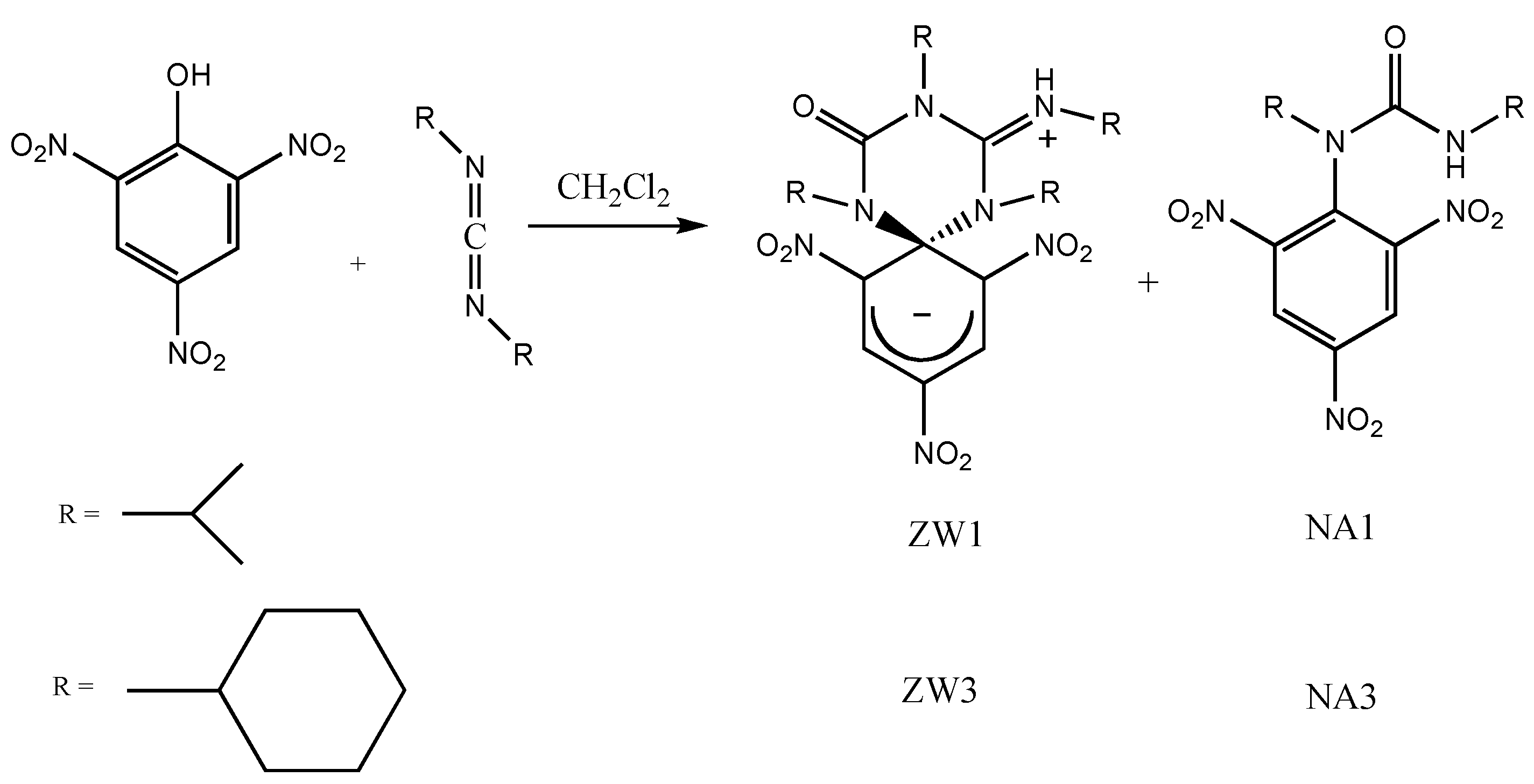
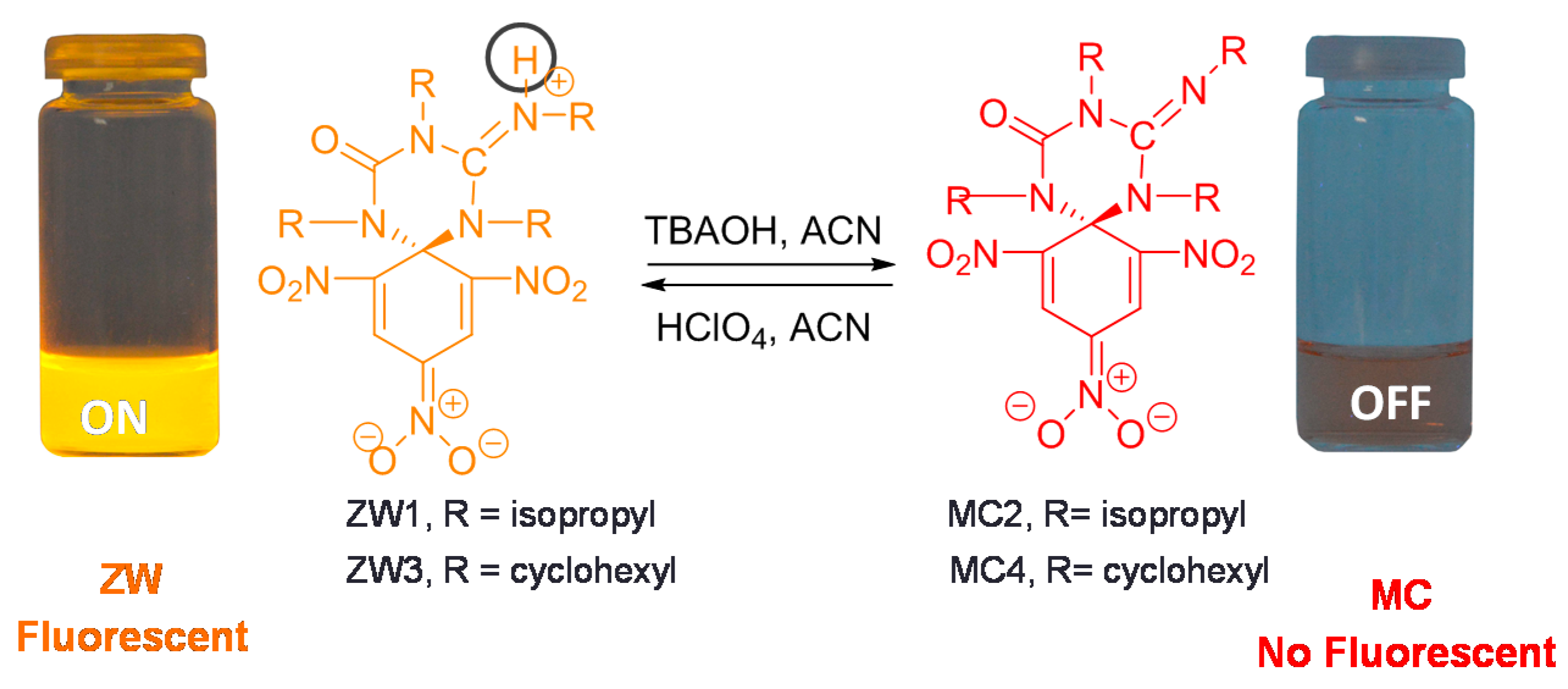
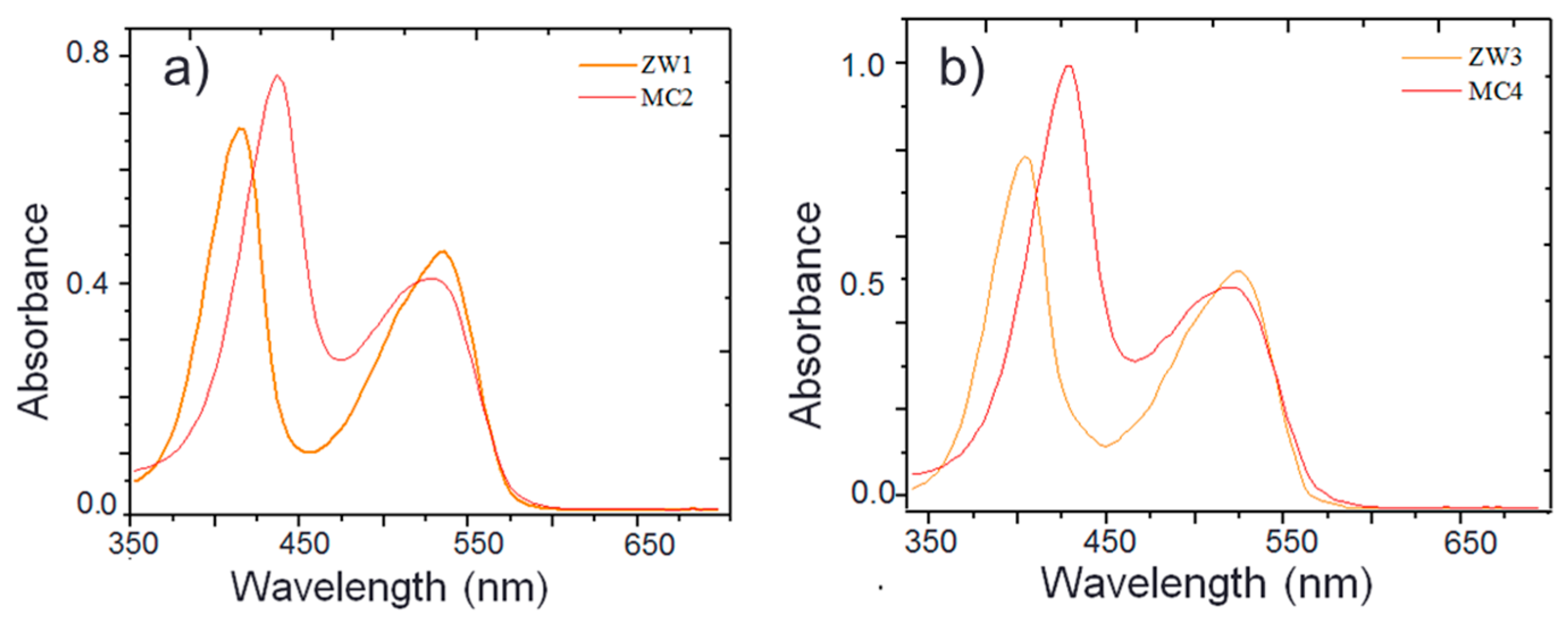
2.1. Synthesis and Photophysical Properties of ZW1 and ZW3
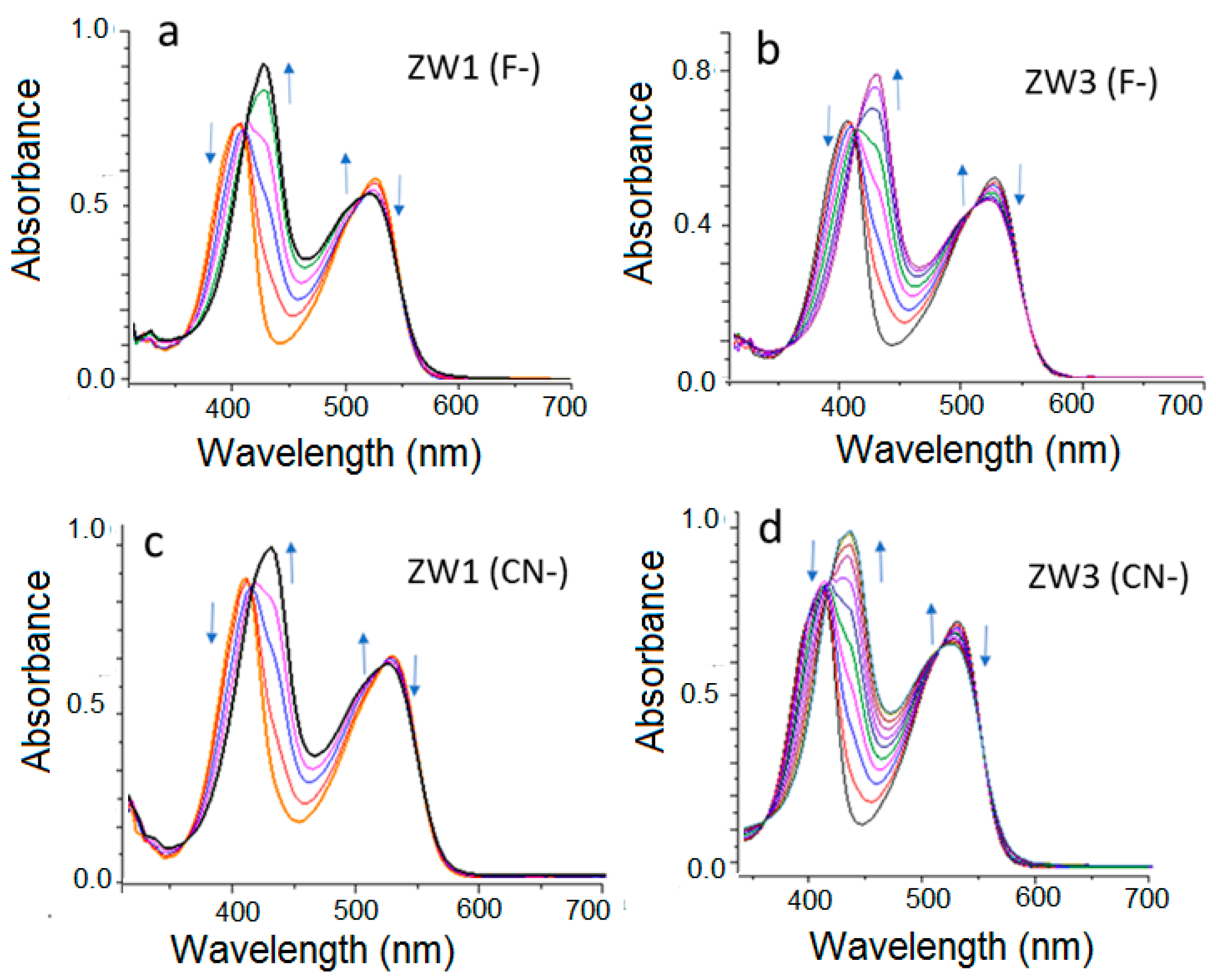
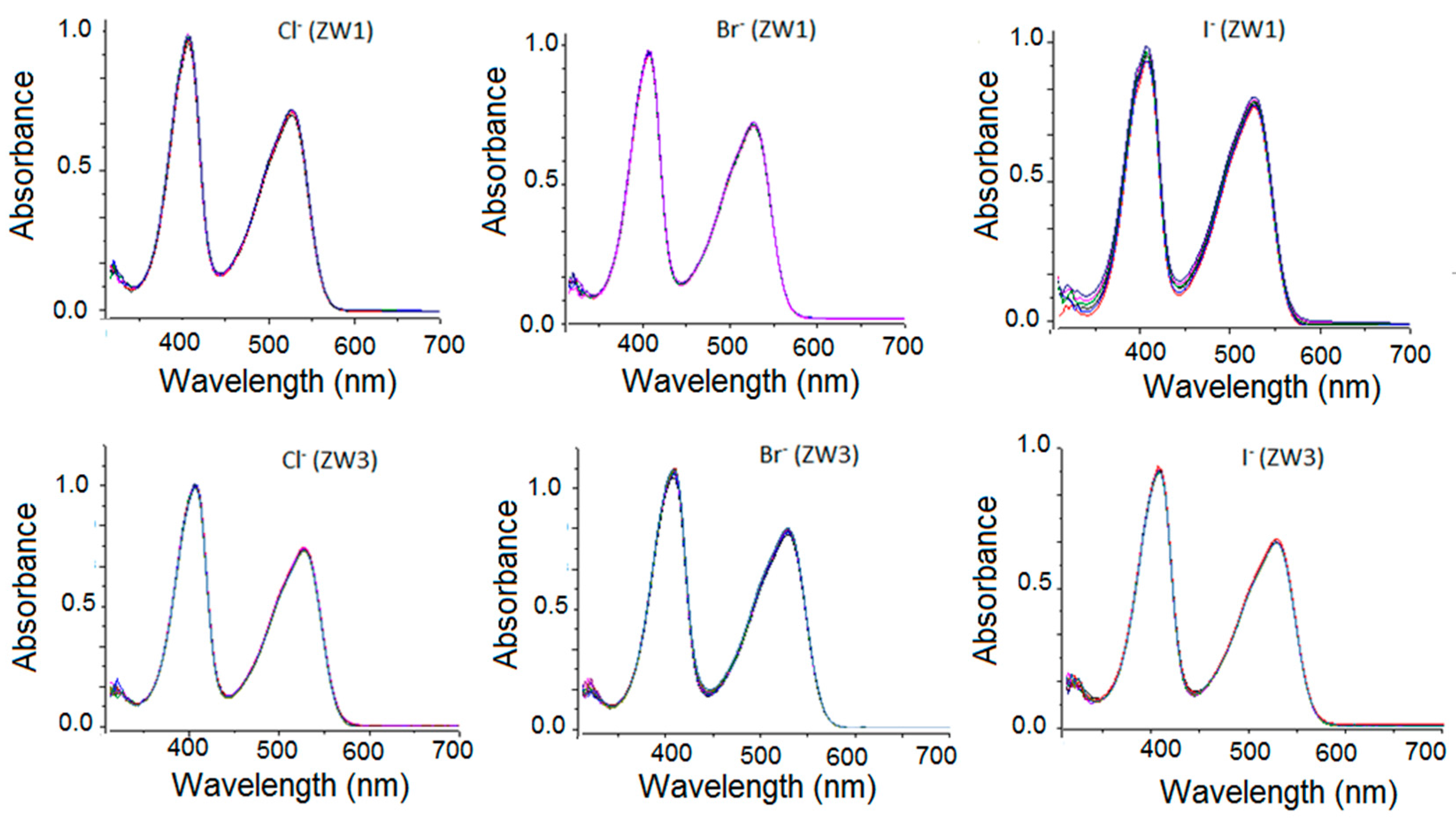
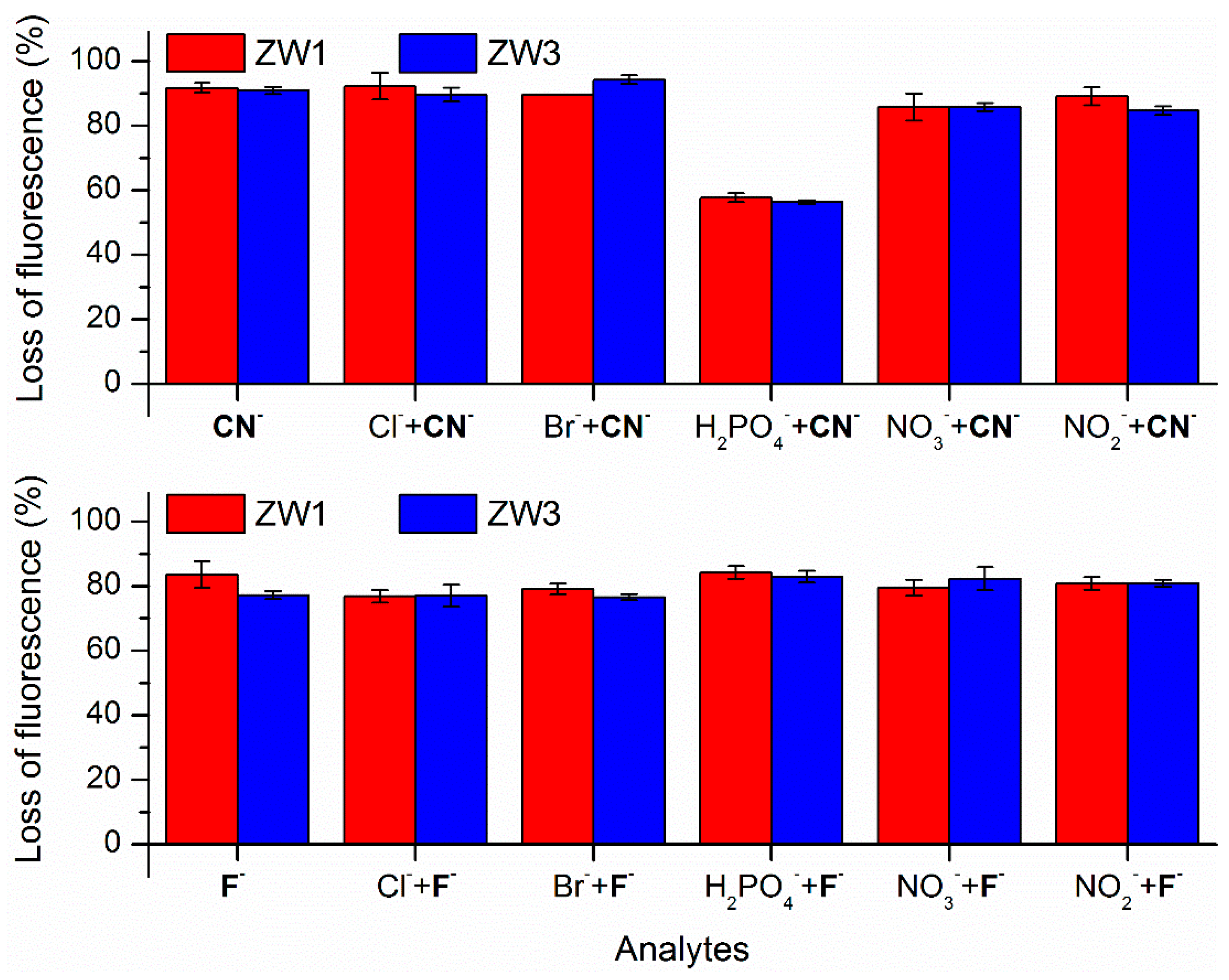
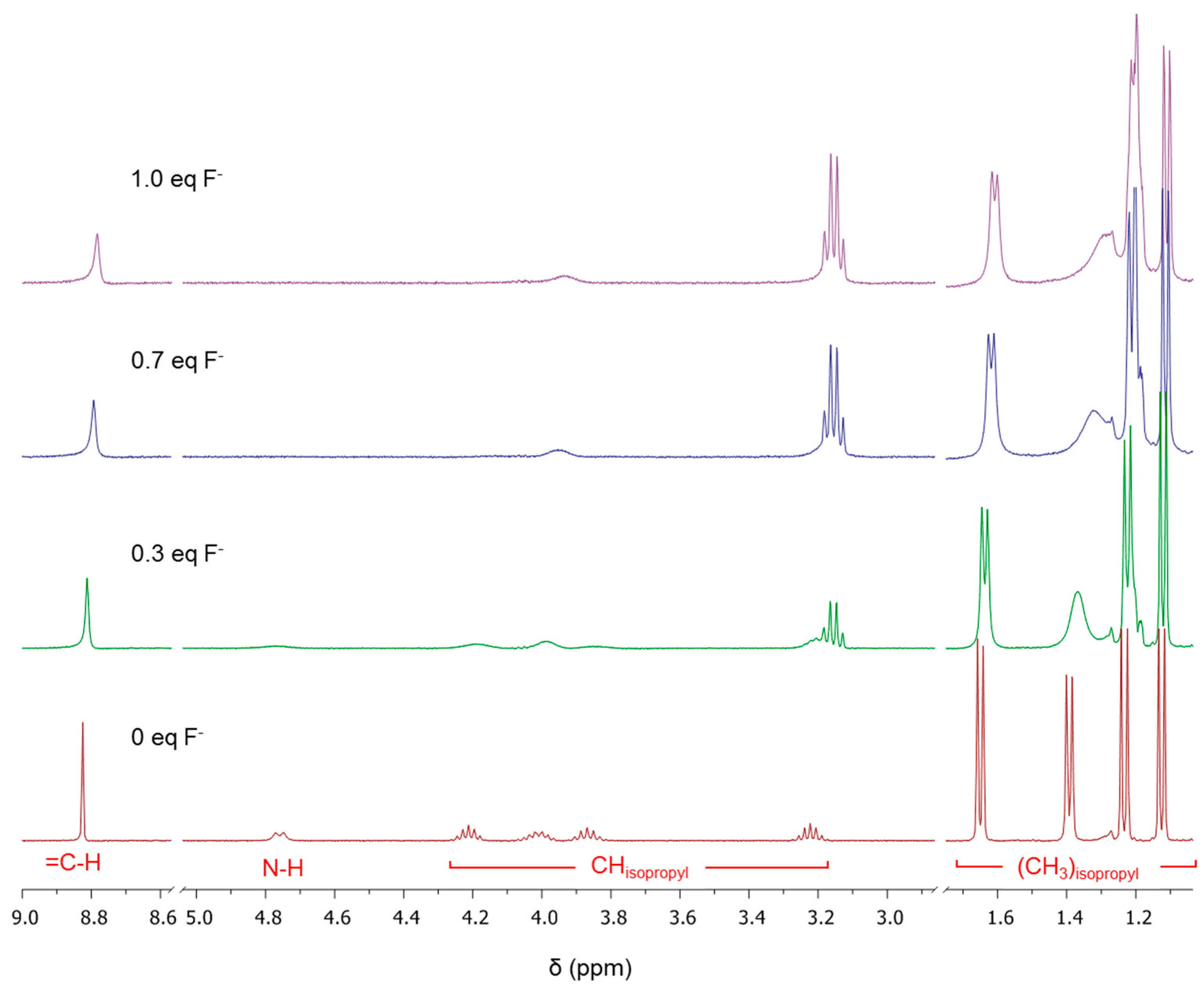
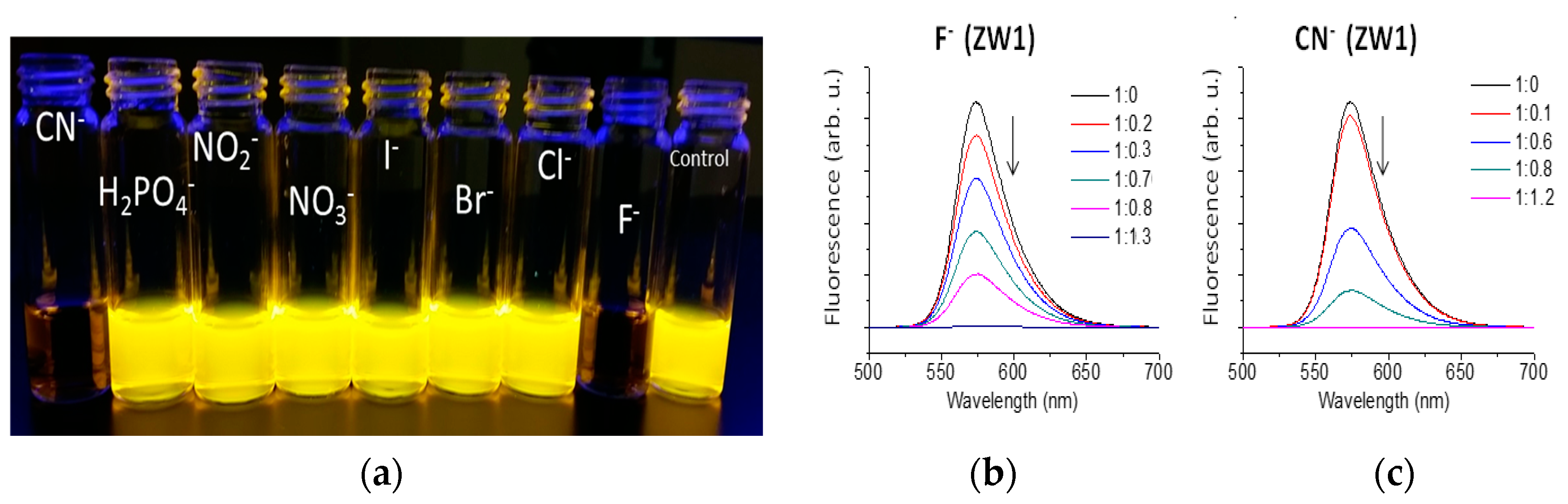
2.2. Using ZW1 or ZW3 for the Detection of F− or CN−
3. Discussion
4. Materials and Methods
4.1. Synthesis of ZW1
4.2. Synthesis of ZW3
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saragi, T.P.I.; Spehr, T.; Siebert, A.; Fuhrmann-Lieker, T.; Salbeck, J. Spiro Compounds for Organic Optoelectronics. Chem. Rev. 2007, 107, 1011–1065. [Google Scholar] [CrossRef] [PubMed]
- Pudzich, R.; Fuhrmann-Lieker, T.; Salbeck, J. Spiro Compounds for Organic Electroluminescence and Related Applications. In Emissive Materials Nanomaterials. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2006; Volume 199, pp. 83–142. [Google Scholar]
- Al-Kaysi, R.O.; Gallardo, I.; Guirado, G. Stable Spirocyclic Meisenheimer Complexes. Molecules 2008, 13, 1282–1302. [Google Scholar] [CrossRef] [PubMed]
- Shimkin, A.A.; Nikalin, D.M.; Shirinian, V.Z.; Krayushkin, M.M.; Vorontsova, L.G.; Metelitsa, A.V.; Minkin, V.I. Synthesis of Novel Photochromic Spiro Compounds based on Thieno[3,2-b]Pyrroles. Mol. Cryst. Liq. Cryst. 2005, 431, 307–313. [Google Scholar] [CrossRef]
- Such, G.; Evans, R.A.; Yee, L.H.; Davis, T.P. Factors Influencing Photochromism of Spiro-Compounds Within Polymeric Matrices. J. Macromol. Sci. Part C 2003, 43, 547–579. [Google Scholar] [CrossRef]
- Molvi, K.I.; Haque, N.; Awen, B.Z.S.; Zameeruddin, M. Synthesis of Spiro Compounds as Medicinal Agents; New Opportunities for Drug Design and Discovery. Part I: A Review. World J. Pharm. Pharm. Sci. 2014, 3, 536–563. [Google Scholar] [CrossRef]
- Zheng, Y.-J.; Tice, C.M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaysi, R.O.; Guirado, G.; Valente, E.J. Synthesis and Characterization of a New Fluorescent Zwitterionic Spirocyclic Meisenheimer Complex of 1,3,5-Trinitrobenzene. Eur. J. Org. Chem. 2004, 2004, 3408–3411. [Google Scholar] [CrossRef]
- Al-Kaysi, R.; Creed, D.; Valente, E. Meisenheimer complex from picric acid and diisopropylcarbodiimide. J. Chem. Crystallogr. 2004, 34, 685–692. [Google Scholar] [CrossRef]
- Al-Kaysi, R.O.; Bourdelande, J.L.; Gallardo, I.; Guirado, G.; Hernando, J. Investigation of an Acid–Base and Redox Molecular Switch: From Bulk to the Single-Molecule Level. Chem. A Eur. J. 2007, 13, 7066–7074. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, R.; Guo, K.; Meng, Q.; Zhang, R.; Kong, X.; Zhang, Z. A gadolinium(iii) complex based dual-modal probe for MRI and fluorescence sensing of fluoride ions in aqueous medium and in vivo. Dalton Trans. 2016, 45, 17616–17623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, R.; Feng, H.; Guo, K.; Meng, Q.; Chi, H.; Zhang, R.; Zhang, Z. Visualization of Fluoride Ions In Vivo Using a Gadolinium(III)-Coumarin Complex-Based Fluorescence/MRI Dual-Modal Probe. Sensors 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.; Song, R.; Guo, K.; Meng, Q.; Feng, H.; Duan, C.; Zhang, Z. Fluoride-specific fluorescence/MRI bimodal probe based on a gadolinium(iii)-flavone complex: Synthesis{,} mechanism and bioimaging application in vivo. J. Mater. Chem. B 2016, 4, 7379–7386. [Google Scholar] [CrossRef]
- Krishnamachari, K.A. Skeletal fluorosis in humans: A review of recent progress in the understanding of the disease. Prog. Food Nutr. Sci. 1986, 10, 279–314. [Google Scholar] [PubMed]
- Mudder, T.; Botz, M. Cyanide and society: A critical review. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 62–74. [Google Scholar]
- Lee, K.S.; Kim, H.J.; Kim, G.H.; Shin, I.; Hong, J.I. Fluorescent chemodosimeter for selective detection of cyanide in water. Org. Lett. 2008, 10, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Chen, X.; Yoon, J. Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dasgupta, P.K. Recent developments in cyanide detection: A review. Anal. Chim. Acta 2010, 673, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Nakamura, M.; Hayashi, N.; Hirai, T. Coumarin–Spiropyran Dyad with a Hydrogenated Pyran Moiety for Rapid, Selective, and Sensitive Fluorometric Detection of Cyanide Anion. Anal. Chem. 2016, 88, 6805–6811. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaysi, R.O.; Müller, A.M.; Ahn, T.; Lee, S.; Bardeen, C.J. Effects of sonication on the size and crystallinity of stable zwitterionic organic nanoparticles formed by reprecipitation in water. Langmuir 2005, 21, 7990–7994. [Google Scholar] [CrossRef] [PubMed]
- Chmyrov, A.; Sandén, T.; Widengren, J. Iodide as a fluorescence quencher and promoter-mechanisms and possible implications. J. Phys. Chem. B 2010, 114, 11282–11291. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.L. The Hard Soft Acids Bases (HSAB) Principle and Organic Chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Bordwell, F.G. Equilibrium Acidities in Dimethyl Sulfoxide Solution. Acc. Chem. Res. 1988, 21, 456–463. [Google Scholar] [CrossRef]
- Sánchez, R.S.; Gras-Charles, R.; Bourdelande, J.L.; Guirado, G.; Hernando, J. Light- and redox-controlled fluorescent switch based on a perylenediimide-dithienylethene dyad. J. Phys. Chem. C 2012, 116, 7164–7172. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound ZW1 and ZW3 are available from the authors. |



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benet, M.; Villabona, M.; Llavina, C.; Mena, S.; Hernando, J.; Al-Kaysi, R.O.; Guirado, G. Fluorescent “Turn-Off” Detection of Fluoride and Cyanide Ions Using Zwitterionic Spirocyclic Meisenheimer Compounds. Molecules 2017, 22, 1842. https://doi.org/10.3390/molecules22111842
Benet M, Villabona M, Llavina C, Mena S, Hernando J, Al-Kaysi RO, Guirado G. Fluorescent “Turn-Off” Detection of Fluoride and Cyanide Ions Using Zwitterionic Spirocyclic Meisenheimer Compounds. Molecules. 2017; 22(11):1842. https://doi.org/10.3390/molecules22111842
Chicago/Turabian StyleBenet, Marina, Marc Villabona, Carles Llavina, Silvia Mena, Jordi Hernando, Rabih O. Al-Kaysi, and Gonzalo Guirado. 2017. "Fluorescent “Turn-Off” Detection of Fluoride and Cyanide Ions Using Zwitterionic Spirocyclic Meisenheimer Compounds" Molecules 22, no. 11: 1842. https://doi.org/10.3390/molecules22111842
APA StyleBenet, M., Villabona, M., Llavina, C., Mena, S., Hernando, J., Al-Kaysi, R. O., & Guirado, G. (2017). Fluorescent “Turn-Off” Detection of Fluoride and Cyanide Ions Using Zwitterionic Spirocyclic Meisenheimer Compounds. Molecules, 22(11), 1842. https://doi.org/10.3390/molecules22111842






