Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application
Abstract
1. Introduction
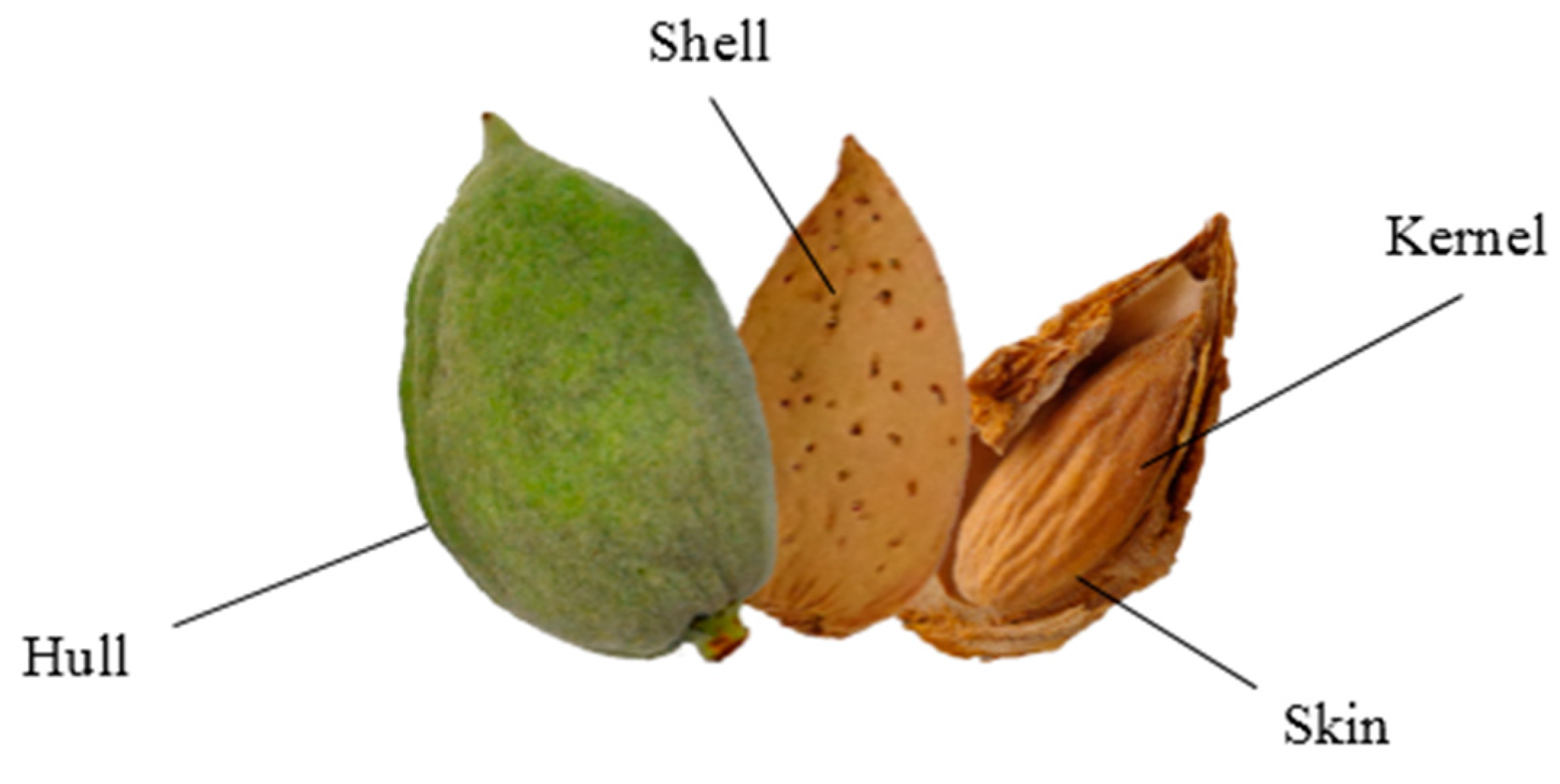
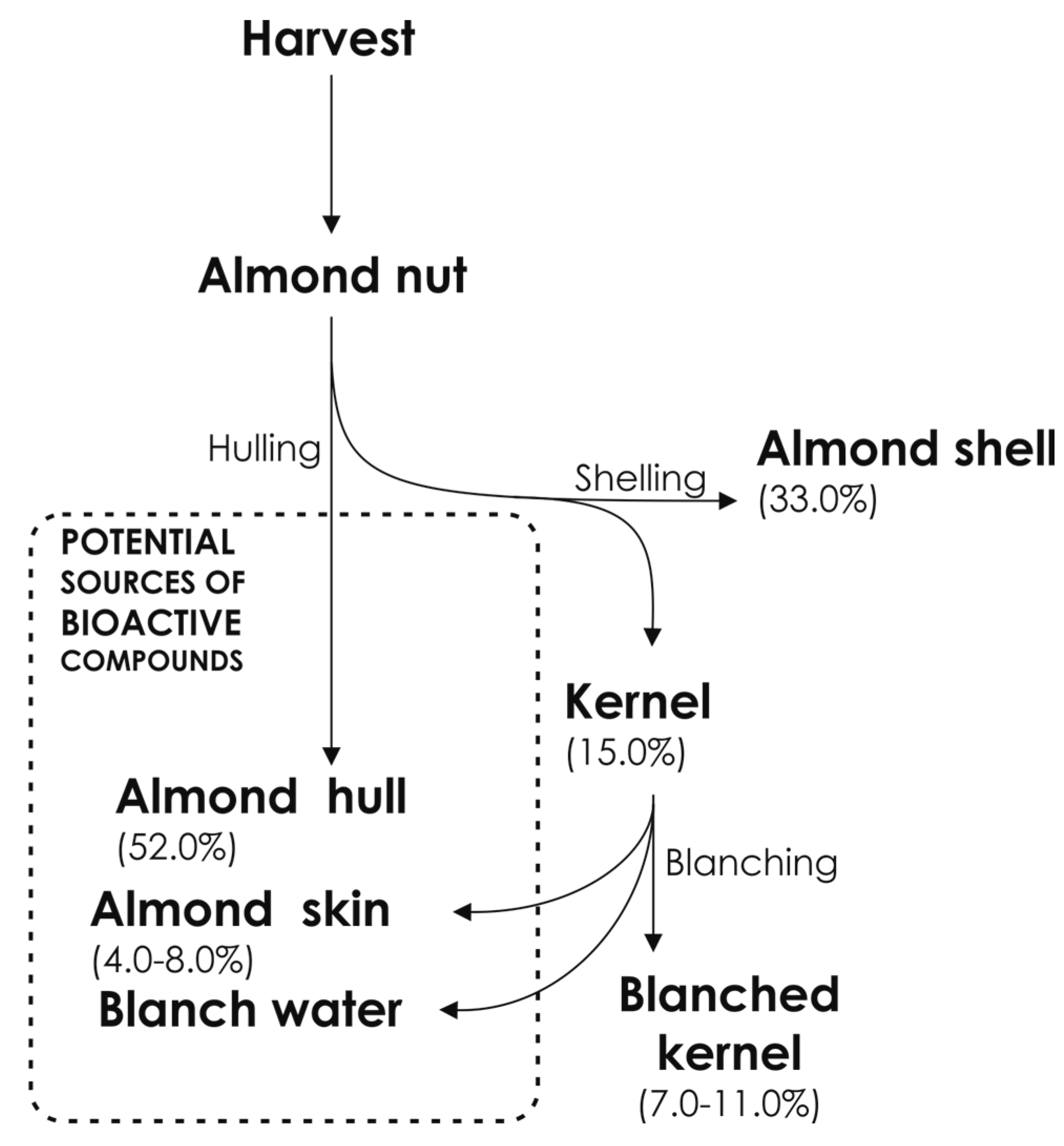
2. Almond By-Products
2.1. Almond Skin
2.2. Almond Shell
2.3. Almond Hull
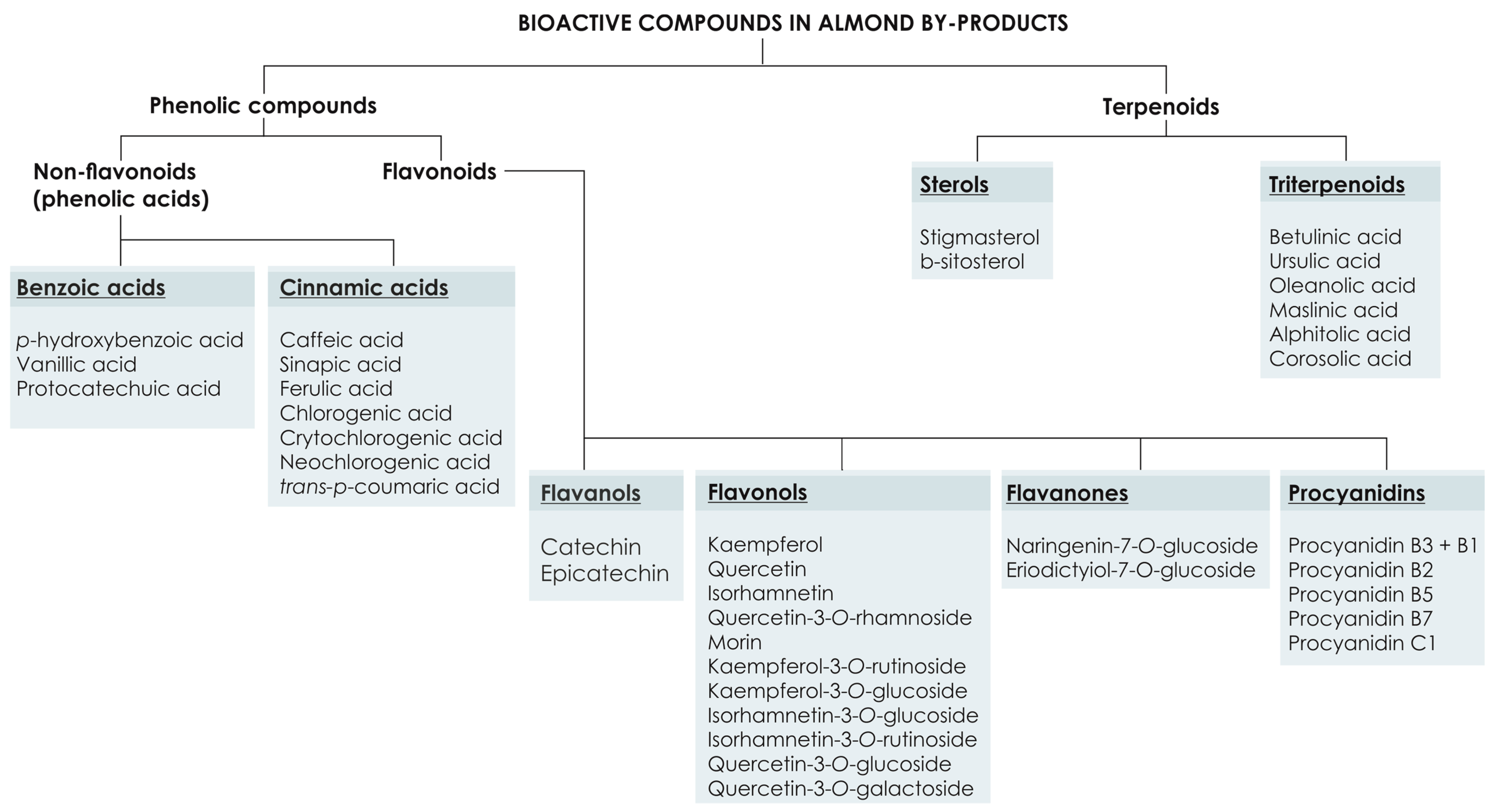
3. Phytochemical Composition of Almond By-Products and Their Radical Scavenging Power
3.1. Phenolic Compounds
3.1.1. Flavonoids
3.1.2. Phenolic Acids
3.2. Phenolic Composition of Almond Skin
3.3. Phenolic Composition of Almond Blanch Water
3.4. Phenolic Composition of Almond Hulls
4. Biological Activities of Almond By-Products
5. Functional Application of Almond By-Products
5.1. Almond Shell as Mulch
5.2. Almond Shell as Natural Colorant
5.3. Almond Shell and Hull as Growing Media
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Nut and Dried Fruit Council. World Tree Nut Production; International Nut and Dried Fruit Council: Reus, Spain, 2016. [Google Scholar]
- Pirayesh, H.; Khazaeian, A. Using almond (Prunus amygdalus L.) shell as a bio-waste resource in wood based composite. Compos. Part B Eng. 2012, 43, 1475–1479. [Google Scholar] [CrossRef]
- Takeoka, G.; Dao, L.; Teranishi, R.; Wong, R.; Flessa, S.; Harden, L.; Edwards, R. Identification of three triterpenoids in almond hulls. J. Agric. Food Chem. 2000, 48, 3437–3439. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M. Almond (Prunus dulcis) breeding. In Breeding Plantation Tree Crops: Temperate Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 1–31. ISBN 9780387712031. [Google Scholar]
- Özcan, M.M.; Ünver, A.; Erkan, E.; Arslan, D. Characteristics of some almond kernel and oils. Sci. Hortic. (Amsterdam) 2011, 127, 330–333. [Google Scholar] [CrossRef]
- Wijeratne, S.S.K.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant polyphenols in almond and its coproducts. J. Agric. Food Chem. 2006, 54, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Sfahlan, A.J.; Mahmoodzadeh, A.; Hasanzadeh, A.; Heidari, R.; Jamei, R. Antioxidants and antiradicals in almond hull and shell (Amygdalus communis L.) as a function of genotype. Food Chem. 2009, 115, 529–533. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Amarowicz, R.; Troszynska, A.; Shahidi, F. Antioxidant activity of almond seed extract and its fractions. J. Food Lipids 2005, 12, 344–358. [Google Scholar] [CrossRef]
- Sabaté, J. Nut consumption, vegetarian diets, ischemic heart disease risk, and all-cause mortality: Evidence from epidemiologic studies. Am. J. Clin. Nutr. 1999, 70, 500S–503S. [Google Scholar] [PubMed]
- Hyson, D.A.; Schneeman, B.O.; Davis, P.A. Almonds and almond oil have similar effects on plasma lipids and LDL oxidation in healthy men and women. J. Nutr. 2002, 132, 703–707. [Google Scholar] [PubMed]
- Sabaté, J.; Haddad, E.; Tanzman, J.S.; Jambazian, P.; Rajaram, S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: A randomized feeding trial. Am. J. Clin. Nutr. 2003, 77, 1379–1384. [Google Scholar] [PubMed]
- Tamizifar, B.; Rismankarzadeh, M.; Vosoughi, A.A.; Rafieeyan, M.; Tamizifar, B.; Aminzade, A. A low-dose almond-based diet decreases LDL-C while preserving HDL-C. Arch. Iran. Med. 2005, 8, 45–51. [Google Scholar]
- Berryman, C.E.; Preston, A.G.; Karmally, W.; Deckelbaum, R.J.; Kris-Etherton, P.M. Effects of almond consumption on the reduction of LDL-cholesterol: A discussion of potential mechanisms and future research directions. Nutr. Rev. 2011, 69, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Grassby, T.; Mandalari, G.; Grundy, M.M.L.; Edwards, C.H.; Bisignano, C.; Trombetta, D.; Smeriglio, A.; Chessa, S.; Ray, S.; Sanderson, J.; et al. In vitro and in vivo modeling of lipid bioaccessibility and digestion from almond muffins: The importance of the cell-wall barrier mechanism. J. Funct. Foods 2017, 37, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Becker, T. Consumer perception of fresh meat quality: A framework for analysis. Br. Food J. 2000, 102, 158–176. [Google Scholar] [CrossRef]
- Abbott, J. Quality measurement of fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 207–225. [Google Scholar] [CrossRef]
- Nanos, G.D.; Kazantzis, I.; Kefalas, P.; Petrakis, C.; Stavroulakis, G.G. Irrigation and harvest time affect almond kernel quality and composition. Sci. Hortic. (Amsterdam) 2002, 96, 249–256. [Google Scholar] [CrossRef]
- Sánchez-Bel, P.; Egea, I.; Martínez-Madrid, M.C.; Flores, B.; Romojaro, F. Influence of irrigation and organic/inorganic fertilization on chemical quality of almond (Prunus amygdalus cv. Guara). J. Agric. Food Chem. 2008, 56, 10056–10062. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Functional Foods: Their Role in Health Promotion and Disease Prevention. J. Food Sci. 2004, 69, 146–149. [Google Scholar] [CrossRef]
- Blomhoff, R.; Carlsen, M.H.; Andersen, L.F.; Jacobs, D.R. Health benefits of nuts: potential role of antioxidants. Br. J. Nutr. 2006, 96, S52–S60. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Lapsley, K.; Blumberg, J. A nutrition and health perspective on almonds. J. Sci. Food Agric. 2006, 86, 2245–2250. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, S.S.K.; Amarowicz, R.; Shahidi, F. Antioxidant activity of almonds and their by-products in food model systems. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 223–230. [Google Scholar] [CrossRef]
- Godini, A. Hull, shell and kernel relationships in almond fresh fruits. In Options Méditerranéennes: Série Etudes; CIHEAM: Paris, France, 1984; pp. 53–56. [Google Scholar]
- Toles, C.A.; Marshall, W.E.; Johns, M.M.; Wartelle, L.H.; McAloon, A. Acid-activated carbons from almond shells: physical, chemical and adsorptive properties and estimated cost of production. Bioresour. Technol. 2000, 71, 87–92. [Google Scholar] [CrossRef]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S.J. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lapsley, K.; Jeong, W.S.; Lachance, P.A.; Ho, C.T.; Rosen, R.T. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Milbury, P.E.; Lapsley, K.; Blumberg, J.B. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2005, 135, 1366–1373. [Google Scholar] [PubMed]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Carmen Gómez-Cordovés, M.; Rybarczyk, A.; Amarowicz, R.; Bartolomé, B. Comparative flavan-3-ol profile and antioxidant capacity of roasted peanut, hazelnut, and almond skins. J. Agric. Food Chem. 2009, 57, 10590–10599. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.L.; Cacciola, F.; Rich, G.T.; Bisignano, C.; Saija, A.; Dugo, P.; et al. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Ledbetter, C.A. Shell cracking strength in almond (Prunus dulcis [Mill.] D.A. Webb.) and its implication in uses as a value-added product. Bioresour. Technol. 2008, 99, 5567–5573. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Alizadeh, A.I. Nutritive value of different varieties of almond (Prunus dulcis) hulls. Res. Opin. Anim. Vet. Sci. 2011, 1, 734–738. [Google Scholar]
- Homedes, J.M.; Roura, E.; Keim, N.L.; Brown, D. Almond hulls in swine diet reduce diet fat. Calif. Agric. 1993, 47, 27–28. [Google Scholar]
- Shahidi, F. Natural Antioxidants: An Overview. In Natural Antioxidants, Chemistry, Health Effects and Applications; Shahidi, F., Ed.; AOCS Press: Champaign, IL, USA, 1997; pp. 1–11. [Google Scholar]
- Sang, S.; Lapsley, K.; Rosen, R.T.; Ho, C.T. New prenylated benzoic acid and other constituents from almond hulls (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): Identification and structure-activity relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: Almond hulls and grape pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant potential of chestnut (Castanea sativa L.) and almond (Prunus dulcis L.) by-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Pazos, M.; Medina, I.; Domínguez, H.; Parajó, J.C. Antioxidant activity of extracts produced by solvent extraction of almond shells acid hydrolysates. Food Chem. 2007, 101, 193–201. [Google Scholar] [CrossRef]
- Mandalari, G.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Rizza, L.; Bonina, F.P.; Trombetta, D.; Tomaino, A. Antioxidant and photoprotective effects of blanch water, a byproduct of the almond processing industry. Molecules 2013, 18, 12426–12440. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P.; Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant Secondary Metabolites: Biosynthesis, Classification, Function and Pharmacological Properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Ioannou, I.; Ghoul, M. Biological Activities and Effects of Food Processing on Flavonoids as Phenolic Antioxidants. In Advances in Applied Biotechnology; Petre, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 101–124. ISBN 978-953-307-820-5. [Google Scholar]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Dao, L.T. Antioxidant constituents of almond [Prunus dulcis (Mill.) D.A. Webb] hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Fu, S.; Sawalha, S.M.S.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC/CE-ESI-TOF-MS methods for the characterization of polyphenols in almond-skin extracts. Electrophoresis 2010, 31, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.Y.O. Quantification of almond skin polyphenols by liquid chromatography-mass spectrometry. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Frison-Norrie, S.; Sporns, P. Identification and quantification of flavonol glycosides in almond seedcoats using MALDI-TOF MS. J. Agric. Food Chem. 2002, 50, 2782–2787. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Mavi, A.; Kilic, H.; Yildirim, A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J. Agric. Food Chem. 2005, 53, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2011, 1, 1–10. [Google Scholar] [CrossRef]
- Chen, C.Y.O.; Blumberg, J.B. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. J. Agric. Food Chem. 2008, 56, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Blumberg, J.B.; Chen, C.Y.O. The influence of roasting, pasteurisation, and storage on the polyphenol content and antioxidant capacity of California almond skins. Food Chem. 2010, 123, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Frison, S.; Sporns, P. Variation in the flavonol glycoside composition of almond seedcoats as determined by maldi-tof mass spectrometry. J. Agric. Food Chem. 2002, 50, 6818–6822. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Bartolomé, B.; Monagas, M.; Garrido, I.; Gómez-Cordovés, C.; Martín-Álvarez, P.J.; Lebrón-Aguilar, R.; Urpí-Sardà, M.; Llorach, R.; Andrés-Lacueva, C. Almond (Prunus dulcis (Mill.) D.A. Webb) polyphenols: From chemical characterization to targeted analysis of phenolic metabolites in humans. Arch. Biochem. Biophys. 2010, 501, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Hughey, C.A.; Janusziewicz, R.; Minardi, C.S.; Phung, J.; Huffman, B.A.; Reyes, L.; Wilcox, B.E.; Prakash, A. Distribution of almond polyphenols in blanch water and skins as a function of blanching time and temperature. Food Chem. 2012, 131, 1165–1173. [Google Scholar] [CrossRef]
- Fisklements, M.; Barrett, D.M. Kinetics of almond skin separation as a function of blanching time and temperature. J. Food Eng. 2014, 138, 11–16. [Google Scholar] [CrossRef]
- Agrawal, A.D. Pharmacological activities of flavonoids: A review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398. [Google Scholar] [CrossRef]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S.J. Antimicrobial potential of polyphenols extracted from almond skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; Genovese, T.; Mazzon, E.; Wickham, M.S.J.; Paterniti, I.; Cuzzocrea, S. Natural almond skin reduced oxidative stress and inflammation in an experimental model of inflammatory bowel disease. Int. Immunopharmacol. 2011, 11, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.K.; Katyal, T. Abatement of detrimental effects of photoaging by Prunus amygdalus skin extract. Int. J. Curr. Pharm. Res. 2011, 3, 57–59. [Google Scholar]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell. Viruses 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Lo Curto, R.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food matrix effects of polyphenol bioaccessibility from almond skin during simulated human digestion. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Fadel, J.G. Quantitative analyses of selected plant by-product feedstuffs, a global perspective. Anim. Feed Sci. Technol. 1999, 79, 255–268. [Google Scholar] [CrossRef]
- Chen, P.; Cheng, Y.; Deng, S.; Lin, X.; Huang, G.; Ruan, R. Utilization of almond residues. Int. J. Agric. Biol. Eng. 2010, 3, 1–18. [Google Scholar] [CrossRef]
- Almond Board of Australia (n.d.). Renewable Energy Production from Almond Waste. Available online: http://www.australianalmonds.com.au (accessed on 3 March 2017).
- Chalker-Scott, L. Impact of mulches on landscape plants and the environment—A review. J. Environ. Hortic. 2007, 25, 239–249. [Google Scholar]
- López, R.; Burgos, P.; Hermoso, J.M.; Hormaza, J.I.; González-Fernández, J.J. Long term changes in soil properties and enzyme activities after almond shell mulching in avocado organic production. Soil Tillage Res. 2014, 143, 155–163. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Martínez, G.A.; Salas, M.D.C. Almond shell waste: Possible local rockwool substitute in soilless crop culture. Sci. Hortic. (Amsterdam) 2005, 103, 453–460. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Mazuela, P.C.; Martínez, G.A. Effect of Substrate Reutilization on Yield and Properties of Melon and Tomato Crops. J. Plant Nutr. 2008, 31, 2031–2043. [Google Scholar] [CrossRef]
- Valverde, M.; Madrid, R.; García, A.L.; del Amor, F.M.; Rincón, L. Use of almond shell and almond hull as substrates for sweet pepper cultivation. Effects on fruit yield and mineral content. Span. J. Agric. Res. 2013, 11, 164–172. [Google Scholar] [CrossRef]
- Heschel, W.; Klose, E. On the suitability of agricultural by-products for the manufacture of granular activated carbon. Fuel 1995, 74, 1786–1791. [Google Scholar] [CrossRef]
- Hayashi, J.I.; Horikawa, T.; Takeda, I.; Muroyama, K.; Nasir Ani, F. Preparing activated carbon from various nutshells by chemical activation with K CO. Carbon N. Y. 2002, 40, 2381–2386. [Google Scholar] [CrossRef]
- Urruzola, I.; Robles, E.; Serrano, L.; Labidi, J. Nanopaper from almond (Prunus dulcis) shell. Cellulose 2014, 21, 1619–1629. [Google Scholar] [CrossRef]
- Işmal, Ö.E.; Yildirim, L. Almond shell as a natural colorant. Indian J. Fibre Text. Res. 2012, 37, 358–363. [Google Scholar]
- Erdem Işmal, Ö.; Yildirim, L.; Özdoǧan, E. Use of almond shell extracts plus biomordants as effective textile dye. J. Clean. Prod. 2014, 70, 61–67. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.; Wei, S.; Yu, H.; Lv, H.; Ge, H. Isolation and purification of phenolic compounds from magnoliae officinalis by preparative high performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 431–440. [Google Scholar] [CrossRef]
- Bailly, M. Production of organic acids by bipolar electrodialysis: Realizations and perspectives. Desalination 2002, 144, 157–162. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds: A review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
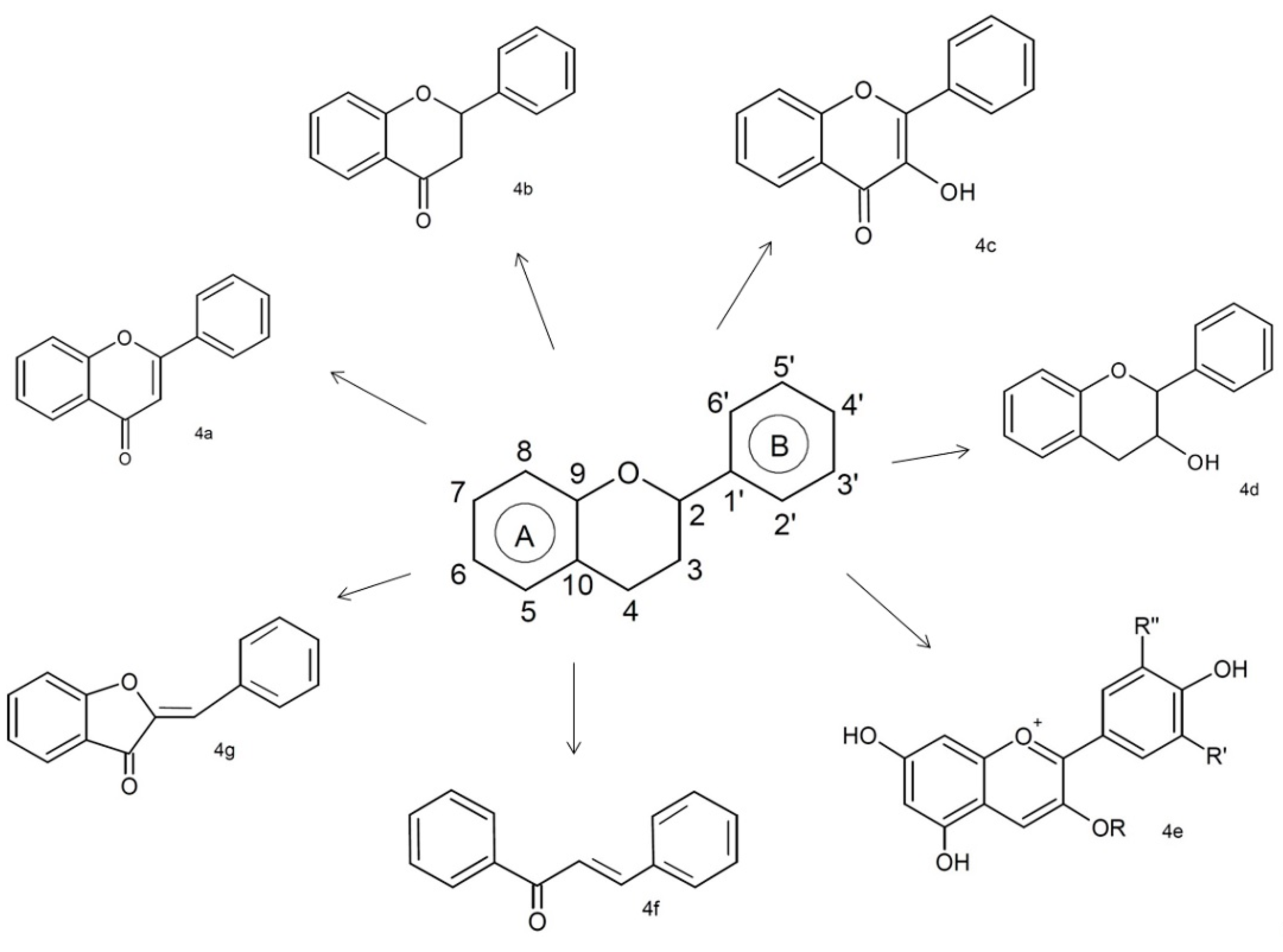
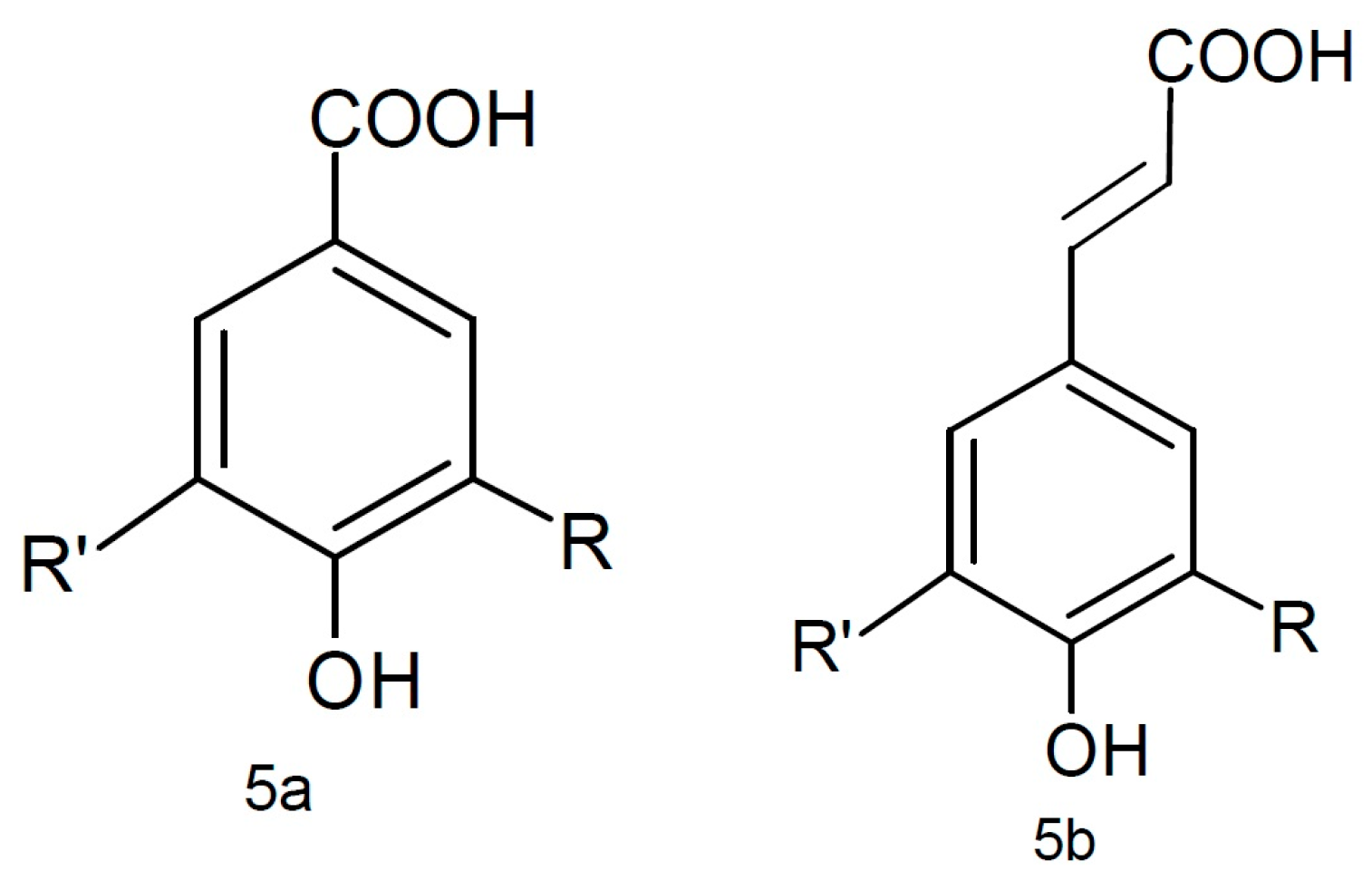
| Phenolic Class | Compound | Radicals | Almond Fruit Parts | Content | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||||
| Cinnamic acids | R1 | R2 | R3 | R4 | ||||||
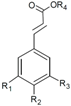 | Caffeic acid | H | OH | OH | H | Hull | Traces | [24] | ||
| Skin | Traces | [24] | ||||||||
| Sinapic acid | CH3O | OH | CH3O | H | Hull | 9.92 a | [24] | |||
| Skin | 9.51 a | [24] | ||||||||
| Ferulic acid | H | OH | CH3O | H | Hull | 2.71 a | [24] | |||
| Skin | 2.19 a | [24] | ||||||||
| Chlorogenic acid | H | OH | OH | C7H11O5 | Hull | 42.52 c | [54] | |||
| Skin | 3.12–10.6 a 1.76–3.77 a + 9571.26 b 62.21 e | [30] [53] [55] [32] [56] | ||||||||
| Crytochlorogenic acid | H | OH | OH | C7H11O5 | Hull | 7.9 c | [54] | |||
| Neochlorogenic acid | H | OH | OH | C7H11O5 | Hull | 3.04 c | [54] | |||
| trans-p-coumaric acid | H | OH | H | H | Hull | 1.34 a | [24] | |||
| Skin | 4.55 a 0.725 a 1.06 a + 367.71 b 31.87 e | [24] [30] [53] [55] [32] [56] | ||||||||
| Phenolic acids | ||||||||||
| Benzoic acids | R1 | R2 | R3 | |||||||
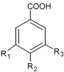 | p-hydroxybenzoic acid | H | OH | H | Skin | + 17.5 d 5.33–6.88 a 3.08–7.18 a + + 6401.20 b 127.17 e | [28] [23] [30] [53] [57] [55] [32] [56] | |||
| Vanillic acid | H | OH | CH3O | Skin | + 18.2 d 7.65–14.5 a 11.1–19.2 a + 5805.23 b 425.15 e | [28] [23] [30] [53] [55] [32] [56] | ||||
| Protocatechuic acid | H | OH | OH | Hull | + + | [37] [6] | ||||
| Skin | + 17.6 d + 14.5–32.0 a 6.69–17.2 a + + 1541.67 b 285.12 e | [28] [23] [6] [30] [53] [57] [55] [32] [56] | ||||||||
| Flavonoids | ||||||||||
| Flavanols | R3 | R5 | R7 | R3’ | R4’ | R5’ | ||||
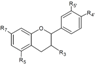 | Catechin | OH | OH | OH | H | OH | OH | Hull | + | [37] |
| Skin | + 35.7 d 36.40–90.10 a 20.1–38.3 a + + 15,569.00 b 183.67 e | [28] [23] [30] [53] [57] [55] [32] [56] | ||||||||
| Epicatechin | OH | OH | OH | H | OH | OH | Skin | 33.9 d 14.8–36.6 a 7.17–26.5 a + + 10,955.6 b 52.4 e | [23] [30] [53] [57] [55] [32] [56] | |
| Flavonoids | ||||||||||
| Flavonols | R3 | R5 | R7 | R4’ | R5’ | R6’ | ||||
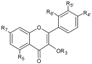 | Quercetin | H | OH | OH | OH | OH | H | Hull | + | [6] |
| Skin | + 100 d 1.43–1.78 a 1.02–4.89 a + + 214.3 b 802.2 e | [6] [23] [30] [53] [57] [55] [32] [56] | ||||||||
| Flavonoids | ||||||||||
| Flavonols | R3 | R5 | R7 | R4’ | R5’ | R6’ | ||||
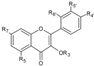 | Quercetin-3-O-glucoside | C6H11O5 | OH | OH | O− | OH | H | Skin | + 24.5 d 1.33–2.41 a 1.71 a + 166.9 e | [28] [23] [30] [53] [55] [56] |
| Quercetin-3-O-galactoside | C6H11O5 | OH | OH | O− | OH | H | Skin | + 41.4 d + 60.6 e | [28] [23] [57] [56] | |
| Quercetin-3-O-rhamnoside | C6H11O4 | OH | OH | OH | OH | H | Skin | + | [55] | |
| Kaempferol | H | OH | OH | OH | H | H | Skin | 100 d 1.71–1.96 a 2.75–12.1 a + + 1250.0 b 3982.9 e | [23] [30] [53] [57] [55] [32] [56] | |
| Kaempferol-3-O-rutinoside | C12H21O9 | OH | OH | OH | H | H | Hull | + | [6] | |
| Skin | + + 12.80–31.80 a 5.26–40.7 a + + 22,949.3 b 10,829.9 e | [58,6] [30] [53] [57] [55] [32] [56] | ||||||||
| Flavonoids | ||||||||||
| Flavonols | R3 | R5 | R7 | R4’ | R5’ | R6’ | ||||
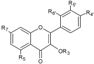 | Kaempferol-3-O-glucoside | C6H11O5 | OH | OH | OH | H | H | Hull | + | [6] |
| Skin | + + 39.0 d + 1.65 a 14.2 a + 39,012.3 b 4080.9 e | [28] [58,23] [6] [30] [53] [57] [32] [56] | ||||||||
| Isorhamnetin | H | OH | OH | OH | CH3O | H | Hull | + | [6] | |
| Skin | 47.2 d + 4.19–4.87 a 5.92–16.0 a + + 4551.9 b | [23] [6] [30] [53] [57] [55] [32] | ||||||||
| Isorhamnetin-3-O-glucoside | C6H11O5 | OH | OH | OH | CH3O | H | Hull | + | [6] | |
| Skin | + + 8.85–15.60 a 5.02–15.3 a + 16,940.9 b 461.3 e | [58] [6] [30] [53] [57] [32] [56] | ||||||||
| Flavonoids | ||||||||||
| Flavonols | R3 | R5 | R7 | R4’ | R5’ | R6’ | ||||
 | Isorhamnetin-3-O-rutinoside | C12H21O9 | OH | OH | OH | CH3O | H | Skin | + 40.4 d 27.60–41.40 a 5.34–58.0 a + + 54,901.1 b 210.8 e | [58] [23] [30] [53] [57] [55] [32] [56] |
| Morin | H | OH | OH | OH | H | OH | Hull | + | [6] | |
| Skin | + | [6] | ||||||||
| Flavonoids | ||||||||||
| Flavanones | R5 | R7 | R4’ | R5’ | ||||||
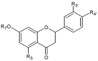 | Naringenin-7-O-glucoside | OH | C6H11O5 | OH | H | Skin | + 16.1 d 6.84–22.10 a 2.34–25.9 a + + 14,288.1 b | [28] [23] [30] [53] [57] [55] [32] | ||
| Eriodictyol-7-O-glucoside | OH | C6H11O5 | OH | OH | Skin | 0.808–1.60 a 2.5–2.65 a + 3382.5 b 1309.2 e | [30] [53] [55] [32] [56] | |||
| Destiny | Use | References |
|---|---|---|
| dairy industry | livestock feed | [80] |
| energy production | energy feedstock | [81] |
| agriculture | compost, soil ameliorants, mulch and/or fertilizers | [82,83,84,85,86,87] |
| particleboard manufacturing | wood-based composite | [2] |
| production of activated carbons | rich source in preparing activated carbons | [26,88,89] |
| nanopaper production | source of nanofibers | [90] |
| natural colorant | wool dyeing | [91,92] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. https://doi.org/10.3390/molecules22101774
Prgomet I, Gonçalves B, Domínguez-Perles R, Pascual-Seva N, Barros AIRNA. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules. 2017; 22(10):1774. https://doi.org/10.3390/molecules22101774
Chicago/Turabian StylePrgomet, Iva, Berta Gonçalves, Raúl Domínguez-Perles, Núria Pascual-Seva, and Ana I. R. N. A. Barros. 2017. "Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application" Molecules 22, no. 10: 1774. https://doi.org/10.3390/molecules22101774
APA StylePrgomet, I., Gonçalves, B., Domínguez-Perles, R., Pascual-Seva, N., & Barros, A. I. R. N. A. (2017). Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules, 22(10), 1774. https://doi.org/10.3390/molecules22101774