Identification for the First Time of Cyclo(d-Pro-l-Leu) Produced by Bacillus amyloliquefaciens Y1 as a Nematocide for Control of Meloidogyne incognita
Abstract
:1. Introduction
2. Results
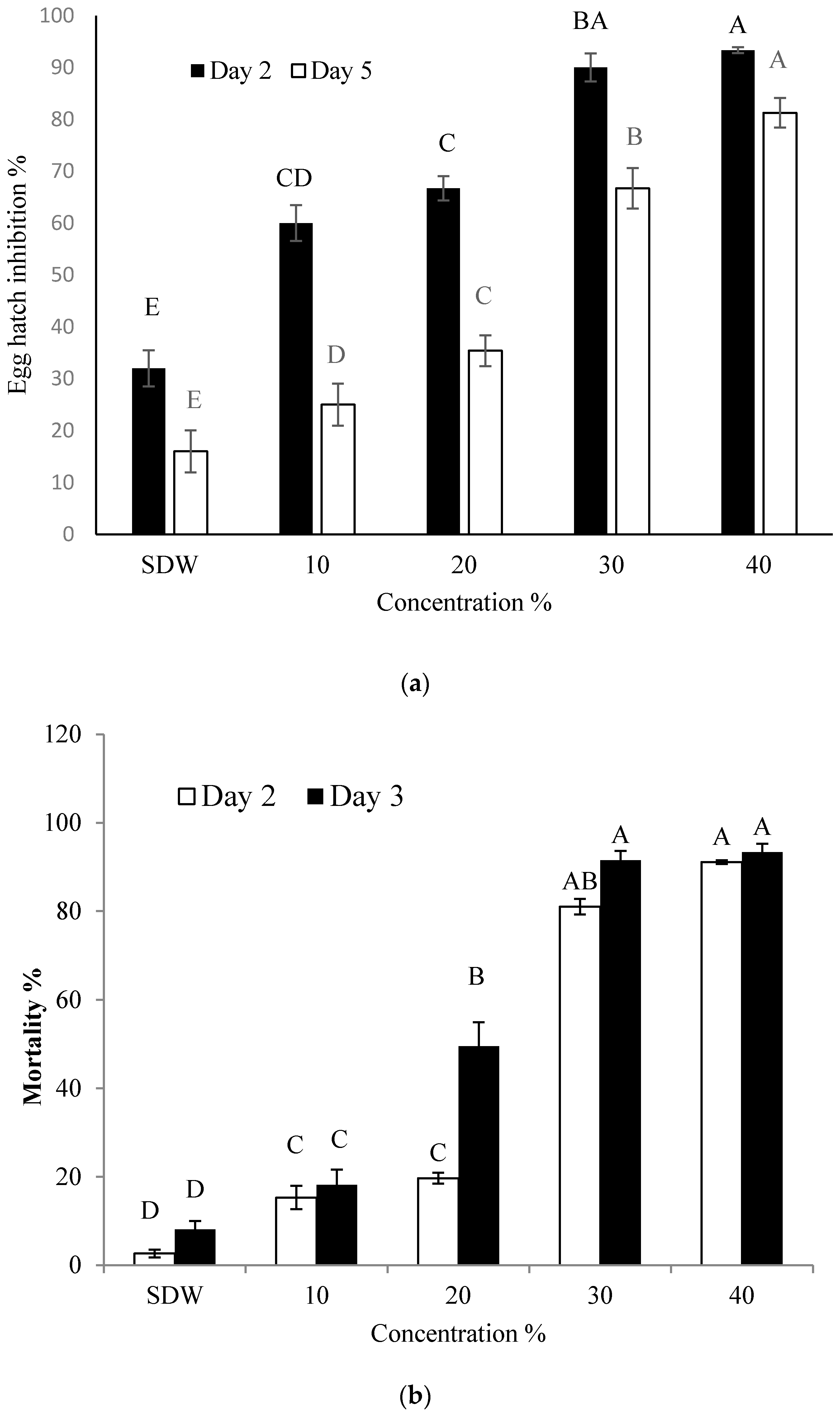
2.1. Effect of BCS on Hatching of Eggs and Mortality of J2 of M. incogita
2.2. Effect of Crude Extract on Hatching of Eggs and J2 Mortality of M. incognita
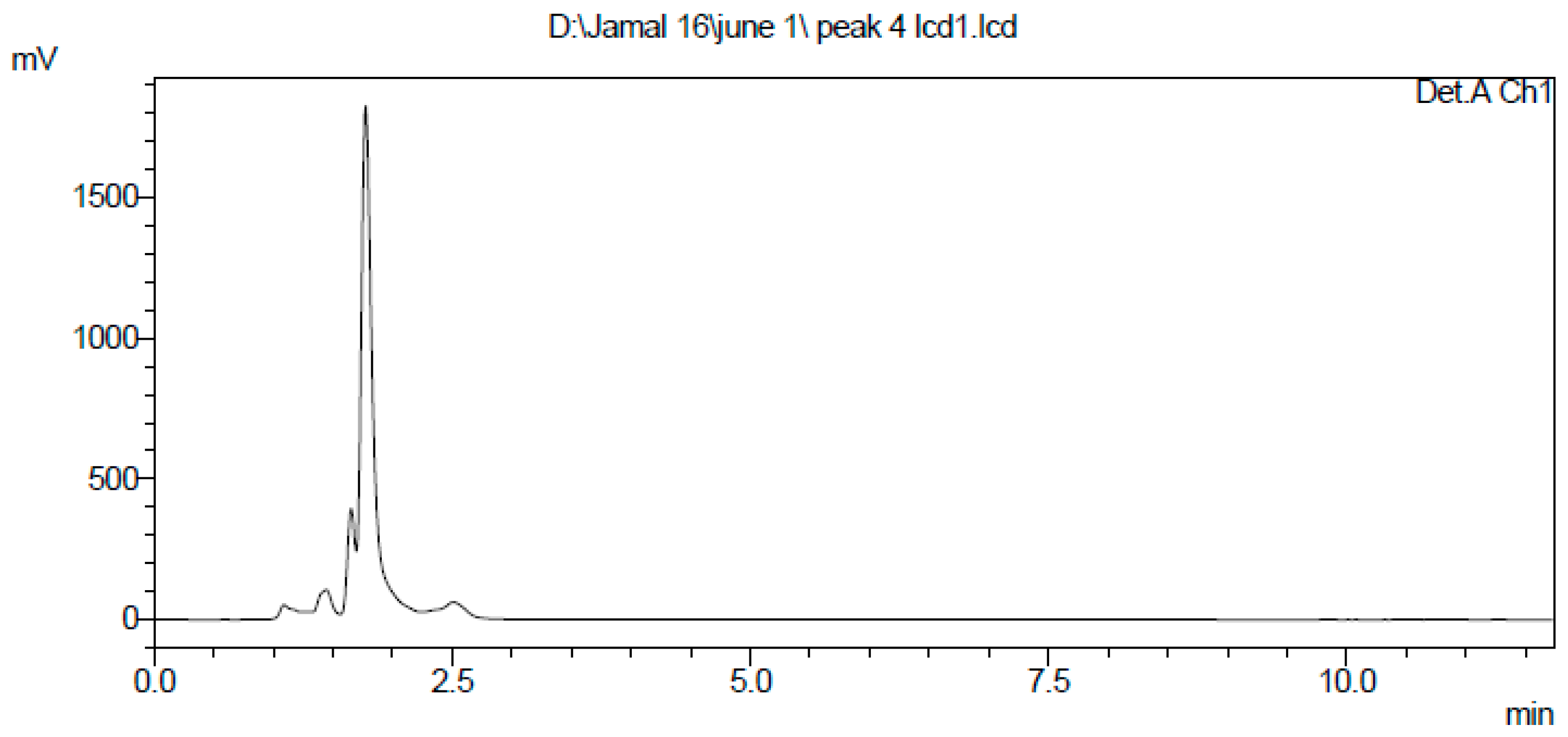
2.3. Extraction and Purification of Nematocidal Compound
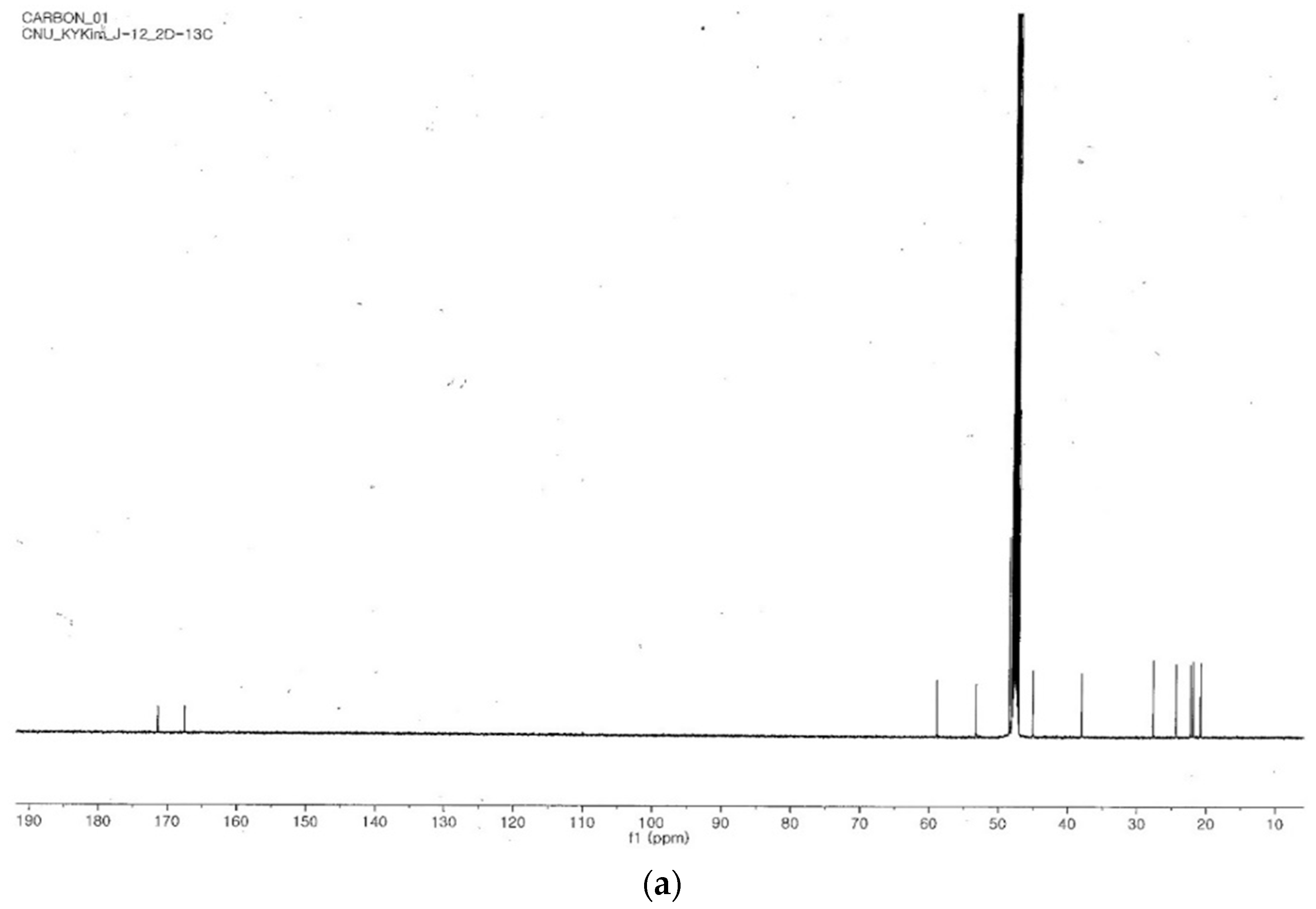
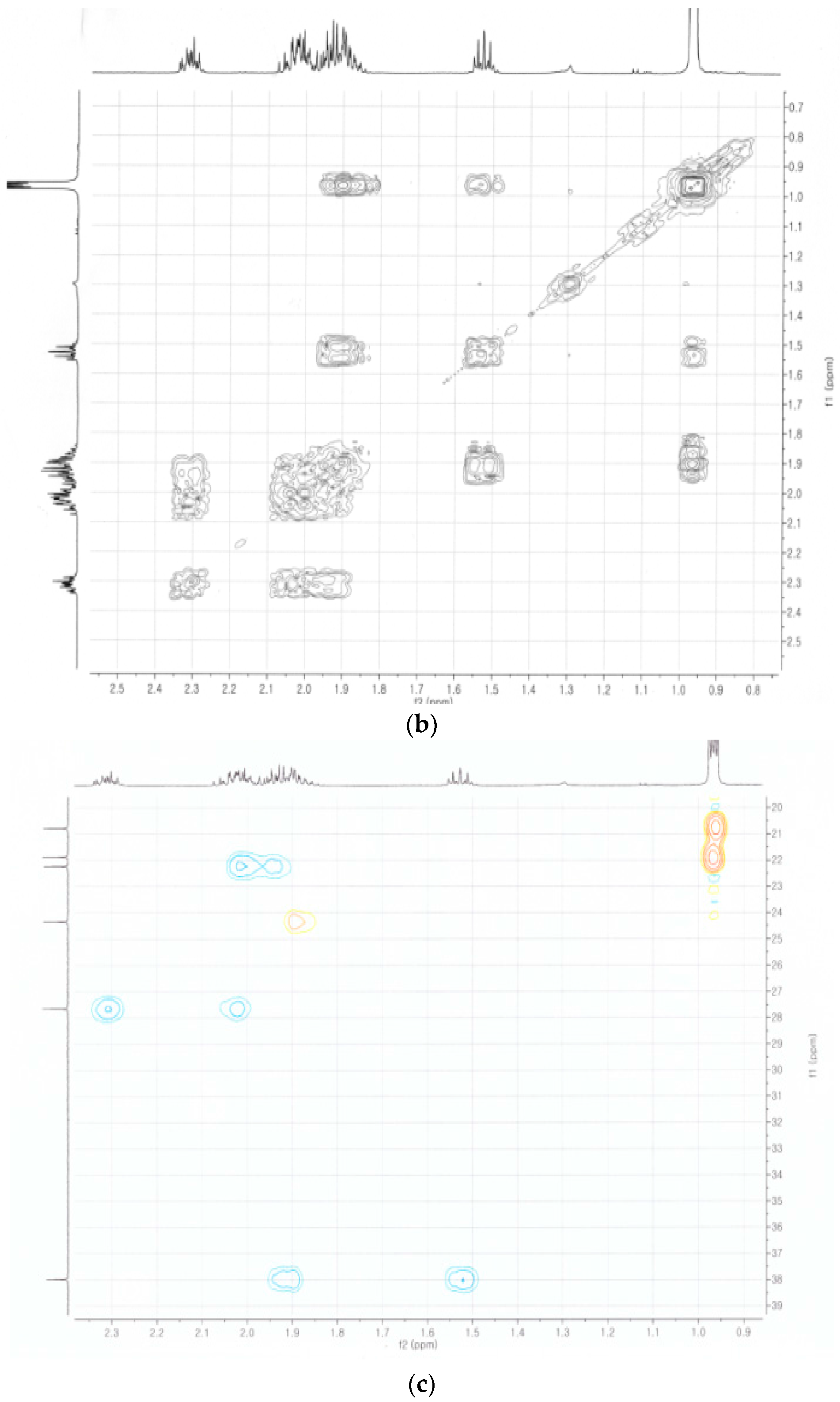
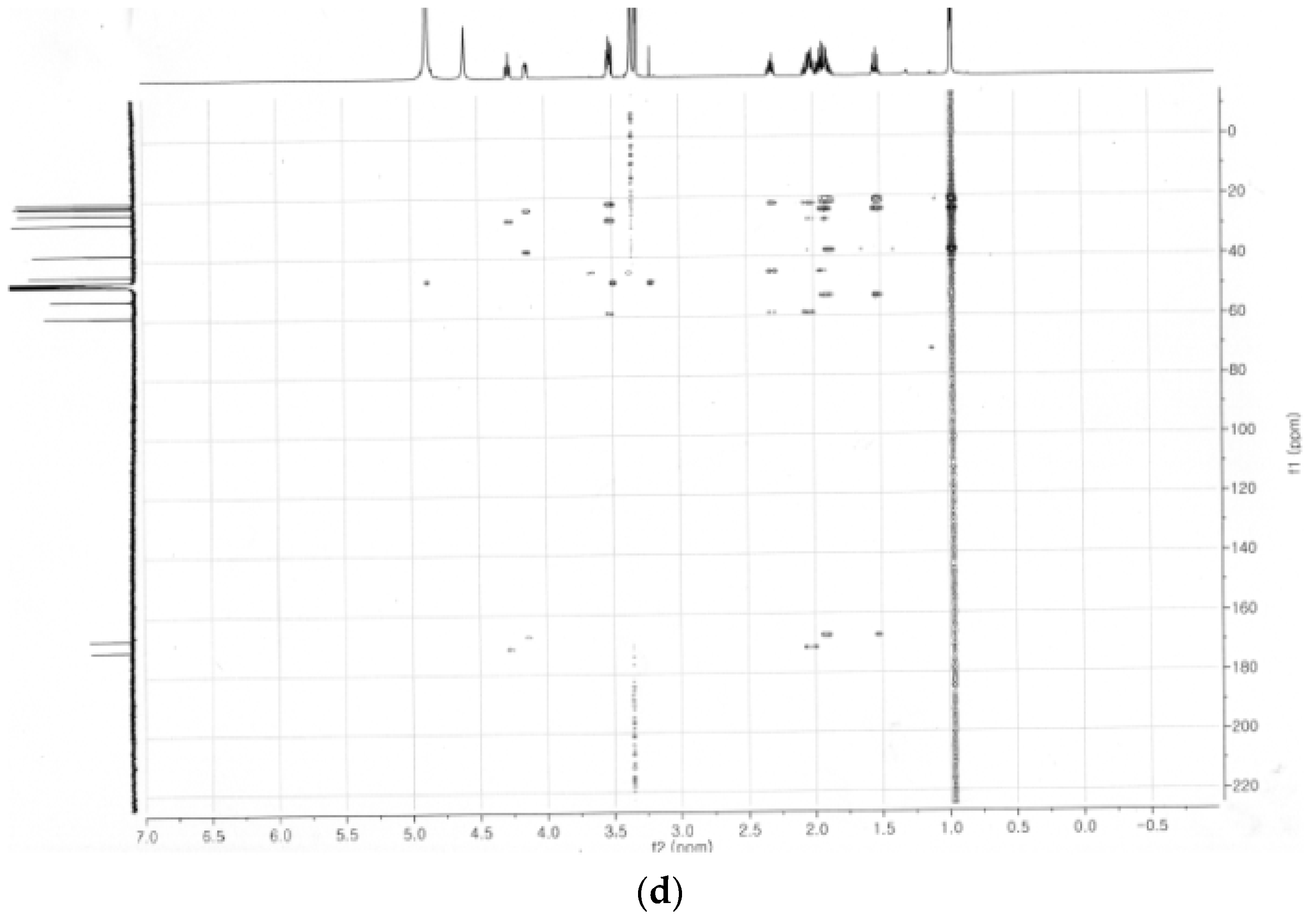
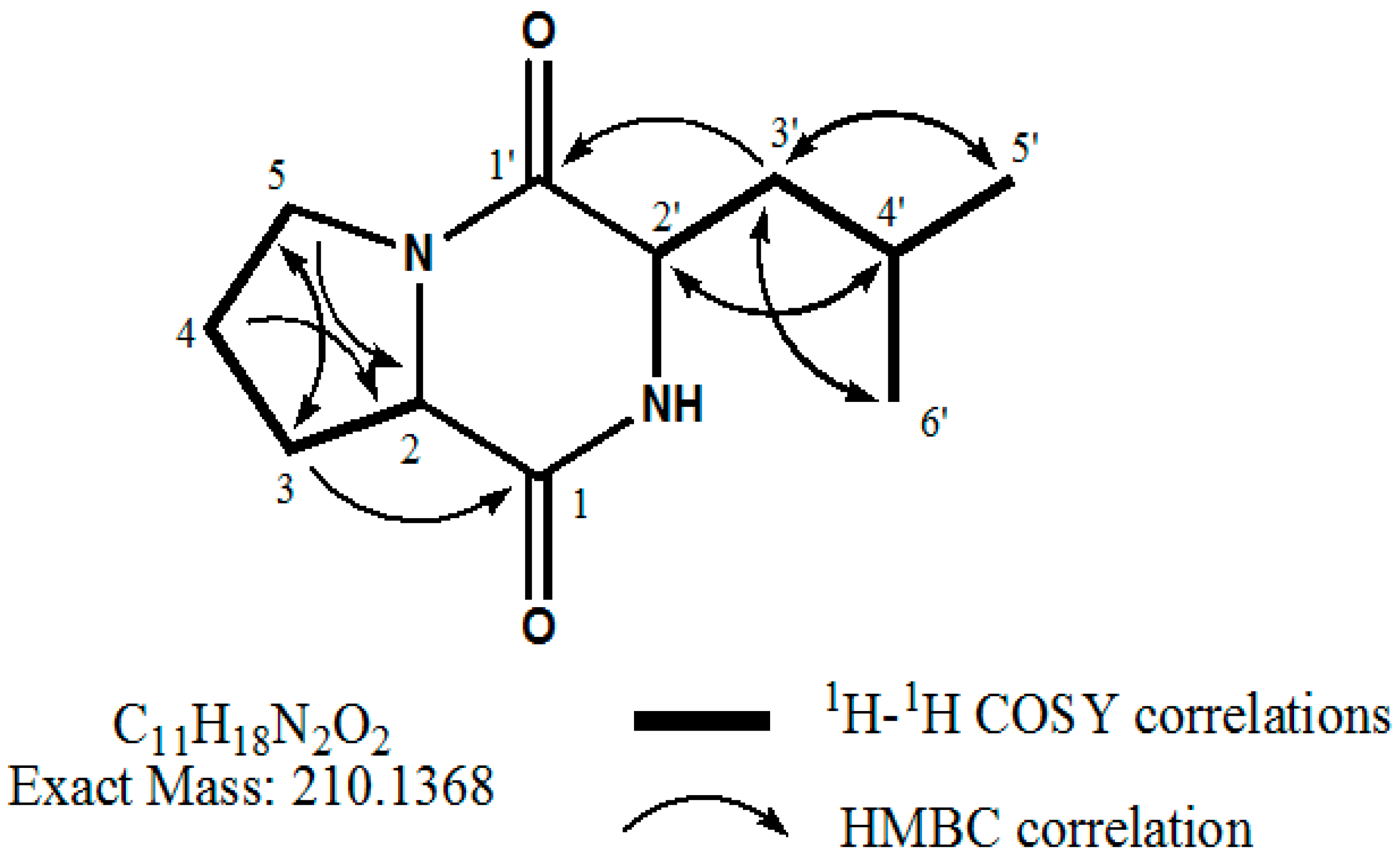
2.4. Identification of the Purified Nematocidal Compound
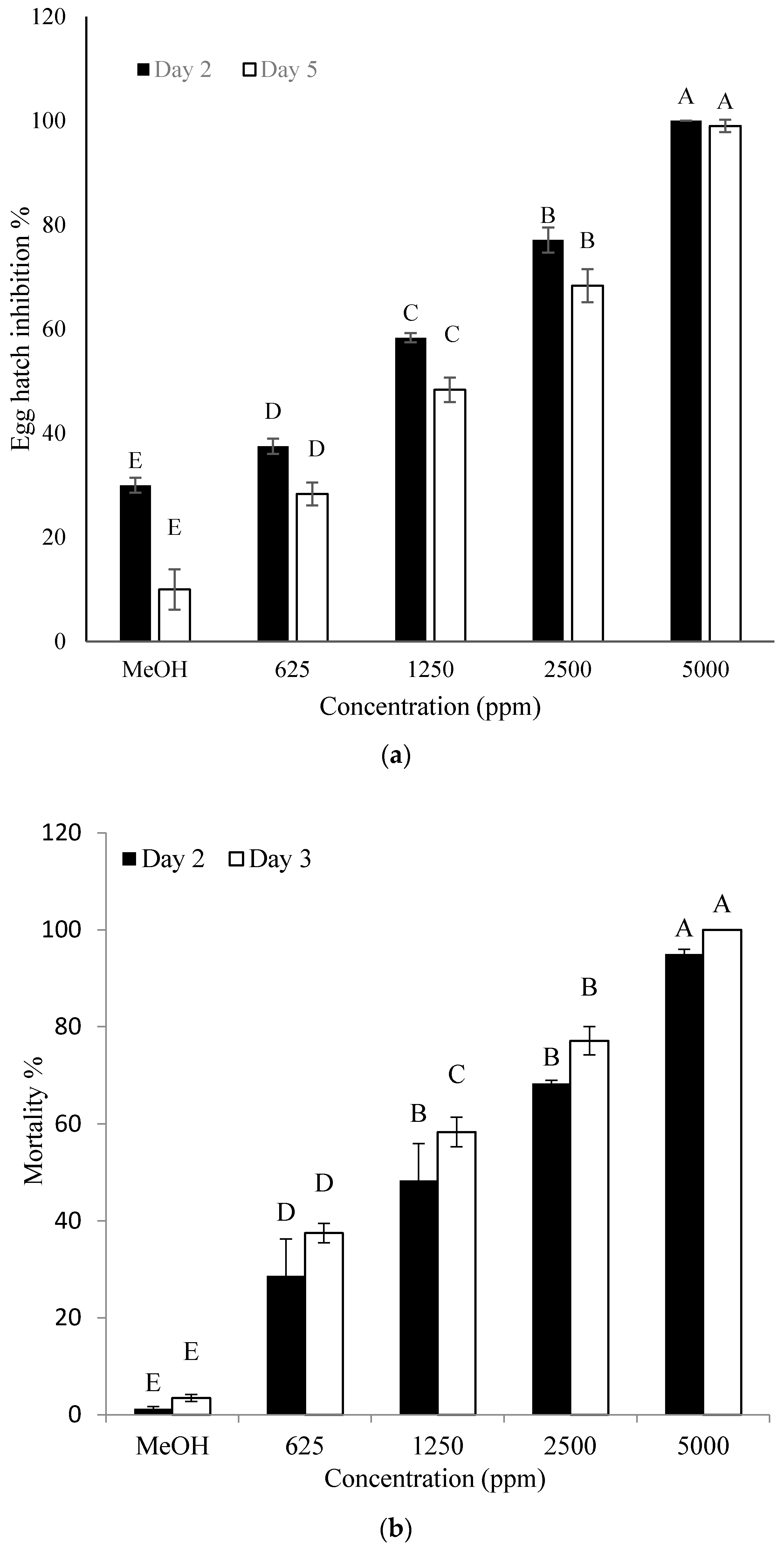
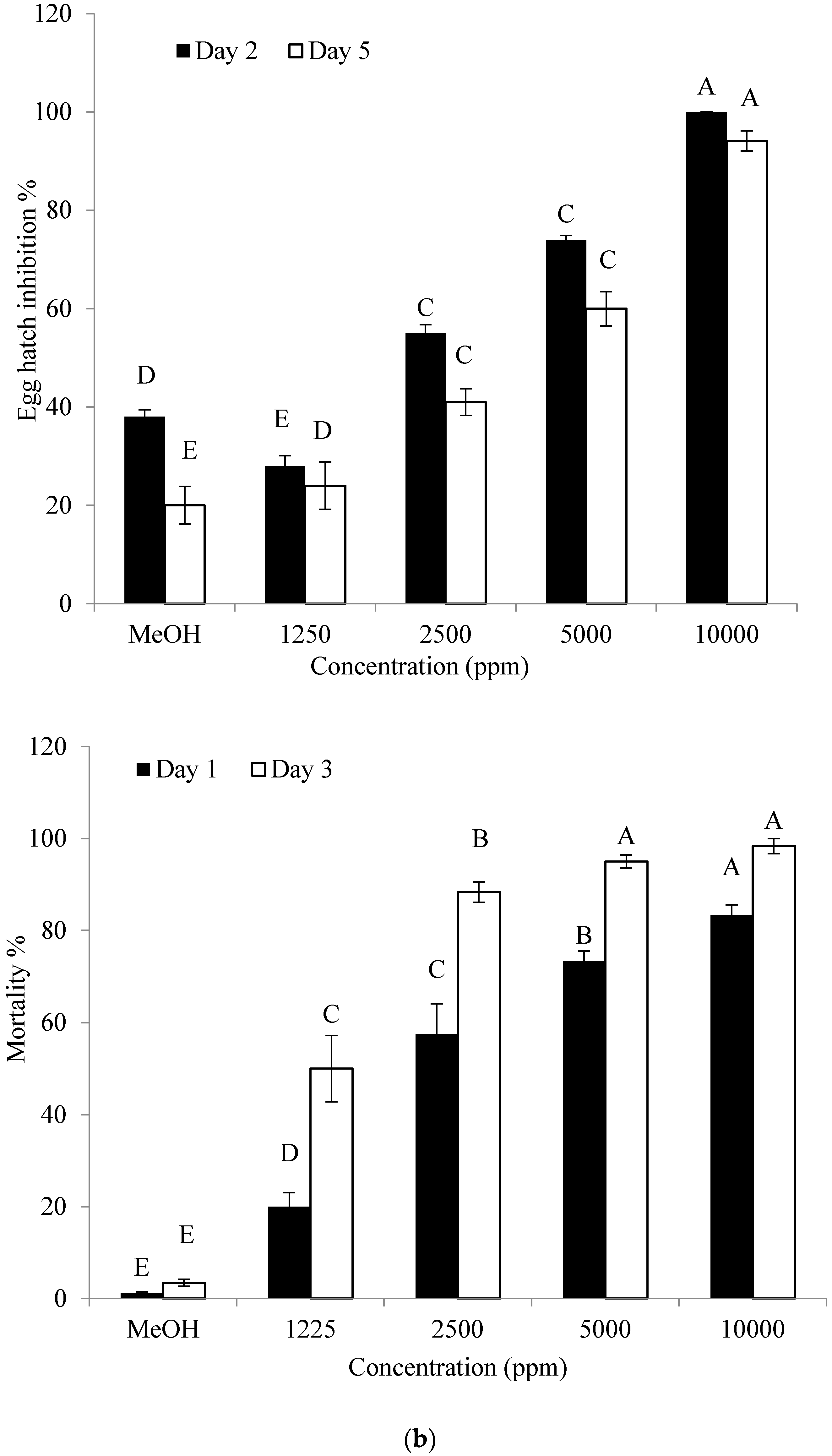
2.5. Effect of the Identified Compound on Hatching of Eggs and J2 Mortality of M. incognita
2.6. Effect of B. amyloliquefaciens Y1 on Incidence of M. incognita and Growth Promotion of Tomato in Pot Assays
3. Discussion
4. Materials and Methods
4.1. Test Microorganisms
4.2. Nematode Inoculum
4.3. Effect of Bacterial Culture Supernatant (BCS) on Hatching of Eggs and J2 Mortality of M. incognita
4.4. Effect of Crude Extract on Hatching of Eggs and J2 Mortality of M.incognita
4.5. Extraction and Purification of the Nematocidal Compound
4.6. Identification of the Purified Nematocidal Compound
4.7. Effect of the Identified Compound on Hatching of Eggs and J2 Mortality of M. incognita
4.8. Effect of B. amyloliquefaciens Y1 on Incidence of M. incognita and Growth Promotion of Tomato in Pot Assays
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species–A diverse group of novel and important plantparasites. Root-Knot Nematodes 2009, 1, 483. [Google Scholar]
- Nicol, J.M.; Turner, S.J.; Coyne, D.; Den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Berlin, Germany, 2011; pp. 21–43. [Google Scholar]
- Aeron, A.; Kumar, S.; Pandey, P.; Maheshwari, D. Emerging role of plant growth promoting rhizobacteria in agrobiology. In Bacteria in Agrobiology: Crop Ecosystems; Springer: Berlin, Germany, 2011; pp. 1–36. [Google Scholar]
- Sahebani, N.; Hadavi, N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol. Biochem. 2008, 40, 2016–2020. [Google Scholar] [CrossRef]
- Sikora, R.A.; Fernandez, E. 9 Nematode Parasites of Vegetables. In Plant Parasitic Nematodes in subtropIcal and Tropical Agriculture; CABI: Oxfordshire, UK, 2005; p. 319. [Google Scholar]
- Walters, S.A.; Wehner, T.C.; Barkel, K.R. Root-knot nematode resistance in cucumber and horned cucumber. HortScience 1993, 28, 151–154. [Google Scholar]
- Khan, Z.; Kim, S.; Jeon, Y.; Khan, H.; Son, S.; Kim, Y. A plant growth promoting rhizobacterium, Paenibacillus polymyxa strain GBR-1, suppresses root-knot nematode. Bioresour. Technol. 2008, 99, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Mahmood, I. Role of bacteria in the management of plant parasitic nematodes: A review. Bioresour. Technol. 1999, 69, 167–179. [Google Scholar] [CrossRef]
- McSpadden Gardener, B.B. Ecology of Bacillus and Paenibacillus spp. in agricultural systems. Phytopathology 2004, 94, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Asaka, O.; Shoda, M. Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis RB14. Appl. Environ. Microbiol. 1996, 62, 4081–4085. [Google Scholar] [PubMed]
- Stabb, E.V.; Jacobson, L.M.; Handelsman, J. Zwittermicin A-producing strains of Bacillus cereus from diverse soils. Appl. Environ. Microbiol. 1994, 60, 4404–4412. [Google Scholar] [PubMed]
- Dolej, S.; Bochow, H. Studies of the mode of action of Bacillus subtilis culture filtrates in the model pathosystem tomato seedling-Fusarium oxysporum f. sp. radicis-lycopersici. Mededelingen-Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen Universiteit Gent (Belgium) 1996, 61, 483–489. [Google Scholar]
- Nakano, M.M.; Zuber, P. Molecular biology of antibiotic production in Bacillus. Crit. Rev. Biotechnol. 1990, 10, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin-a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29–3. J. Antibiot. 1986, 39, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, K.Y. Antagonistic Potential of Bacillus pumilus L1 Against Root-Knot Nematode, Meloidogyne arenaria. J. Phytopathol. 2016, 164, 29–39. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Ma, L.; Zhang, K.; Duan, C.; Mo, M. Characterisation of volatiles produced from Bacillus megaterium YFM3. 25 and their nematocidal activity against Meloidogyne incognita. Eur. J. Plant Pathol. 2010, 126, 417–422. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Qureshi, S.A.; Sultana, V.; Ehteshamul-Haque, S.; Ghaffar, A. Biological control of root rot-root knot disease complex of tomato. Plant Soil 2000, 227, 163–169. [Google Scholar] [CrossRef]
- Xia, Y.; Xie, S.; Ma, X.; Wu, H.; Wang, X.; Gao, X. The purL gene of Bacillus subtilis is associated with nematocidal activity. FEMS Microbiol. Lett. 2011, 322, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.R.; Kiewnick, S.; Sikora, R.A. In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Sci. Technol. 2008, 18, 377–389. [Google Scholar] [CrossRef]
- Yu, Z.; Xiong, J.; Zhou, Q.; Luo, H.; Hu, S.; Xia, L.; Sun, M.; Li, L.; Yu, Z. The diverse nematocidal properties and biocontrol efficacy of Bacillus thuringiensis Cry6A against the root-knot nematode Meloidogyne hapla. J. Invertebr. Pathol. 2015, 125, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Qiuhong, N.; Xiaowei, H.; Baoyu, T.; Jinkui, Y.; Jiang, L.; Lin, Z.; Keqin, Z. Bacillus sp. B16 kills nematodes with a serine protease identified as a pathogenic factor. Appl. Microbiol. Biotechnol. 2006, 69, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Chet, I.; Spiegel, Y. Control of the rootknot nematode Meloidogyne javanica by Bacillus cereus. Biocontrol Sci. Technol. 1993, 3, 115–126. [Google Scholar] [CrossRef]
- Krebs, B.; Junge, H.; Ockhardt, A.; Hoding, B.; Heubner, D.; Erben, U. Bacillus subtilis-an effective biocontrol agent. Pesticide Sci. 1993, 37, 427–429. [Google Scholar] [CrossRef]
- Falcäo, L.; Silva-Werneck, J.; Vilarinho, B.; da Silva, J.; Pomella, A.; Marcellino, L. Antimicrobial and plant growth-promoting properties of the cacao endophyte Bacillus subtilis ALB629. J. Appl. Microbiol. 2014, 116, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Ryu, C.-M. Bacterial endophytes as elicitors of induced systemic resistance. In Microbial Root Endophytes; Springer: Berlin, Germany, 2006; pp. 33–52. [Google Scholar]
- Zhao, L.; Xu, Y.; Lai, X.-H.; Shan, C.; Deng, Z.; Ji, Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz. J. Microbiol. 2015, 46, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Jamal, Q.; Lee, Y.S.; Jeon, H.D.; Park, Y.S.; Kim, K.Y. Isolation and Biocontrol Potential of Bacillus amyloliquefaciens Y1 against Fungal Plant Pathogens. Korean J. Soil Sci. Fertil. 2015, 48, 485–491. [Google Scholar] [CrossRef]
- Quyen, V.T.; Ngan, T.B.; Mai Huong, D.T.; Bourguet Kondracki, M.L.; Longeon, A.; Murphy, B.M.; Cuong, P.V. Secondary metabolites from Micromonospora ectrinospora G017. Viet. J. Chem. 2015, 53, 1–10. [Google Scholar]
- Kavitha, P.; Jonathan, E.; Nakkeeran, S. Effects of crude antibiotic of Bacillus subtilis on hatching of eggs and mortality of juveniles of Meloidogyne incognita. Nematol. Medit. 2012, 40, 203–206. [Google Scholar]
- Lee, Y.S.; Park, Y.S.; Anees, M.; Kim, Y.C.; Kim, Y.H.; Kim, K.Y. Nematicidal activity of Lysobacter capsici YS1215 and the role of gelatinolytic proteins against root-knot nematodes. Biocontrol Sci. Technol. 2013, 23, 1427–1441. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an Antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Products 1996, 59, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C. Bioactive cyclic dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef]
- Degrassi, G.; Aguilar, C.; Bosco, M.; Zahariev, S.; Pongor, S.; Venturi, V. Plant growth-promoting Pseudomonas putida WCS358 produces and secretes four cyclic dipeptides: Cross-talk with quorum sensing bacterial sensors. Curr. Microbiol. 2002, 45, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.-H. Isolation and characterization of Streptomyces sp. KH-614 producing anti-VRE (vancomycin-resistant enterococci) antibiotics. J. Gen. Appl. Microbiol. 2002, 48, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.-S.; Song, Y.; Sakuno, E.; Nakajima, H.; Nakagawa, H.; Yabe, K. Cyclo (l-leucyl-l-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl. Environ. Microbiol. 2004, 70, 7466–7473. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, F.; Clarke, C.; Ryan, L.; Ulmer, H.; Schober, T.; Ström, K.; Sjögren, J.; Van Sinderen, D.; Schnürer, J.; Arendt, E. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Kumar, S.N.; Mohandas, C.; Nambisan, B. Purification of an antifungal compound, cyclo (l-Pro-d-Leu) for cereals produced by Bacillus cereus subsp. thuringiensis associated with entomopathogenic nematode. Microbiol. Res. 2013, 168, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Jamal, Q.; Cho, J.Y.; Moon, J.H.; Kim, K.Y. Purification and antifungal characterization of cyclo(d-Pro-l-Val) from Bacillus amyloliquefaciens Y1 against Fusarium graminearum to control head blight in wheat. Biocatal. Agric. Biotechnol. 2017, 10, 140–147. [Google Scholar] [CrossRef]
- Benitez, L.B.; Velho, R.V.; Lisboa, M.P.; da Costa Medina, L.F.; Brandelli, A. Isolation and characterization of antifungal peptides produced by Bacillus amyloliquefaciens LBM5006. J. Microbiol. 2010, 48, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Burkett-Cadena, M.; Kokalis-Burelle, N.; Lawrence, K.S.; Van Santen, E.; Kloepper, J.W. Suppressiveness of root-knot nematodes mediated by rhizobacteria. Biol. Control 2008, 47, 55–59. [Google Scholar] [CrossRef]
- Cladera-Olivera, F.; Caron, G.R.; Motta, A.S.; Souto, A.A.; Brandelli, A. Bacteriocin-like substance inhibits potato soft rot caused by Erwinia carotovora. Can. J. Microbiol. 2006, 52, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Sutyak, K.; Wirawan, R.; Aroutcheva, A.; Chikindas, M. Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J. Appl. Microbiol. 2008, 104, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Ramezani Moghaddam, M.; Mahdikhani Moghaddam, E.; Baghaee Ravari, S.; Rouhani, H. The nematocidal potential of local Bacillus species against the root-knot nematode infecting greenhouse tomatoes. Biocontrol Sci. Technol. 2014, 24, 279–290. [Google Scholar] [CrossRef]
- Insunza, V.; Alström, S.; Eriksson, K. Root bacteria from nematicidal plants and their biocontrol potential against trichodorid nematodes in potato. Plant Soil 2002, 241, 271–278. [Google Scholar] [CrossRef]
- Kong, Q.; Shan, S.; Liu, Q.; Wang, X.; Yu, F. Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int. J. Food Microbiol. 2010, 139, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Sopheareth, M.; Chan, S.; Naing, K.W.; Lee, Y.S.; Hyun, H.N.; Kim, Y.C.; Kim, K.Y. Biocontrol of Late Blight (Phytophthora capsici) disease and growth promotion of pepper by Burkholderia cepacia MPC-7. Plant Pathol. J. 2013, 29, 67–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
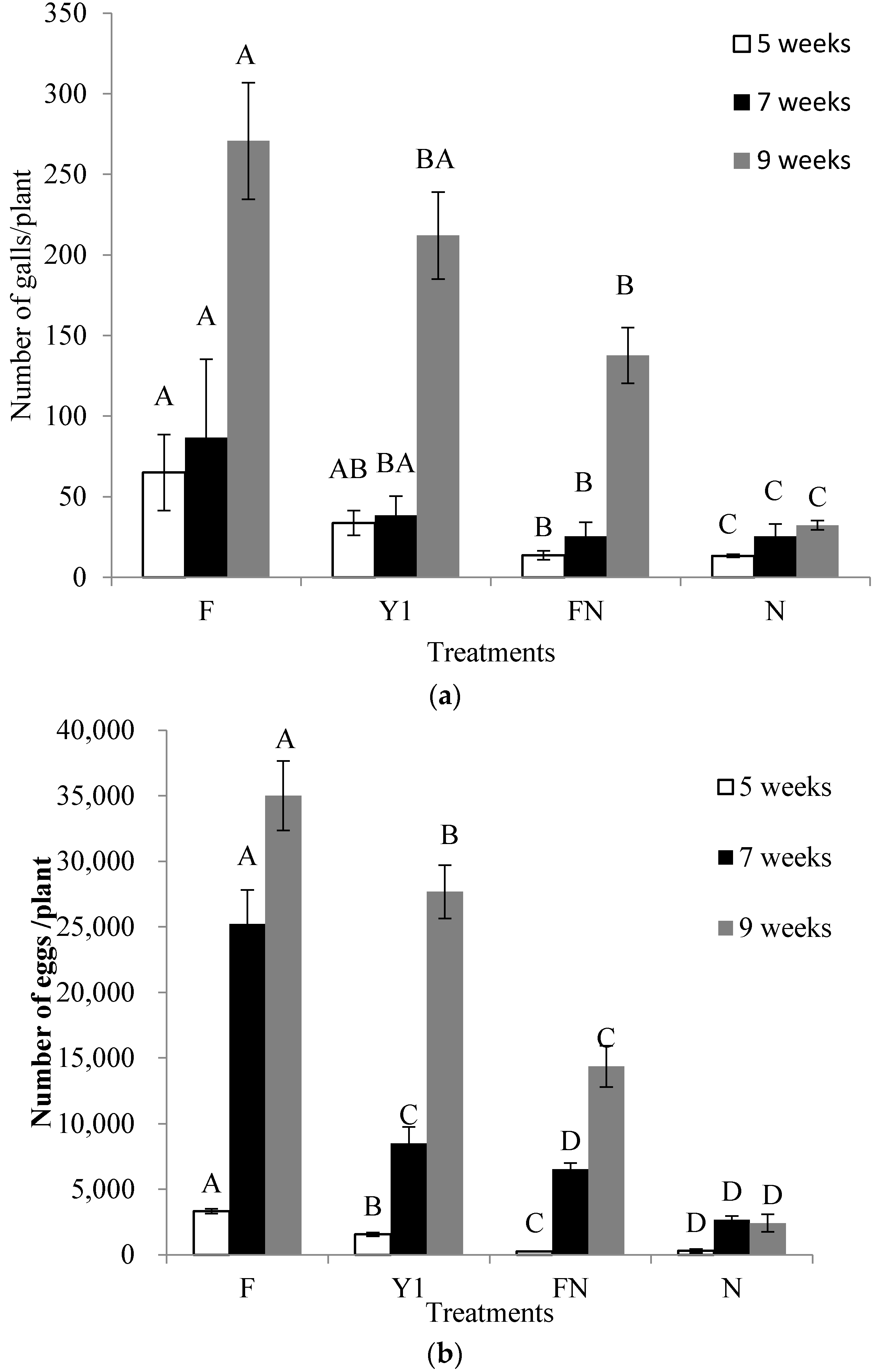
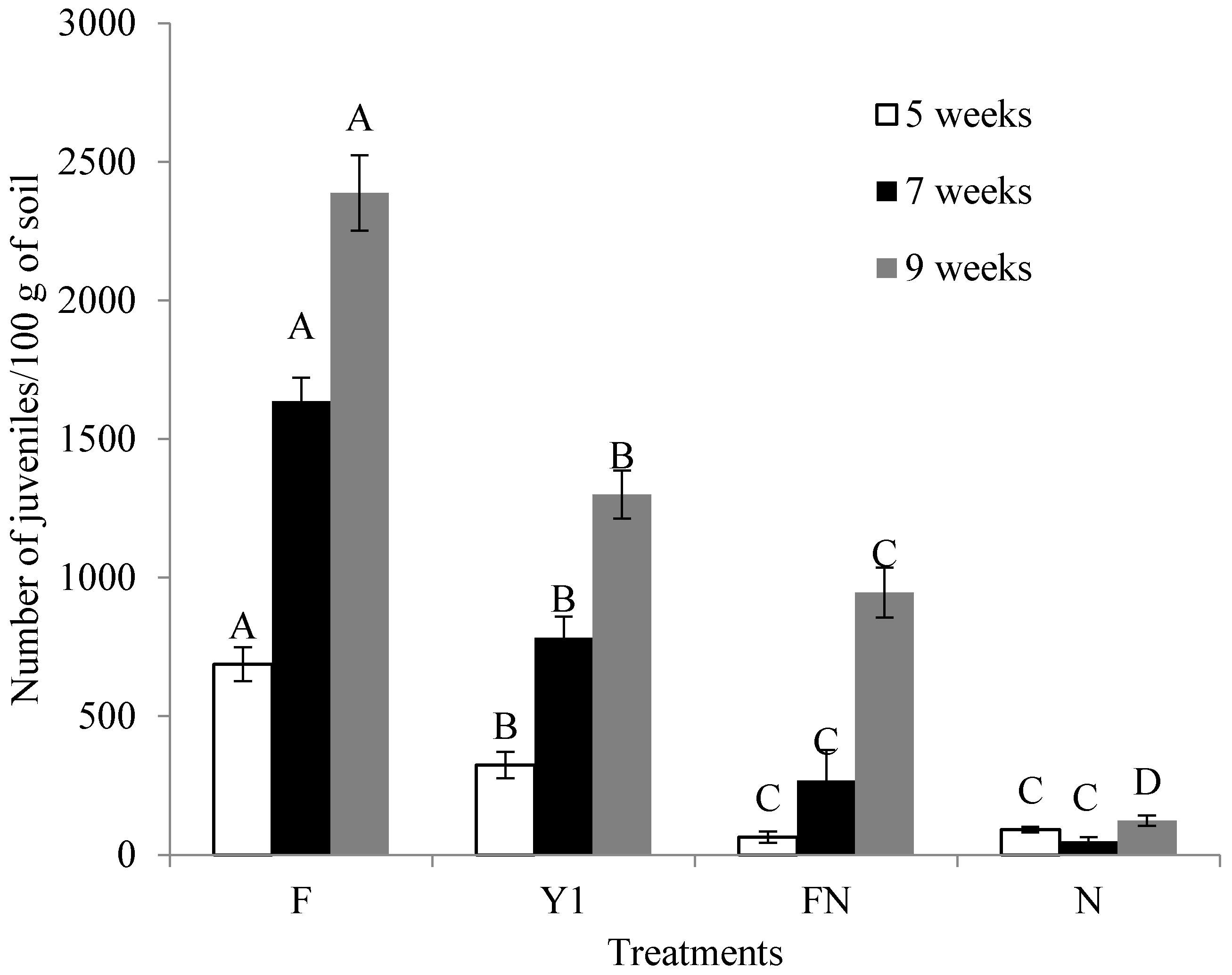
| Weeks after NI | Treatments | Plant Height (cm) | Shoot Fresh Weight (g) | Shoot Dry Weight (g) |
|---|---|---|---|---|
| 5 | F | 30.3 ± 0.8 b | 13.3 ± 0.9 bc | 1.5 ± 0.2 ba |
| Y1 | 35.6 ± 0.8 a | 17.2 ± 1.1 ba | 1.8 ± 0.1 ba | |
| FN | 35 ± 0.5 a | 18.6 ± 1.6 a | 2.1 ±0.1 a | |
| N | 28.6 ± 1.8 ba | 11.6 ± 1.8 c | 1.3 ± 0.3 b | |
| 7 | F | 34.1 ± 2.1 ba | 16.2 ± 1.4 bc | 1.4 ± 0.1 c |
| Y1 | 37.6 ± 0.3 a | 21.3 ± 2.4 a | 2.5 ± 0.1 a | |
| FN | 33.3 ± 1.2 ba | 17.4 ± 2.1 ba | 2.1 ± 0.1 b | |
| N | 28.6 ± 2.1 b | 13.4 ± 1.8 c | 1.3 ± 0.5 c | |
| 9 | F | 36.6 ± 1.2 ba | 21.6 ± 1.7 b | 2.4 ± 0.1 b |
| Y1 | 40.1 ± 1 a | 27.6 ± 1.3 a | 3.2 ± 0.1 a | |
| FN | 35.3 ± 1.5 b | 20.9 ± 2.1 b | 2.4 ± 0.1 b | |
| N | 34.3 ± 1.2 b | 15.1 ± 0.5 c | 1.3 ± 0.2 c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, Q.; Cho, J.-Y.; Moon, J.-H.; Munir, S.; Anees, M.; Kim, K.Y. Identification for the First Time of Cyclo(d-Pro-l-Leu) Produced by Bacillus amyloliquefaciens Y1 as a Nematocide for Control of Meloidogyne incognita. Molecules 2017, 22, 1839. https://doi.org/10.3390/molecules22111839
Jamal Q, Cho J-Y, Moon J-H, Munir S, Anees M, Kim KY. Identification for the First Time of Cyclo(d-Pro-l-Leu) Produced by Bacillus amyloliquefaciens Y1 as a Nematocide for Control of Meloidogyne incognita. Molecules. 2017; 22(11):1839. https://doi.org/10.3390/molecules22111839
Chicago/Turabian StyleJamal, Qaiser, Jeong-Yong Cho, Jae-Hak Moon, Shahzad Munir, Muhammad Anees, and Kil Yong Kim. 2017. "Identification for the First Time of Cyclo(d-Pro-l-Leu) Produced by Bacillus amyloliquefaciens Y1 as a Nematocide for Control of Meloidogyne incognita" Molecules 22, no. 11: 1839. https://doi.org/10.3390/molecules22111839
APA StyleJamal, Q., Cho, J.-Y., Moon, J.-H., Munir, S., Anees, M., & Kim, K. Y. (2017). Identification for the First Time of Cyclo(d-Pro-l-Leu) Produced by Bacillus amyloliquefaciens Y1 as a Nematocide for Control of Meloidogyne incognita. Molecules, 22(11), 1839. https://doi.org/10.3390/molecules22111839





