Abstract
Reactions of O-peracylated C-(1-bromo-β-d-glucopyranosyl)formamides with thioamides furnished the corresponding glucopyranosylidene-spiro-thiazolin-4-one. While O-debenzoylations under a variety of conditions resulted in decomposition, during O-deacetylations the addition of MeOH to the thiazolinone moiety was observed, and with EtOH and water similar adducts were isolated or detected. The structure and stereochemistry of the new compounds were established by means of NMR and electronic circular dichroism (ECD) data supported by time-dependent density functional theory ECD (TDDFT-ECD) calculations. TDDFT-ECD calculations could efficiently distinguish the proposed epimeric products having different absolute configuration in the spiro heterocyclic ring.
1. Introduction
During the past two decades, several glucopyranosylidene-spiro heterocycles have emerged as potent inhibitors of glycogen phosphorylase (GP) [1,2,3,4,5]. The liver isoform of GP is a validated target in the search for new therapeutic possibilities against type 2 diabetes mellitus [6], nevertheless, its inhibition is considered to have potential in other important pharmacological fields such as possible medication and/or prevention of cardiovascular disorders [7,8], cerebral ischemias [9,10] and tumorous growth [11,12,13,14,15,16].
The first spirocyclic GP inhibitor (GPI), a glucopyranosylidene-spiro-hydantoin (A, X = O in Chart 1) was disclosed in the mid-nineties [17] followed by the more easily synthesized thiohydantoin counterpart (A, X = S) [18,19]. Both compounds were active as GPIs in the low micromolar concentration range. Glucopyranosylidene-spiro-oxathiazoles (B, X = S) [20,21] and isoxazolines (B, X = CH2) [22] identified some ten years later proved to be submicromolar GPIs and still belong in the top ten most efficient glucose-derived inhibitors of the enzyme. For the strong binding of compounds A to the enzyme, X-ray crystallography of the enzyme-inhibitor complexes revealed the importance of the H-bond donor β-NH, as well as that of the α-carbonyl groups highlighted in red [23,24]. Similar studies with compounds B concluded that bulky substituents such as the 2-naphthyl moiety (marked in blue) better contributed to the binding even in the absence of the hydrogen bonding β-NH and the carbonyl in the α-position [22]. An attempt was made to unify these properties by the study of compounds C [3]. Surprisingly, these derivatives proved rather weak inhibitors and this was attributed to the =N–C=O linker between the spirocycle and the aryl groups resulting in an unfavorable orientation of the latter for the binding. To circumvent this problem the synthesis of compounds D was envisaged, and the results of these studies are presented in this paper.
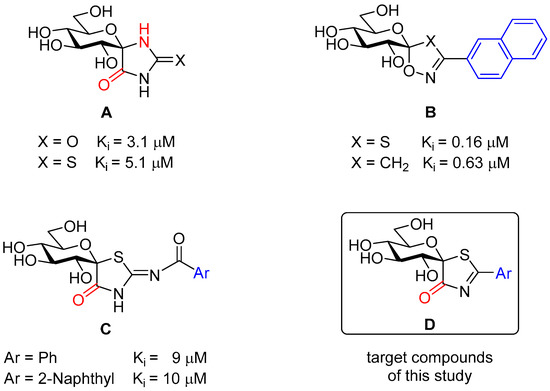
Chart 1.
Selected spirobicyclic inhibitors of rabbit muscle glycogen phosphorylase A, B and C (Ki dissociation constant of the enzyme-inhibitor complex) and the target compounds of the present study (D).
2. Results and Discussion
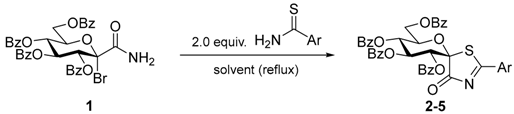
In our previous study dealing with the preparation of glucopyranosylidene-spiro-iminothiazolidinone C [3] bromo-amide derivative 1 was found to be the substrate of choice in reactions with thiourea, therefore its transformation with thiobenzamide was investigated first (Table 1) to find the best conditions for providing the present target compounds. In refluxing EtOH, that was advantageous earlier, only 6 [25] could be obtained (entry 1). Since acetone also proved to be a suitable solvent previously, the higher boiling diethylketone was tried next, but 7 [26] could be isolated as the only product (entry 2). Much less time was needed for the complete consumption of the starting 1 in pyridine or DMF (entries 3 and 4), however, only the formation of 8 [27] or 9 [28] was observed, respectively. Following this, less reactive solvents such as toluene and m-xylene (entries 5 and 6) were tested affording similar yields of the expected compound 2 with a significantly shorter reaction time for the latter. In ether type solvents (entries 7–9) and nitromethane (entry 10) the yields of 2 remained the same, therefore, the use of m-xylene was further optimized. Applying an inert atmosphere (entry 11) or addition of a silver salt (entry 12) left the yield practically unchanged, while microwave heating resulted in a considerable increase in the yield of 2 (entry 13). This was further improved by raising the temperature (entry 14), however even these conditions did not allow diminishing the amount of thiobenzamide (entry 15). Other thioamides gave the expected spiro derivatives 3–5 in acceptable yields (entries 16–18). The formation of the depicted molecules 2–5 with an inversion of configuration at the newly formed spiro carbon is consistent with a presumable bimolecular nucleophilic substitution mechanism and concurs with earlier observations on the stereochemical outcome of the synthesis of glucopyranosylidene-spiro-iminothiazolidinone C [3].

Table 1.
Synthesis of spiro-thiazolinones 2–5.
Attempts to remove the benzoyl protecting groups [29] under a series of base (NaOMe/MeOH, 1 day; KCN MeOH, 4 weeks decomposition) or acid catalyzed (AcCl/MeOH, 4 weeks no reaction) transesterification conditions or under basic hydrolysis conditions (4 equiv. LiOH/MeOH, 0 °C decomposition) proved unsuccesful.
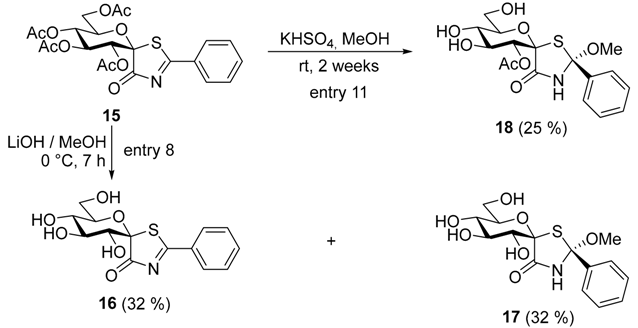
After this experience the use of the more readily removable O-acetyl protecting groups was envisaged. To this end, the O-benzoyl groups of 10 [28] were removed by using the Zemplén protocol and the resulting amide 11 was treated with Ac2O under acidic catalysis (Scheme 1). Since N-acetylation also took place with an excess of the acetylating agent to give 12, four equivalents of Ac2O in AcOH had to be used to yield the expected 13 [30]. This compound was subjected to radical-mediated bromination conditions [31,32] to afford 14 [19] which could be transformed to thiazolinone 15 with thiobenzamide. It is to be noted, that in this latter transformation the conditions used to get the O-perbenzoylated 2–5 resulted in only 14–18% yields due to decomposition, but at lower temperature in toluene 46% of 15 could be achieved.
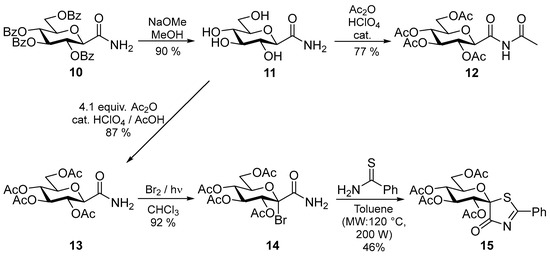
Scheme 1.
Synthesis of spiro-thiazolinone 15 with O-acetyl protecting groups.
For the removal of the O-acetyl protective groups [29] of 15 a wide range of conditions recommended in the literature were attempted (Table 2). Under classical Zemplén conditions or transesterifications catalyzed by K2CO3 or KCN (entries 1–3, respectively) only the formation of multicomponent mixtures could be observed. Similar outcomes were achieved by using nucleophilic ester cleaving reagents (entries 4 and 5) or the non-nucleophilic base DBU (entry 6). However, LiOH in MeOH (entry 7) gave a mixture of 16 and 17, and these compounds were obtained in better yields with a substoichiometric amount of LiOH (entry 8). From the attempted acid catalyzed transesterification conditions (entries 9–11), it was only the use of KHSO4 (entry 11) that resulted in a moderate yield of 18 with the 2’-O-acetyl group remaining in the molecule.

Table 2.
Observations during experiments for deacetylation [29] of spiro-thiazolinone 15.
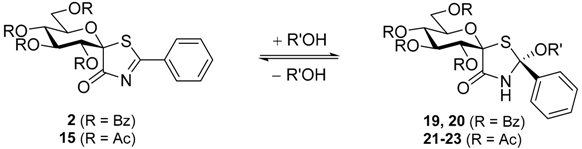
The presence of the MeOH addition products 17 and 18 in the deacetylation mixtures prompted us to investigate the reaction of 2 and 15 with MeOH and EtOH without any other additive (Table 3). It turned out that both alcohols added to the thiazolinone moiety to furnish 19–22 (entries 1–4), respectively, as the major stereoisomers indicated by the characteristic signals in the NMR spectra (see detailed structural elucidation below). Removal of the solvent alcohol at 30 °C allowed us to characterize these addition products. The presence of minor components (<10%) could be observed in the 1H-NMR spectra (see Supporting Information), however, their separation and purification proved infeasible due to the same chromatographic mobility of the substances. Therefore, the formation of both possible diastereomeric addition products cannot be excluded. This addition reaction proved to be reversible since by heating of 19 (at 80 °C) or 21 (at 60 °C) in toluene thiazolinones 2 and 15 were recovered, respectively. To the best of our knowledge, similar alcohol addition was reported only for 2-perfluoroalkyl-5-methoxycarbonylmethylene-thiazoline-4-one type compounds [33,34]. Our findings indicate that the presence of strongly electron withdrawing substituents, such as the perfluoroalkyl groups are not required for the ROH addition.

Table 3.
Addition of alcohols and water onto spiro-thiazolinones 2 and 15.
The addition of water was also tested with 15 (entry 5) and the formation of 23 was observed in a mixture with the starting compound (15:23~1:0.4). The appearance of 23 made it clear that a similar addition may occur during the enzymatic tests necessarily carried out under aqueous conditions, thereby thwarting the study of these compounds as enzyme inhibitors.
Structural elucidation of the O-perbenzoylated spirocycles 2–5 was carried out by NMR and MS methods. The 13C-NMR spectra contained signals in the range of 194–195 ppm and 187–188 ppm for C-2 and C-4, respectively, while similar resonances for 15 appeared at 194.7 ppm (C-2) and 187.0 ppm (C-4) (for numbering see Chart 2). Molecular masses determined by MS corroborated the formation of the spirocycles 2–5 and 15. The configuration of the 1’,5-spiro carbon in 2 was established by determining the three-bond heteronuclear coupling constant between the axial H-2’ (4C1 conformation of the sugar ring followed from the vicinal proton–proton couplings in the 1H-NMR spectra, see experimental) and C-4. The observed 6 Hz value indicated trans diaxial arrangement of these nuclei thereby proving the (R) configuration of the spiro center. For 3–5, the similarities of the chemical shifts for the sugar ring protons lead to the assumption that the spiro configuration is the same.
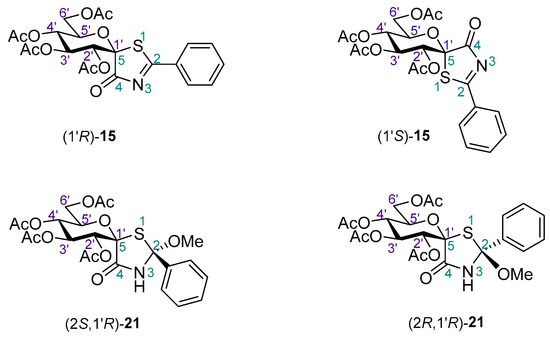
Chart 2.
Possible stereoisomers of 15 and 21.
Both 1H- and 13C-NMR spectra of 17–22 exhibited characteristic signals for the OMe and OEt groups (see experimental section for specifics). The 1H-NMR spectra showed resonances for the exchangable NH protons (6.6–7.2 ppm), and in the 13C-NMR spectra signals appeared for C-2 in the range of 90–100 ppm instead of the 194–195 ppm resonances of the starting materials.
Since no NMR method seemed suitable to determine the configuration of C-2 in the addition products 17–22, ECD measurements and TDDFT-ECD calculations were also performed for 21. These studies were extended to 15 in order to confirm the NMR results independently (Chart 2 shows the studied stereoisomeric structures).
For the determination of the absolute configuration of the C-1’ chirality center, the solution TDDFT-ECD method was utilized on the (1’R) and (1’S) diastereomers of 15 (Chart 2) [35,36]. Based on the literature data for other acetylated glucopyranose derivatives, the ECD spectrum was expected to be governed by the aglycon part containing the C-1’ chirality center as part of a 2-phenylthiazolin-4-one unit [37]. The initial conformers generated for both stereoisomers with the OPLS-2005 force field were reoptimized at the B3LYP/6-31G(d) in vacuo and the ωB97XD/TZVP [35,38] PCM/MeCN levels (see Supporting Information). ECD calculations were performed with various functionals (B3LYP, BH&HLYP, CAM-B3LYP and PBE0) and the TZVP basis set for the low-energy conformers over 1% Boltzmann population. The computed averaged ECD spectrum of the (1’R) diastereomer reproduced all the transitions of the experimental ECD spectrum well (Figure 1a). In contrast, the (1’S) diastereomer had a much poorer mirror-image agreement of the experimental Cotton effects (CEs) below 325 nm (Figure 1b) confirming the decisive role of the C-1’ chirality center in the ECD pattern. The highest-wavelength positive n-π* CE could not be reproduced by the computed ECD of the (1’S) epimer, since similar to the other transitions, opposite CE should have been calculated. Based on the good agreement of the (1’R) diastereomer, the (1’R) absolute configuration of 15 was unambiguously determined in concord with the NMR analysis of 2.
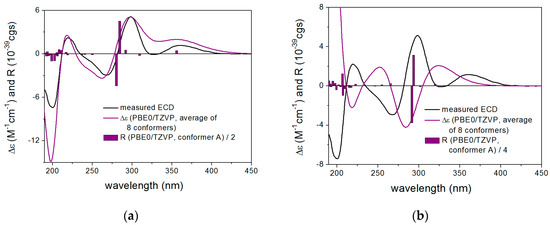
Figure 1.
Experimental ECD spectrum of 15 in MeCN compared with the Boltzmann-weighted PBE0/TZVP PCM/MeCN ECD spectra of (a) (1’R)-15 and (b) (1’S)-15 both computed for the ωB97XD/TZVP PCM/MeCN conformers. The bars represent the rotational strength values of the lowest-energy conformers.
In the case of 21, both the C-1’ and the C-2 chirality centers were expected to contribute to the ECD spectrum and ECD calculations were performed for the (2R,1’R) and (2S,1’R) diastereomers (Chart 2). The computed ECD spectra of (2R,1’R)-21 and (2S,1’R)-21 showed opposite CEs in the 190–290 nm spectral range with acceptable mirror image agreement (Figure 2a) or good reproduction of the experimental ECD spectrum (Figure 2b), respectively. However, the computed ECD of (2S,1’R)-21 had two well-separated negative CEs, the shape of which were much more similar to the experimental ECD than that of the broad positive computed CE of (2R,1’R)-21. Thus, the good overall agreement of (2S,1’R)-21 allowed the unambiguous elucidation of the absolute configuration at C-2 as (S).
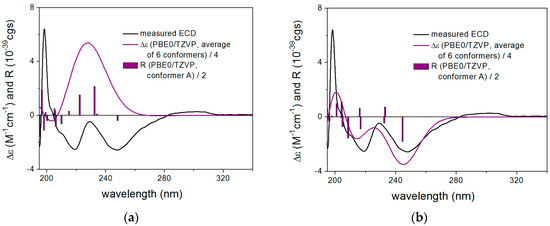
Figure 2.
Experimental ECD spectrum of 21 in MeCN compared with the Boltzmann-weighted PBE0/TZVP PCM/MeCN ECD spectra of (a) (2R,1’R)-21 and (b) (2S,1’R)-21 both computed for the ωB97XD/TZVP PCM/MeCN conformers. The bars represent the rotational strength values of the lowest-energy conformers.
Compounds 19–22 were formed as preponderant diastereomers (e.g., (2S,1’R)-21 in Chart 2) although nucleophilic attack of the alcohol could have been expected from both sides of the planar thiazolinone ring. This observation may be explained by a steric hindrance of the C-2 center by the 2’-OAc or 2’-OBz groups facilitating the addition reaction from the opposite side. This can be visualized by the computed conformers of (1’R)-15 (Figure S1b in the Supporting Information).
3. Conclusions
Glucopyranosylidene-spiro-thiazolinones were prepared in O-perbenzoylated and O-peracetylated forms by reacting the corresponding C-(1-bromo-β-d-glucopyranosyl)formamides with arenethiocarboxamides in m-xylene under microwave heating conditions. While O-debenzoylations could not be achieved, during the O-deacetylation by the Zemplén protocol addition of MeOH onto the thiazolinone moiety was observed. This addition could be extended to EtOH and even water and the reactions proved to be reversible. Electronic circular dichroism spectroscopy aided by TDDFT-ECD computations could differentiate the possible diastereomers and confirmed the absolute configuration assigned by NMR analysis of the new compounds and also allowed the determination of the absolute configuration of the new stereogenic center formed upon the addition of alcohols. The formation of the water addition product under aqueous conditions prevented the test of these compounds as enzyme inhibitors.
4. Experimental
4.1. General Methods
Melting points were measured on a Kofler hot-stage and are uncorrected. Optical rotations were determined with a Perkin-Elmer 241 polarimeter at r.t. NMR spectra and were recorded with Bruker 360 (360/90 MHz for 1H/13C) or Bruker 400 (400/100 MHz for 1H/13C) or Avance DRX 500 (500/125 MHz for 1H/13C) spectrometers (Bruker, Karlsruhe, Germany; Billerica, MA, USA). Chemical shifts are referenced to the internal TMS (1H), or to the residual solvent signals (13C). In the NMR spectra complete signal assignments are based on COSY, HSQC, and HSQMBC correlations. Mass spectra were obtained by a Bruker micrOTOF-Q instrument. TLC was performed on DC-Alurolle Kieselgel 60 F254 (Merck, Darmstadt, Germany), and the plates were visualised under UV light and by gentle heating (generally no spray reagent was used but, if more intense charring was necessary, the plate was sprayed with the following solution: abs. EtOH (95 mL), cc.H2SO4 (5 mL) anisaldehyde (1 mL)). For column chromatography Kieselgel 60 (Merck, particle size 0.063–0.200 mm) was used. Organic solutions were concentrated in vacuo at 40–60 °C (water bath). Xylene and toluene were distilled from P4O10 and stored over sodium wires. MeOH was purified by distillation after refluxing for a couple of hours with magnesium turnings and iodine. CHCl3 was distilled from P4O10 and stored over 4Å molecular sieves. Thioamides were purchased from Sigma-Aldrich (Steinheim, Germany). C-(2,3,4,6-tetra-O-benzoyl-1-bromo-β-d-glucopyranosyl)formamide (1) and C-(2,3,4,6-tetra-O-benzoyl-β-d-glucopyranosyl)formamide (10) were synthesized according to published procedures [28]. Microwave-assisted reactions were carried out using a CEM-Discover Focused microwave synthesis system (2450 MHz).
General procedure I for the synthesis of (1’R)-1’,5’-anhydro-2’,3’,4’,6’-tetra-O-benzoyl-d-glucitol-spiro-[1’,5]-2-arylthiazolin-4-ones (2–5). C-(2,3,4,6-Tetra-O-benzoyl-1-bromo-1-deoxy-β-d-glucopyranosyl)formamide (1) [28] and the corresponding thioamide (2.0 equiv.) were mixed in dry xylene (10 mL/mmol), in a sealed vial and the reaction mixture was stirred for 1.5 h at 140 °C (200 W). Then the mixture was evaporated, and the residue was purified by column chromatography.
General procedure II for the preparation of (2S,1’R)-2’,3’,4’,6’-tetra-O-acyl-1’,5’-anhydro-d-glucitol-spiro-[1’,5]-2-alkoxy-2-phenylthiazolidin-4-ones (19–22). The peracetylated or the perbenzoylated spiro thiazolinone (2 or 15) was dissolved in the corresponding alcohol (30 mL/mmol) and the solution was stirred at rt for ~1 day. When the starting material was consumed (TLC, 1:3 EtOAc/toluene) the solvent was evaporated at diminished pressure and 30 °C water bath temp.
4.2. Characterization of the Compounds
(1’R)-1’,5’-Anhydro-2’,3’,4’,6’-tetra-O-benzoyl-d-glucitol-spiro-[1’,5]-2-phenylthiazolin-4-one (2). Prepared from compound 1 (0.50 g, 0.71 mmol) and thiobenzamide (0.19 g, 1.42 mmol) according to general procedure I. Purified by column chromatography (1:5 EtOAc/hexane) to give 0.28 g (53%) orange amorphous solid. Rf = 0.42 (2:3 EtOAc/hexane); [α]D +87 (c 0.50, CHCl3). 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.09–7.93 (6H, m, aromatics), 7.86–7.78 (4H, m, aromatics), 7.60 (1H, t, J = 7.5 Hz, aromatics), 7.55–7.31 (10H, m, aromatics), 7.26–7.20 (4H, m, aromatics), 6.86 (1H, pseudo t, J = 9.7 Hz, H-3’), 6.33 (1H, d, J = 9.7 Hz, H-2’), 5.95 (1H, pseudo t, J = 10.0 Hz, H-4’), 5.39 (1H, ddd, J = 10.1, 4.1, 2.3 Hz, H-5’), 4.67 (1H, dd, J = 12.6, 2.3 Hz, H-6’a), 4.51 (1H, dd, J = 12.6, 4.1 Hz, H-6’b); 13C-NMR (100 MHz, CDCl3) δ (ppm): 195.3 (C=N), 187.7 (CON, 3JH-2’,CON = 6.0 Hz, from HSQMBC at 125 MHz), 166.1, 166.3, 165.2, 164.8 (C=O), 136.3–127.9 (aromatics), 94.5 (C-1’), 71.8 (C-5’), 70.74, 70.65 (C-2’, C-3’), 68.6 (C-4’), 62.6 (C-6’). ESI-MS positive mode (m/z): calcd for C42H31NNaO10S ([M + Na]+): 764.156. Found: 764.155.
(1’R)-1’,5’-Anhydro-2’,3’,4’,6’-tetra-O-benzoyl-d-glucitol-spiro-[1’,5]-2-(1-naphthyl)thiazolin-4-one (3). Prepared from compound 1 (0.40 g, 0.57 mmol) and naphthalene-1-carbothioamide (0.21 g, 1.14 mmol) according to general procedure I. Purified by column chromatography (1:5 EtOAc/hexane) to give 0.15 g (32%) orange amorphous solid. Rf = 0.40 (2:3 EtOAc/hexane); [α]D +107 (c 0.47, CHCl3); 1H-NMR (400 MHz, CDCl3) δ (ppm): 9.06 (1H, d, J = 8.6 Hz, aromatics), 8.09–7.97 (6H, m, aromatics), 7.88–7.81 (4H, m, aromatics), 7.62–7.33 (12H, m, aromatics), 7.28–7.20 (4H, m, aromatics), 6.86 (1H, pseudo t, J = 9.7 Hz, H-3’), 6.35 (1H, d, J = 9.7 Hz, H-2’), 5.96 (1H, pseudo t, J = 10.0 Hz, H-4’), 5.45 (1H, ddd, J = 10.1, 4.2, 2.6 Hz, H-5’), 4.69 (1H, dd, J = 12.6, 2.6 Hz, H-6’a), 4.52 (1H, dd, J = 12.6, 4.2 Hz, H-6’b); 13C-NMR (100 MHz, CDCl3) δ (ppm): 195.6 (C=N), 188.1 (CON), 166.2, 165.4, 165.3, 164.9 (C=O), 136.6–124.6 (aromatics), 93.8 (C-1’), 71.9 (C-5’), 70.9, 70.7 (C-2’, C-3’), 68.7 (C-4’), 62.7 (C-6’). ESI-MS positive mode (m/z): calcd for C46H33NNaO10S ([M + Na]+): 814.172. Found: 814.171.
(1’R)-1’,5’-Anhydro-2’,3’,4’,6’-tetra-O-benzoyl-d-glucitol-spiro-[1’,5]-2-(2-naphthyl)thiazolin-4-one (4). Prepared from compound 1 (0.50 g, 0.71 mmol) and naphthalene-2-carbothioamide (0.27 g, 1.42 mmol) according to general procedure I. Purified by column chromatography (1:4 EtOAc/hexane) to give 0.23 g (40%) orange amorphous solid. Rf = 0.40 (2:3 EtOAc/hexane); [α]D +62 (c 0.63, CHCl3); 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.55 (1H, s, aromatics), 8.08–7.97 (5H, m, aromatics), 7.89–7.78 (6H, m, aromatics), 7.62–7.30 (11H, m, aromatics), 7.27–7.19 (4H, m, aromatics), 6.89 (1H, pseudo t, J = 9.7 Hz, H-3’), 6.36 (1H, d, J = 9.7 Hz, H-2’), 5.97 (1H, pseudo t, J = 10.0 Hz, H-4’), 5.42 (1H, ddd, J = 10.1, 4.1, 2.5 Hz, H-5’), 4.69 (1H, dd, J = 12.6, 2.5 Hz, H-6’a), 4.51 (1H, dd, J = 12.6, 4.1 Hz, H-6’b); 13C-NMR (100 MHz, CDCl3) δ (ppm): 194.9 (C=N), 187.6 (CON), 166.1, 165.4, 165.3, 165.0 (C=O), 137.1–123.9 (aromatics), 94.6 (C-1’), 71.9 (C-5’), 70.8, 70.7 (C-2’, C-3’), 68.7 (C-4’), 62.7 (C-6’). ESI-MS positive mode (m/z): calcd for C46H33NNaO10S ([M + Na]+): 814.172. Found: 814.169.
(1’R)-1’,5’-Anhydro-2’,3’,4’,6’-tetra-O-benzoyl-d-glucitol-spiro-[1’,5]-2-(4-methylphenyl)thiazolin-4-one (5). Prepared from compound 2 (0.40 g, 0.57 mmol) and 4-methylbenzenethioamide (0.17 g, 1.14 mmol) according to general procedure I. Purified by column chromatography (1:4 EtOAc/hexane) to give 0.23 g (53%) orange amorphous solid. Rf = 0.31 (1:2 EtOAc/hexane); [α]D +87 (c 0.61, CHCl3); 1H-NMR (360 MHz, CDCl3) δ (ppm): 8.08–8.03 (2H, m, aromatics), 8.00–7.89 (4H, m, aromatics), 7.85–7.77 (4H, m, aromatics), 7.60–7.30 (9H, m, aromatics), 7.27–7.20 (5H, m, aromatics), 6.83 (1H, pseudo t, J = 9.7 Hz, H-3’), 6.29 (1H, d, J = 9.7 Hz, H-2’), 5.92 (1H, pseudo t, J = 9.9 Hz, H-4’), 5.37 (1H, ddd, J = 10.0, 4.0, 2.3 Hz, H-5’), 4.65 (1H, dd, J = 12.6, 2.3 Hz, H-6’a), 4.48 (1H, dd, J = 12.6, 4.0 Hz, H-6’b), 2.38 (3H, s, CH3); 13C-NMR (90 MHz, CDCl3) δ (ppm): 194.9 (C=N), 187.8 (CON), 166.1, 166.4, 165.3, 164.9 (C=O), 148.3–128.0 (aromatics), 94.4 (C-1’), 71.8 (C-5’), 70.8, 70.7 (C-2’, C-3’), 68.7 (C-4’), 62.7 (C-6’), 22.1 (CH3). ESI-MS positive mode (m/z): calcd for C43H33NNaO10S ([M + Na]+): 778.172. Found: 778.171.
C-(β-D-Glucopyranosyl)formamide (11). C-(2,3,4,6-tetra-O-benzoyl-β-d-glucopyranosyl) formamide [28] (10, 5.0 g, 8.03 mmol) was dissolved in dry MeOH (100 mL) and 10 drops of a 1 M methanolic NaOMe solution were added. The reaction mixture was stirred at r.t. and monitored by TLC (3:7 MeOH/CHCl3, ~1 day). After complete conversion of 10 the mixture was neutralised with acetic acid and the product precipitated as a white solid. The material was filtered off (1.20 g). The filtrate was concentrated and the residue was crystallized again from methanol to give 0.30 g of 11. Yield: 90%. M.p.: 204–206 °C; Rf = 0.15 (1:2 MeOH/CHCl3); [α]D +30 (c 0.53, H2O); 1H-NMR (400 MHz, D2O) δ (ppm): 3.95–3.85 (2H, m, H-1 and/or H-2 and/or H-3 and/or H-4 and/or H-5 and/or H-6a), 3.76 (1H, dd, J = 12.2, 4.0 Hz, H-6b), 3.60–3.41 (4H, m, H-1 and/or H-2 and/or H-3 and/or H-4 and/or H-5 and/or H-6a); 13C-NMR (100 MHz, D2O) δ (ppm): 174.7 (C=O), 80.0, 78.6, 77.5, 72.3, 69.8 (C-1–C-5), 61.4 (C-6). ESI-MS positive mode (m/z): calcd for C7H13NNaO6S ([M + Na]+): 230.064. Found: 230.063.
N-Acetyl-C-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)formamide (12). Compound 11 (0.30 g, 1.45 mmol) was dissolved in acetic anhydride (5 mL, 52.89 mmol) and one drop of HClO4 was added and the mixture was stirred at rt for 1 h. The resulting solution was poured into ice-water (50 mL), which was then extracted with CHCl3 (3 × 20 mL). The unified CHCl3 phases were washed with NaHCO3 (2 × 20 mL), then with water (1 × 20 mL). The organic phase was dried over MgSO4, concentrated under diminished pressure, and the residual syrup crystallized on addition of diethyl ether to give 0.46 g (77%) white solid. M.p.: 135–137 °C; Rf = 0.34 (1:1 Acetone/hexane); [α]D +8 (c 0.59, CHCl3); 1H-NMR (360 MHz, CDCl3) δ (ppm): 8.78 (1H, s, NH), 5.30 (1H, pseudo t, J = 9.3 Hz, H-2 or H-3 or H-4), 5.19–5.07 (2H, m, H-2 and/or H-3 and/or H-4), 4.29 (1H, dd, J = 12.5, 4.7 Hz, H-6a ), 4.17 (1H, dd, J = 12.5, 1.1 Hz, H-6b), 4.00 (1H, d, J = 9.8 Hz, H-1), 3.82 (1H, ddd, J = 9.8, 4.7, 1.1 Hz, H-5), 2.42 (3H, s, NHCOCH3), 2.12, 2.07, 2.05, 2.03 (4 × 3H, 4 s, CH3); 13C-NMR (90 MHz, CDCl3) δ (ppm): 171.9, 170.6, 170.0, 169.7, 169.4, 166.0 (C=O), 76.4, 76.0, 73.0, 68.9, 67.8 (C-1–C-5), 61.8 (C-6), 25.4 (NHCOCH3), 20.8, 20.6 × 3 (4 × CH3). ESI-MS positive mode (m/z): calcd for C17H23NNaO11 ([M + Na]+): 440.116. Found: 440.116.
C-(2,3,4,6-Tetra-O-acetyl-β-d-glucopyranosyl)formamide (13). Compound 11 (1.50 g, 7.24 mmol) was suspended in glacial acetic acid (20 mL) and acetic anhydride (2.8 mL, 29.68 mmol) and one drop of HClO4 were added. The reaction mixture was stirred at room temperature. After disappearance of the starting material (TLC, 2:1 Acetone/hexane, 2 h) the resulting solution was poured into ice-water (100 mL), which was then extracted with CHCl3 (3 × 50 mL). The unified CHCl3 phases were washed with NaHCO3 (2 × 50 mL), then with water (1 × 50 mL). The organic phase was dried over MgSO4, concentrated under diminished pressure, and the residue was crystallized from ether–hexane mixture to give 2.35 g (87%) of 13. The compound characterization data are identical with those reported [30].
C-(2,3,4,6-Tetra-O-acetyl-1-bromo-β-d-glucopyranosyl)formamide (14). Compound 13 (2.30 g, 6.10 mmol) was dissolved in anhydrous CHCl3 (130 mL) and bromine (1 mL, 19.5 mmol) and some K2CO3 were added (as the acid scavenger). The mixture was placed in an Erlenmeyer flask above a heat lamp (375 W, white, distance from the lamp ~1–2 cm) and refluxed until TLC showed complete transformation (1:1 Acetone/hexane, ~2 h). It was then washed with 5% aq. Na2SO3 (3 × 30 mL) and NaHCO3 (2 × 30 mL) solutions. Drying (MgSO4) and evaporation of the solvent gave 2.56 g (92%) yellowish foam, which was sufficiently pure for the nextstep. The compound characterization data are identical with those reported [19].
(1’R)-1’,5’-Anhydro-2’,3’,4’,6’-Tetra-O-acetyl-d-glucitol-spiro-[1’,5]-2-phenylthiazolin-4-one (15). Compound 14 (0.35 g, 0.77 mmol) and thiobenzamide (0.21 g, 1.54 mmol) were mixed in dry toluene (3 mL) in a sealed vial and the reaction mixture was stirred for 2 h at 120 °C (200 W). Then the solvent was evaporated, and the residual syrup was purified by column chromatography (1:3 EtOAc/hexane) to give 0.32 g (46%) orange amorphous solid. Rf = 0.45 (1:1 EtOAc/hexane); [α]D +63 (c 0.52, CHCl3); ECD (MeCN) λmax (Δε) 360 (+1.16), 327 (−0.08), 299 (+5.14), 268 (−2.93), 242sh (−0.61), 220 (+2.21), 201 (−7.41) nm; 1H-NMR (360 MHz, CDCl3) δ (ppm): 8.11 (2H, d, J = 7.4 Hz, aromatics), 7.76 (1H, t, J = 7.5 Hz, aromatics), 7.57 (2H, t, J = 7.8 Hz, aromatics), 6.12 (1H, pseudo t, J = 9.6 Hz, H-3’ or H-4’), 5.75 (1H, d, J = 9.7 Hz, H-2’), 5.26 (1H, pseudo t, J = 9.9 Hz, H-4’ or H-3’), 4.88 (1H, ddd, J = 10.3, 4.0, 2.0 Hz, H-5’), 4.29 (1H, dd, J = 12.7, 4.1 Hz, H-6’a), 4.09 (1H, dd, J = 12.7, 2.0 Hz, H-6’b), 2.08 × 2, 2.01, 1.94 (4 × 3H, 4 s, CH3); 13C-NMR (90 MHz, CDCl3) δ (ppm): 194.7 (C=N), 187.0 (CON), 170.5, 169.6, 169.5, 169.1 (C=O), 136.4, 131.3, 129.3, 129.2 (aromatics), 94.0 (C-1’), 71.2, 70.3, 69.8, 67.4 (C-2’–C-5’), 61.6 (C-6’), 20.7, 20.5 × 2, 20.4 (4 × CH3). ESI-MS positive mode (m/z): calcd for C22H23NNaO10S ([M + Na]+): 516.093. Found: 516.095.
(1’R)-1’,5’-Anhydro-d-glucitol-spiro-[1’,5]-2-phenylthiazolin-4-one (16) and (2S,1’R)-1’,5’-anhydro-d-glucitol-spiro-[1’,5]-2-methoxy-2-phenylthiazolidin-4-one (17). Compound 15 (100 mg, 0.20 mmol) was dissolved in dry MeOH (10 mL), cooled in an ice-bath and LiOH (2.5 mg, 0.10 mmol) was added. The mixture was stirred until TLC had indicated disappearance of the starting material (3:7 MeOH/CHCl3, 7 h). Then the mixture was neutralised with a cation exchange resin Amberlyst 15 (H+ form) and the resin was filtered off and the solvent was removed. The purification by column chromatography (1:10 MeOH/CHCl3) provided 46 mg yellow syrup as a mixture of 16 (32%) and 17 (32%). Based on 1H-NMR spectrum the ratio of thiazolinone to thiazolidinone was ~ 1:1. Rf = 0.50 (3:7 MeOH/CHCl3); 1H-NMR (360 MHz, CD3OD) δ (ppm): 8.11 (d, J = 7.6 Hz, aromatics), 7.77 (t, J = 7.4 Hz, aromatics), 7.72–7.65 (m, aromatics), 7.60 (t, J = 7.7 Hz, aromatics), 7.36–7.24 (m, aromatics), 4.44–4.27 (m, 2 × H-3’, 2 × H-5’), 3.95 (d, J = 9.3 Hz, H-2’), 3.88–3.80 (m, 2 × H-6’a), 3.74–3.67 (m, 2 × H-6’b), 3.62 (s, OCH3), 3.49–3.35 (m, H-2’, 2 × H-4’); 13C-NMR (90 MHz, CD3OD) δ (ppm): 197.0, 191.0 (thiazolinone C-2, C-4), 173.8 (HNC=O), 144.1–126.6 (aromatics), 99.3, 98.6, 94.0 (thiazolidinone C-1’, C-2, thiazolinone C-1’), 78.7, 77.9, 75.4, 74.9, 74.7, 73.9, 71.2, 70.7 (2 × C-2’–C-5’), 62.9, 62.6 (2 × C-6’), 51.3 (OCH3). ESI-MS positive mode (m/z): calcd for compound 16 C14H15NNaO6S ([M + Na]+): 348.051, found: 348.052; and calcd for compound 17 C15H19NNaO7S ([M + Na]+): 380.077, found: 380.079.
(2S,1’R)-2’-O-Acetyl-1’,5’-anhydro-d-glucitol-spiro-[1’,5]-2-methoxy-2-phenylthiazolidin-4-one (18). Compound 15 (75 mg, 0.15 mmol) was dissolved in dry MeOH (5 mL) and KHSO4 (20 mg, 0.15 mmol) was added. The reaction mixture was kept at r.t. until completion of the transformation (TLC, 1:5 MeOH/CHCl3, 14 days). Then the solvent was removed in vacuo and the purification by column chromatography (1:10 MeOH/CHCl3) gave 15 mg yellow syrup of 18 (25%). Rf = 0.30 (1:5 MeOH/CHCl3); [α]D +50 (c 0.75, MeOH); 1H-NMR (360 MHz, CD3OD) δ (ppm): 7.57–7.51 (2H, m, aromatics), 7.39–7.32 (3H, m, aromatics), 5.01 (1H, d, J = 9.6 Hz, H-2’), 4.54 (1H, pseudo t, J = 9.3 Hz, H-3’ or H-4’), 4.28 (1H, ddd, J = 9.9, 5.0, 2.0 Hz, H-5’), 3.86 (1H, dd, J = 12.1, 2.0 Hz, H-6’a), 3.72 (1H, dd, J = 12.0, 5.0 Hz, H-6’b), 3.61 (3H, s, OCH3), 3.46 (1H, pseudo t, J = 9.5 Hz, H-4’ or H-3’), 2.00 (3H, s, CH3); 13C-NMR (90 MHz, CD3OD) δ (ppm): 172.8, 171.3 (C=O), 144.1, 129.8, 129.4, 126.2 (aromatics), 98.6, 92.1 (C-1’, C-2), 79.1, 74.5, 73.2, 71.1 (C-2’–C-5’), 62.8 (C-6’), 51.6 (OCH3), 21.1 (CH3). ESI-MS positive mode (m/z): calcd for C17H21NNaO8S ([M + Na]+): 422.088. Found: 422.088.
(2S,1’R)-1’,5’-Anhydro-2’,3’,4’,6’-tetra-O-benzoyl-d-glucitol-spiro-[1’,5]-2-methoxy-2-phenylthiazolidin-4-one (19). Prepared from 2 (30 mg, 0.04 mmol) in MeOH according to general procedure II. Orange syrup, Rf = 0.38 (1:1:3 EtOAc/hexane/toluene); 1H-NMR (360 MHz, CDCl3) δ (ppm): 8.06–7.95 (6H, m, aromatics), 7.81 (2H, d, J = 7.6 Hz, aromatics), 7.63–7.23 (14H, m, aromatics), 7.03 (1H, t, J = 7.5 Hz, aromatics), 6.87 (1H, pseudo t, J = 9.7 Hz, H-3’ or H-4’), 6.72 (2H, t, J = 7.8 Hz, aromatics), 6.65 (1H, s, NH), 5.89 (1H, d, J = 9.7 Hz, H-2’), 5.73 (1H, pseudo t, J = 10.0 Hz, H-4’ or H-3’), 5.19 (1H, ddd, J = 10.2, 5.8, 2.9 Hz, H-5’), 4.60 (1H, dd, J = 12.2, 2.9 Hz, H-6’a), 4.53 (1H, dd, J = 12.2, 5.8 Hz, H-6’b), 3.53 (3H, s, OCH3); 13C-NMR (100 MHz, CDCl3) δ (ppm): 170.4 (C-4), 166.1, 165.6, 165.5, 164.7 (C=O), 140.6 (q, Ph), 133.6–125.5 (aromatics), 97.2, 90.5 (C-1’, C-2), 73.3, 71.4, 71.1, 69.4 (C-2’–C-5’), 63.5 (C-6’), 51.3 (OCH3). ESI-MS positive mode (m/z): calcd for C43H35NNaO11S ([M + Na]+): 796.182. Found: 796.180.
The following experiment indicated the reversibility of the methanol addition: Compound 19 was dissolved in dry toluene (10 mL/mmol) and heated at 80 °C. After 3 h TLC (1:1:3 EtOAc/hexane/toluene) indicated complete transformation to give, after removal of the solvent, compound 2 in a quantitative yield.
(2S,1’R)-1’,5’-Anhydro-2’,3’,4’,6’-tetra-O-benzoyl-d-glucitol-spiro-[1’,5]-2-ethoxy-2-phenylthiazolidin-4-one (20). Prepared from 2 (65 mg, 0.09 mmol) in MeOH according to general procedure II. Orange syrup, Rf = 0.40 (1:1:3 EtOAc/hexane/toluene); 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.08–7.95 (6H, m, aromatics), 7.80 (2H, d, J = 7.6 Hz, aromatics), 7.64–7.22 (14H, m, aromatics), 7.01 (1H, t, J = 7.2 Hz, aromatics), 6.95 (1H, br s, NH), 6.88 (1H, pseudo t, J = 9.7 Hz, H-3’ or H-4’), 6.71 (2H, t, J = 7.9 Hz, aromatics), 5.89 (1H, d, J = 9.7 Hz, H-2’), 5.72 (1H, pseudo t, J = 9.9 Hz, H-4’ or H-3’), 5.23 (1H, ddd, J = 10.0, 5.4, 2.9 Hz, H-5’), 4.55 (2H, m, H-6’a, H-6’b), 4.18–4.07 (1H, m, OCH2CH3), 3.67–3.62 (1H, m, OCH2CH3), 1.12 (3H, t, J = 7.0 Hz, OCH2CH3); 13C-NMR (100 MHz, CDCl3) δ (ppm): 170.3 (C-4), 166.1, 165.5, 165.5, 164.7 (C=O), 140.9 (q, Ph), 133.6–125.4 (aromatics), 96.3, 90.5 (C-1’, C-2), 73.3, 71.4, 71.1, 69.4 (C-2’–C-5’), 63.7 (C-6’), 59.7 (OCH2CH3), 14.5 (OCH2CH3). ESI-MS positive mode (m/z): calcd for C44H37NNaO11S ([M + Na]+): 810.198. Found: 810.195.
(2S,1’R)-2’,3’,4’,6’-Tetra-O-acetyl-1’,5’-anhydro-d-glucitol-spiro-[1’,5]-2-methoxy-2-phenylthiazolidin-4-one (21). Prepared from 15 (85 mg, 0.17 mmol) in MeOH according to general procedure II. Orange syrup; Rf = 0.28 (1:2 EtOAc/toluene); ECD (MeCN) λmax (Δε) 302 (+0.29), 267sh (−0.84), 248 (−2.57), 219 (−2.52), 209sh (−1.29), 203sh (+0.45) nm; 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.51 (2H, dd, J = 6.7, 2.9 Hz, aromatics), 7.35–7.29 (3H, m, aromatics), 7.12 (1H, br s, NH), 6.09 (1H, pseudo t, J = 9.6 Hz, H-3’ or H-4’), 5.27 (1H, d, J = 9.6 Hz, H-2’), 5.13 (1H, pseudo t, J = 9.9 Hz, H-4’ or H-3’), 4.68 (1H, ddd, J = 10.2, 4.8, 2.2 Hz, H-5’), 4.19 (1H, dd, J = 12.4, 5.0 Hz, H-6’a), 4.09 (1H, dd, J = 12.4, 2.2 Hz, H-6’b), 3.56 (3H, s, OCH3), 2.04, 2.02, 1.97, 1.95 (4 × 3H, 4 s, 4 × CH3); 13C-NMR (100 MHz, CDCl3) δ (ppm): 170.8, 170.3, 170.0, 169.7, 169.2 (C=O, C-4), 141.5, 129.2, 125.5, 124.9 (aromatics), 97.3, 90.1 (C-1’, C-2), 72.5, 71.2, 70.3, 68.0 (C-2’–C-5’), 62.3 (C-6’), 51.1 (OCH3), 20.6 (3), 20.5 (4 × CH3). ESI-MS positive mode (m/z): calcd for C23H27NNaO11S ([M + Na]+): 548.120. Found: 548.120.
The following experiment indicated the reversibility of the methanol addition: Compound 21 was dissolved in dry toluene (10 mL/mmol) and heated at 60 °C. After 2 h TLC (1:2 EtOAc/toluene) indicated complete transformation to give, after removal of the solvent, compound 15 in a quantitative yield.
(2S,1’R)-2’,3’,4’,6’-Tetra-O-acetyl-1’,5’-anhydro-d-glucitol-spiro-[1’,5]-2-ethoxy-2-phenylthiazolidin-4-one (22). Prepared from 15 (30 mg, 0.06 mmol) in MeOH according to general procedure II. Orange syrup; Rf = 0.26 (1:3 EtOAc/toluene); 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.54 (2H, dd, J = 6.8, 2.9 Hz, aromatics), 7.36–7.31 (3H, m, aromatics), 6.79 (1H, br s, NH), 6.10 (1H, pseudo t, J = 9.5 Hz, H-3’ or H-4’), 5.28 (1H, d, J = 9.7 Hz, H-2’), 5.13 (1H, pseudo t, J = 9.9 Hz, H-4’ or H-3’), 4.70 (1H, ddd, J = 10.3, 5.0, 2.2 Hz, H-5’), 4.19 (1H, dd, J = 12.4, 5.1 Hz, H-6’a), 4.11 (1H, dd, J = 12.4, 2.2 Hz, H-6’b), 4.10–4.08 (1H, m, OCH2CH3), 3.69–3.64 (1H, m, OCH2CH3), 2.07, 2.04, 1.99, 1.96 (4 × 3H, 4 s, CH3), 1.31 (3H, t, J = 7.0 Hz, OCH2CH3); 13C-NMR (100 MHz, CDCl3) δ (ppm): 170.7, 170.0, 169.9, 169.7, 169.1 (C=O, C-4), 141.7, 129.4, 128.7, 125.1 (aromatics), 96.2, 90.0 (C-1’, C-2), 72.8, 71.3, 70.4, 68.1 (C-2’–C-5’), 62.5 (C-6’), 59.9 (OCH2CH3), 20.8, 20.7 (3) (4 × CH3), 14.9 (OCH2CH3). ESI-MS positive mode (m/z): calcd for C24H29NNaO11S ([M + Na]+): 562.135. Found: 562.134.
(1’R)-2’,3’,4’,6’-Tetra-O-acetyl-1’,5’-anhydro-d-glucitol-spiro-[1’,5]-2-phenylthiazolin-4-one (15) and (1’R)-2’,3’,4’,6’-tetra-O-acetyl-1’,5’-anhydro-d-glucitol-spiro-[1’,5]-2-hydroxy-2-phenylthiazolidin-4-one (23). Water (0.1 mL) was added to the solution of spiro thiazolinone 15 (50 mg, 0.10 mmol) in toluene (3 mL) and the mixture was stirred for 6 days at rt, then the solvent was evaporated. Based on 1H-NMR spectrum the ratio of thiazolinone to thiazolidinone was ~1:0.4. Yellow syrup, Rf = 0.45 (1:1 EtOAc/hexane); 1H-NMR (400 MHz, CDCl3) δ (ppm): 8.11 (d, J = 7.4 Hz, aromatics), 7.76 (t, J = 7.5 Hz, aromatics), 7.58–7.53 (m, aromatics), 7.36–7.32 (m, aromatics), 6.82 (s, NH), 6.12 (2 × pseudo t, J = 9.6 Hz, 2 × H-3’), 5.74 (d, J = 9.7 Hz, thiazolinone H-2’), 5.30 (d, J = 9.7 Hz, thiazolidinone H-2’), 5.25 (pseudo t, J = 9.9 Hz, thiazolinone H-4’), 5.15 (pseudo t, J = 9.9 Hz, thiazolidinone H-4’), 4.87 (ddd, J = 10.3, 4.1, 2.1 Hz, thiazolinone H-5’), 4.73 (ddd, J = 10.3, 5.1, 2.2 Hz, thiazolidinone H-5’), 4.27 (dd, J = 12.8, 4.2 Hz, thiazolinone H-6’a), 4.21 (dd, J = 12.4, 5.1 Hz, thiazolidinone H-6’a), 4.13 (dd, J = 12.4, 2.2 Hz, thiazolidinone H-6’b), 4.08 (dd, J = 12.7, 2.0 Hz, thiazolinone H-6’b), 2.08, 2.08, 2.07, 2.05, 2.00, 2.00, 1.97, 1.93 (8 s, 8 × CH3). ESI-MS positive mode (m/z): calcd for compound 15 C22H23NNaO10S ([M + Na]+): 516.093, found: 516.091; and calcd for compound 23 C22H25NNaO11S ([M + Na]+): 534.104, found: 534.103.
4.3. Computational Methods
Mixed torsional/low-frequency mode conformational searches were carried out by means of the Macromodel 10.8.011 software [39] using OPLS-2005 (Optimized Potential for Liquid Simulations) Force Field [40,41] with the implicit solvent model for CHCl3 applying a 21 kJ/mol energy window yielding 53–151 conformers. Geometry optimizations [B3LYP/6-31G(d) in vacuo and ωB97XD/TZVP [35,38] with a PCM solvent model for MeCN] and TDDFT calculations were performed with Gaussian 09 using various functionals (B3LYP, BH&HLYP, CAM-B3LYP and PBE0) and TZVP basis set [42]. ECD spectra were generated as the sum of Gaussians with 3000 cm−1 half-height width (corresponding to ca. 15 nm at 220 nm), using dipole-velocity computed rotational strengths [43]. Boltzmann distributions were estimated from the ZPVE-corrected B3LYP/6-31G(d) energies in the gas-phase calculations and from the ωB97XD/TZVP energies in the solvated ones. The MOLEKEL software package was used for visualization of the results [44].
Supplementary Materials
The following are available online. Copies of 1H and 13C spectra of new compounds; depictions of low energy conformers of (1’R)-15, (1’S)-15, (2R,1’R)-21, (2S,1’R)-21 (Figures S1–S4).
Acknowledgments
This work was supported by the National Research, Development and Innovation Office of Hungary (NKFIH K109450, K120181, PD121020 and PD121406). The Governmental Information-Technology Development Agency (KIFÜ) is thanked for CPU time.
Author Contributions
K.E.S. carried out the experiments; S.K. designed and carried out experiments; A.M. recorded the ECD spectra and performed the calculations; T.K. evaluated the ECD spectra and wrote the paper; L.S. conceived the research and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Somsák, L. Glucose derived inhibitors of glycogen phosphorylase. Compt. Rend. Chim. 2011, 14, 211–223. [Google Scholar] [CrossRef]
- Tite, T.; Tomas, L.; Docsa, T.; Gergely, P.; Kovensky, J.; Gueyrard, D.; Wadouachi, A. Synthesis of N-aryl spiro-sulfamides as potential glycogen phosphorylase inhibitors. Tetrahedron Lett. 2012, 53, 959–961. [Google Scholar] [CrossRef]
- Czifrák, K.; Páhi, A.; Deák, S.; Kiss-Szikszai, A.; Kövér, K.E.; Docsa, T.; Gergely, P.; Alexacou, K.-M.; Papakonstantinou, M.; Leonidas, D.D.; et al. Glucopyranosylidene-spiro-iminothiazolidinone, a New Bicyclic Ring System: Synthesis, Derivatization, and Evaluation for Inhibition of Glycogen Phosphorylase by Enzyme Kinetic and Crystallographic Methods. Bioorg. Med. Chem. 2014, 22, 4028–4041. [Google Scholar] [CrossRef] [PubMed]
- Docsa, T.; Marics, B.; Németh, J.; Hüse, C.; Somsák, L.; Gergely, P.; Peitl, B. Insulin sensitivity is modified by a glycogen phosphorylase inhibitor: glucopyranosylidene-spiro-thiohydantoin in streptozotocin-induced diabetic rats. Curr. Top. Med. Chem. 2015, 15, 2390–2394. [Google Scholar] [CrossRef] [PubMed]
- Goyard, D.; Kónya, B.; Chajistamatiou, A.S.; Chrysina, E.D.; Leroy, J.; Balzarin, S.; Tournier, M.; Tousch, D.; Petit, P.; Duret, C.; et al. Glucose-derived spiro-isoxazolines are anti-hyperglycemic agents against type 2 diabetes through glycogen phosphorylase inhibition. Eur. J. Med. Chem. 2016, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Donnier-Maréchal, M.; Vidal, S. Glycogen phosphorylase inhibitors: A patent review (2013–2015). Expert Opin. Ther. Patents 2016, 26, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Tracey, W.; Treadway, J.; Magee, W.; McPherson, R.; Levy, C.; Wilder, D.; Li, Y.; Yue, C.; Zavadoski, W.; Gibbs, E.; et al. A novel glycogen phosphorylase inhibitor, CP-368296, reduces myocardial ischemic injury. Diabetes 2003, 52, A135. [Google Scholar]
- Tracey, W.R.; Treadway, J.L.; Magee, W.P.; Sutt, J.C.; McPherson, R.K.; Levy, C.B.; Wilder, D.E.; Yu, L.J.; Chen, Y.; Shanker, R.M.; Mutchler, A.K.; et al. Cardioprotective effects of ingliforib, a novel glycogen phosphorylase inhibitor. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1177–H1184. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, H. Pharmacological manipulation of brain glycogenolysis as a therapeutic approach to cerebral ischemia. Mini Rev. Med. Chem. 2010, 10, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Qian, Y.S.; Tang, X.Z.; Huang, M.H.; Huang, L.F.; Li, Y.M.; Sun, H.B. Maslinic Acid, a Natural Inhibitor of Glycogen Phosphorylase, Reduces Cerebral Ischemic Injury in Hyperglycemic Rats by GLT-1 Up-Regulation. J. Neurosci. Res. 2011, 89, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Schnier, J.B.; Nishi, K.; Monks, A.; Gorin, F.A.; Bradbury, E.M. Inhibition of glycogen phosphorylase (GP) by CP-91,149 induces growth inhibition correlating with brain GP expression. Biochem. Biophys. Res. Commun. 2003, 309, 126–134. [Google Scholar] [CrossRef]
- Geschwind, J.-F.; Georgiades, C.S.; Ko, Y.H.; Pedersen, P.L. Recently elucidated energy catabolism pathways provide opportunities for novel treatments in hepatocellular carcinoma. Expert Rev. Anticanc. Ther. 2004, 4, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Go, V.L.W.; Lee, W.-N.P. Glycogen phosphorylase inhibitor CP-320626 inhibits pancreatic cancer cell proliferation via inhibiting pentose cycle metabolism and glycolysis. Pancreas 2003, 27, 368–420. [Google Scholar]
- Favaro, E.; Bensaad, K.; Chong, M.G.; Tennant, D.A.; Ferguson, D.J.P.; Snell, C.; Steers, G.; Turley, H.; Li, J.-L.; Günther, U.L.; et al. Glucose Utilization via Glycogen Phosphorylase Sustains Proliferation and Prevents Premature Senescence in Cancer Cells. Cell Metab. 2012, 16, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Zois, C.E.; Favaro, E.; Harris, A.L. Glycogen metabolism in cancer. Biochem. Pharmacol. 2014, 92, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Bichard, C.J.F.; Mitchell, E.P.; Wormald, M.R.; Watson, K.A.; Johnson, L.N.; Zographos, S.E.; Koutra, D.D.; Oikonomakos, N.G.; Fleet, G.W.J. Potent Inhibition of Glycogen Phosphorylase by a Spirohydantoin of Glucopyranose: First Pyranose Analogues of Hydantocidin. Tetrahedron Lett. 1995, 36, 2145–2148. [Google Scholar] [CrossRef]
- Ősz, E.; Somsák, L.; Szilágyi, L.; Kovács, L.; Docsa, T.; Tóth, B.; Gergely, P. Efficient inhibition of muscle and liver glycogen phosphorylases by a new glucopyranosylidene-spiro-thiohydantoin. Bioorg. Med. Chem. Lett. 1999, 9, 1385–1390. [Google Scholar] [CrossRef]
- Somsák, L.; Kovács, L.; Tóth, M.; Ősz, E.; Szilágyi, L.; Györgydeák, Z.; Dinya, Z.; Docsa, T.; Tóth, B.; Gergely, P. Synthesis of and a Comparative Study on the Inhibition of Muscle and Liver Glycogen Phosphorylases by Epimeric Pairs of d-Gluco- and d-Xylopyranosylidene-spiro-(thio)hydantoins and N-(d-Glucopyranosyl) Amides. J. Med. Chem. 2001, 44, 2843–2848. [Google Scholar] [CrossRef] [PubMed]
- Somsák, L.; Nagy, V.; Vidal, S.; Czifrák, K.; Berzsényi, E.; Praly, J.-P. Novel design principle validated: glucopyranosylidene-spiro-oxathiazole as new nanomolar inhibitor of glycogen phosphorylase, potential antidiabetic agent. Bioorg. Med. Chem. Lett. 2008, 18, 5680–5683. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Benltifa, M.; Vidal, S.; Berzsényi, E.; Teilhet, C.; Czifrák, K.; Batta, G.; Docsa, T.; Gergely, P.; Somsák, L.; et al. Glucose-based spiro-heterocycles as potent inhibitors of glycogen phosphorylase. Bioorg. Med. Chem. 2009, 17, 5696–5707. [Google Scholar] [CrossRef] [PubMed]
- Benltifa, M.; Hayes, J.M.; Vidal, S.; Gueyrard, D.; Goekjian, P.G.; Praly, J.-P.; Kizilis, G.; Tiraidis, C.; Alexacou, K.-M.; Chrysina, E.D.; et al. Glucose-based Spiro-isoxazolines: A New Family of Potent Glycogen Phosphorylase Inhibitors. Bioorg. Med. Chem. 2009, 17, 7368–7380. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, M.; Noble, M.E.M.; Watson, K.A.; Garman, E.F.; Krülle, T.M.; Fuente, C.; Fleet, G.W.J.; Oikonomakos, N.G.; Johnson, L.N. The structure of a glycogen phosphorylase glucopyranose spirohydantoin complex at 1.8 A resolution and 100 K: The role of the water structure and its contribution to the binding. Protein Sci. 1998, 7, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Oikonomakos, N.G.; Skamnaki, V.T.; Ősz, E.; Szilágyi, L.; Somsák, L.; Docsa, T.; Tóth, B.; Gergely, P. Kinetic and crystallographic studies of glucopyranosylidene spirothiohydantoin binding to glycogen phosphorylase b. Bioorg. Med. Chem. 2002, 10, 261–268. [Google Scholar] [CrossRef]
- Nagy, V.; Czifrák, K.; Bényei, A.; Somsák, L. Synthesis of some O-, S-, and N-glycosides of hept-2-ulopyranosonamides. Carbohydr. Res. 2009, 344, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Páhi, A.; Czifrák, K.; Kövér, K.E.; Somsák, L. Anomeric Spirocycles by Solvent Incorporation: Reactions of O-Peracylated (Glyculopyranose and Glyculopyranosyl Bromide)onamide Derivatives with Ketones. Carbohydr. Res. 2015, 403, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kun, S.; Deák, S.; Czifrák, K.; Juhász, L.; Somsák, L.; Apelt, O. Preparation of 2,6-anhydro-hept-2-enonic acid derivatives and their 3-deoxy counterparts. In Carbohydrate Chemistry: Proven Synthetic Methods; Vogel, C., Murphy, P.V., Eds.; CRC Press: Boca Raton, FL, USA, 2017; Volume 4, pp. 79–90. [Google Scholar]
- Somsák, L.; Nagy, V. A new, scalable preparation of a glucopyranosylidene-spiro-thiohydantoin: one of the best inhibitors of glycogen phosphorylases. Tetrahedron Asymm. 2000, 11, 1719–1727. [Google Scholar] [CrossRef]
- Wuts, P.G.M.; Greene, T.W. Greene’s Protective Groups in Organic Synthesis, 4th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Myers, R.W.; Lee, Y.C. Synthesis of diazomethyl β-d-galactopyranosyl and β-d-glucopyranosyl ketones. Potential affinity-labeling reagents for carbohydrate-binding proteins. Carbohydr. Res. 1986, 152, 143–158. [Google Scholar] [CrossRef]
- Somsák, L.; Ferrier, R.J. Radical-mediated Brominations at Ring Positions of Carbohydrates. Adv. Carbohydr. Chem. Biochem. 1991, 49, 37–92. [Google Scholar] [CrossRef] [PubMed]
- Somsák, L.; Czifrák, K. Radical-mediated brominations at ring-positions of carbohydrates–35 years later. Carbohydr. Chem. 2013, 39, 1–37. [Google Scholar] [CrossRef]
- Rudnichenko, A.V.; Timoshenko, V.M.; Shermolovich, Y.G. Cycloaddition reactions of polyfluoroalkylthioncarboxylic acid derivatives with dimethyl acetylenedicarboxylate (DMAD). J. Fluorine Chem. 2004, 125, 439–444. [Google Scholar] [CrossRef]
- Rudnichenko, A.V.; Timoshenko, V.M.; Chernega, A.N.; Nesterenko, A.M.; Shermolovich, Y.G. Addition reactions of 2-polyfluoroalkyl substituted 1,3-thiazolin-4-one derivatives. J. Fluorine Chem. 2004, 125, 1351–1356. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Xu, D.-X.; Mándi, A.; Kurtán, T.; Li, T.-J.; Schulz, B.; Zhang, W. Structure, Absolute Configuration, and Conformational Study of 12-Membered Macrolides from the Fungus Dendrodochium sp. Associated with the Sea Cucumber Holothuria nobilis Selenka. J. Org. Chem. 2013, 78, 7030–7047. [Google Scholar] [CrossRef] [PubMed]
- Nicu, V.P.; Mándi, A.; Kurtán, T.; Polavarapu, P.L. On the Complementarity of ECD and VCD Techniques. Chirality 2014, 26, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger LLC. MacroModel 10.8.011; Schrödinger LLC: New York, NY, USA, 2015. [Google Scholar]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.L.; Beard, H.S.; Cao, Y.; Cho, A.E.; Damm, W.; Farid, R.; Felts, A.K.; Halgren, T.A.; Mainz, D.T.; Maple, J.R.; et al. Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comput. Chem. 2005, 26, 1752–1780. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision E.01; Gaussian, Inc.: Wallingford, CT, USA; Oxfordshire, UK, 2013. [Google Scholar]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Varetto, U. MOLEKEL; 5.4; Swiss National Supercomputing Centre: Manno, Switzerland, 2009. [Google Scholar]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).