Synthesis of Siloxyalumoxanes and Alumosiloxanes Based on Organosilicon Diols
Abstract
:1. Introduction
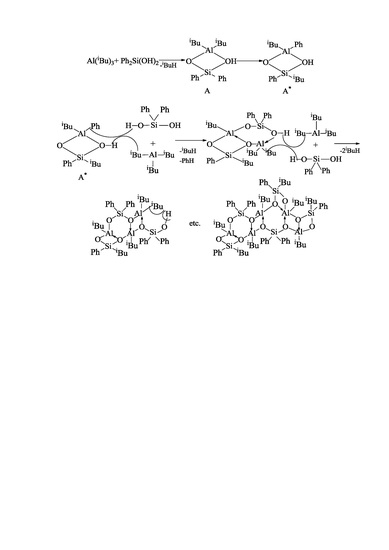
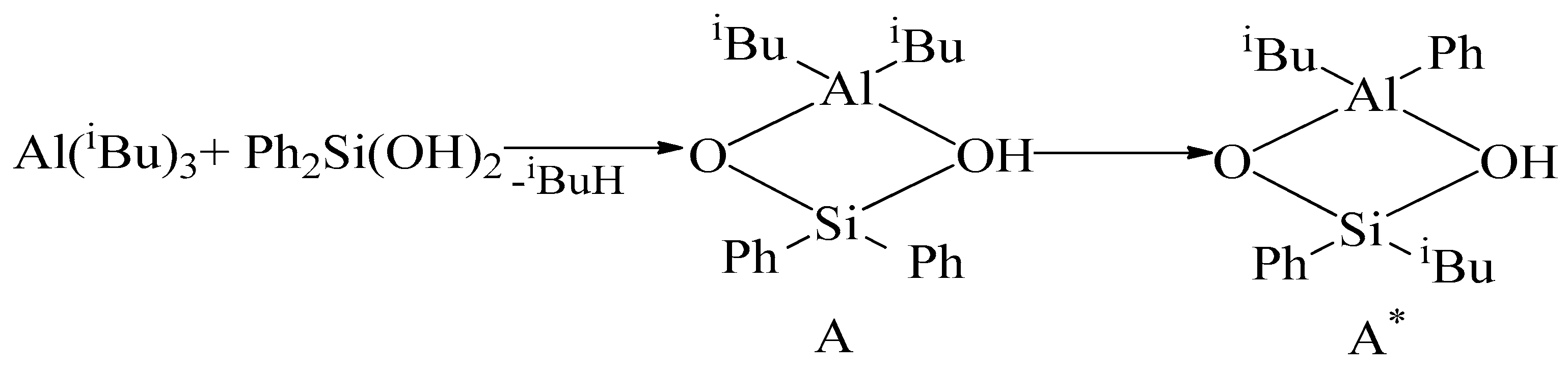
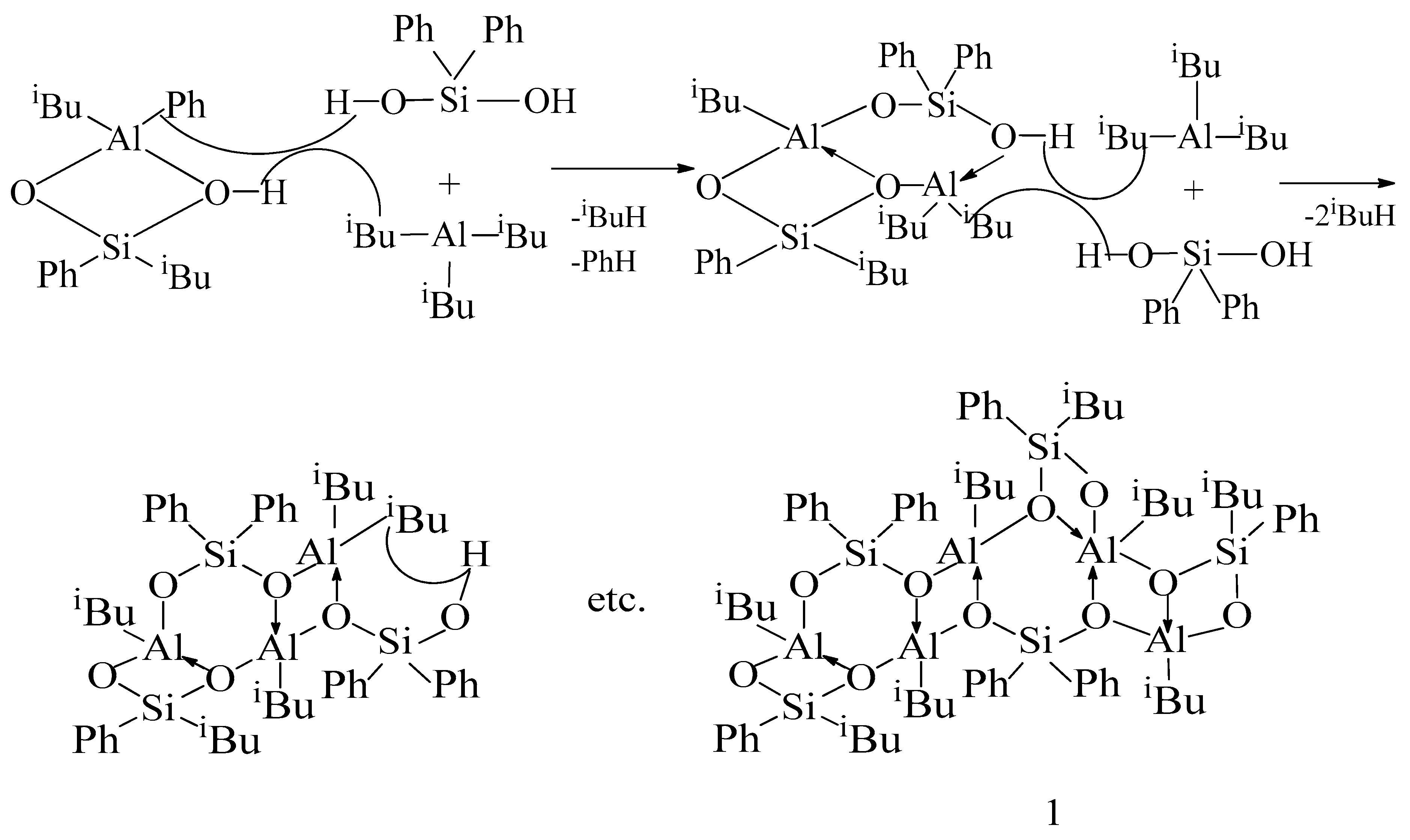
2. Results and Discussion
3. Experimental
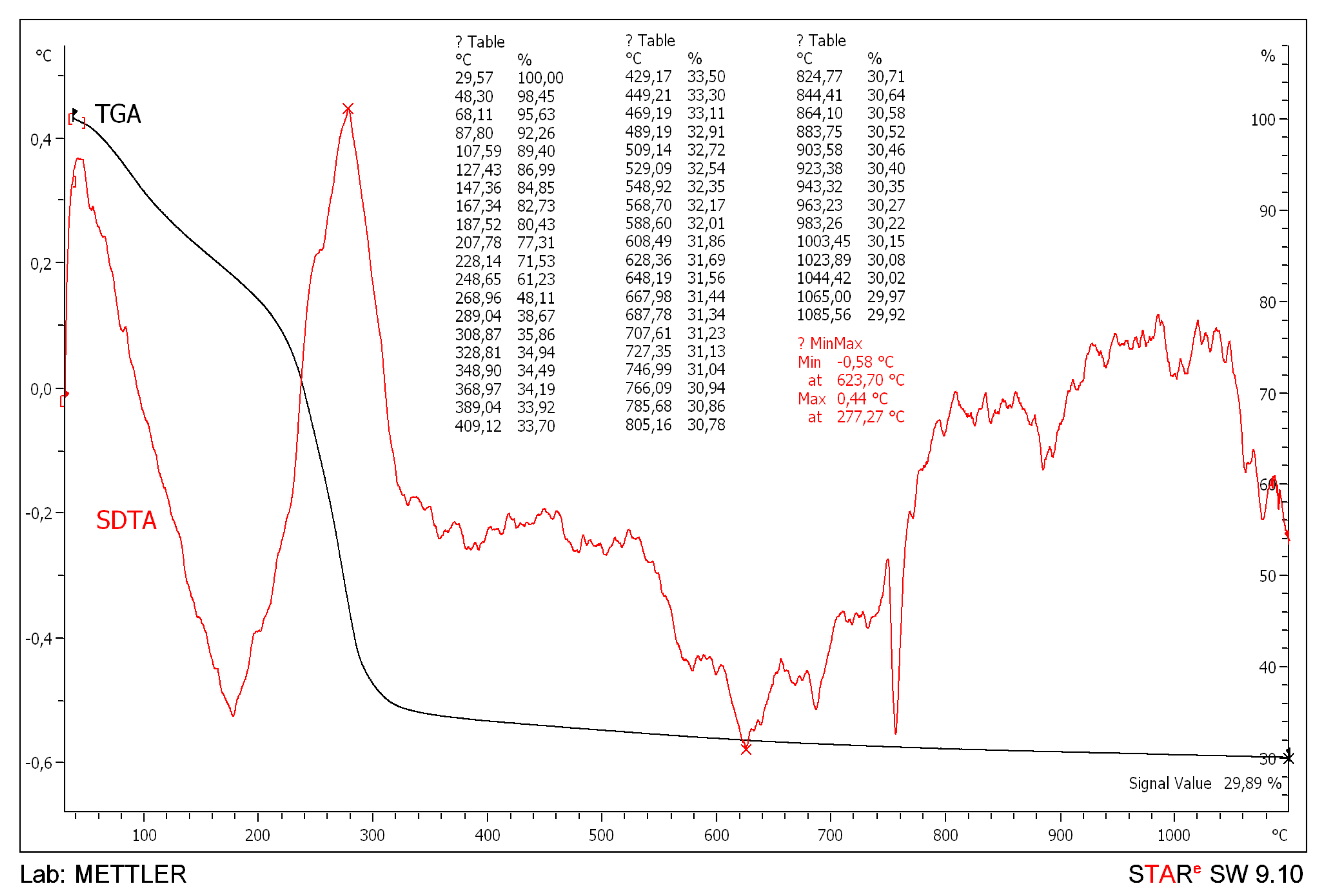
3.1. General Methods
3.2. Synthetic Procedures
3.2.1. General Procedure for the Preparation of Organosiloxyalumoxanes
3.2.2. General Procedure for the Preparation of Organoalumosiloxanes and Organoalumoxanesiloxanes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Korneev, N.N.; Shcherbakova, G.I.; Kolesov, V.S.; Sakharovskaya, G.B.; Shevchenko, E.I.; Nikitin, V.S.; Bazhenova, L.I.; Nikishina, I.S. Triisobutylaluminium and isobutylalumoxane interaction with hydroxyl-containing organosilicon compounds. Zhurnal Obshchei Khimii 1987, 57, 330–335. [Google Scholar]
- Bochkarev, V.N.; Belokon, A.I. Structural and special isomerism of non classic mono- and dicyclic alumoxanes. Zhurnal Obshchei Khimii 1984, 54, 2553–2559. [Google Scholar]
- Gershkokhen, S.L.; Chaplina, I.V.; Poletaeva, I.L. Study of composition and structure of aluminium alkyl partial hydrolysis products. Zhurnal Obshchei Khimii 1984, 54, 2714–2720. [Google Scholar]
- Korneev, N.N.; Shcherbakova, G.I.; Bochkarev, V.N.; Belokon, A.I.; Kisin, A.V.; Apalkova, G.M. Ttiisobutyl interaction with tetraethoxysilane. Zhurnal Obshchei Khimii. 1985, 55, 589–593. [Google Scholar]
- Bonamico, M.; Dessy, G. The crystal structure of aluminosiloxanes X-ray analisis of compound of formula C8H24Al3Br5O6Si4. J. Chem. Soc. A 1968, 2, 291–297. [Google Scholar] [CrossRef]
- Shcherbakova, G.I. Preparation and Studying the Properties of Organoaluminium Oligomers Containing Elementoxane Fragments. Master’s Thesis, State Research Institute for Chemistry and Technology of Organoelement Compounds (GNIIChTEOS), Moscow, Russia, 1988; pp. 95–112. [Google Scholar]
- Pasynkiewicz, S. Alumoxanes: Synthesis, structures, complexes and reactions. Polyhedron 1990, 9, 429–453. [Google Scholar] [CrossRef]
- Korneev, N.N.; Khrapova, I.M.; Polonskii, A.V.; Ivanova, N.I.; Kisin, A.V.; Kolesov, V.S. Study of methylalumoxanes structure and composition peculiarities. 27A1 and 1H-NMR spectra of trimethylaluminium and its limited hydrolysis products. Izvestiya Akademii Nauk RF Chem. 1993, 8, 1453–1457. [Google Scholar]
- Harlan, C.J.; Mason, M.R.; Barron, A.R. Tret-Butylaluminum hydroxides and oxides: structural relationship between alkylalumoxanes and alumina gels. Organometallics 1994, 13, 2957–2969. [Google Scholar] [CrossRef]
- Landry, C.C.; Pappè, N.; Mason, M.R.; Apblett, A.W.; Tyler, A.N.; MacInnes, A.N.; Barron, A.R. From minerals to materials: Synthesis of alumoxanes from the reaction of boehmite with carboxylic acids. J. Mater. Chem. 1995, 5, 331–341. [Google Scholar] [CrossRef]
- Koide, Y.; Barron, A.R. [Al5(tBu)5(μ3-O)2(μ3-OH)2(μ-OH)2(μ-O2CPh)2]: A model for the interaction of carboxylic acids with boehmite. Organometallics 1995, 14, 4026–4029. [Google Scholar] [CrossRef]
- Landry, C.C.; Pappè, N.; Mason, M.R.; Apblett, A.W.; Barron, A.R. Reaction of boehmite with carboxylic acids: A new synthetic route to alumoxanes. In Inorganic and Organometallic Polymers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; Chapter 13; pp. 149–164. [Google Scholar] [CrossRef]
- Barron, A.R. New method for the determination of the trialkylaluminum content in alumoxanes. Organometallics 1995, 14, 3581–3583. [Google Scholar] [CrossRef]
- Henderson, M.A.; Trefz, T.K.; Collins, S.; Wang, M.Y.; McIndoe, J.S. Characterization of isobutylaluminoxanes by electrospray ionization mass spectrometry. Organometallics 2013, 32, 2079–2083. [Google Scholar] [CrossRef]
- Lewinski, J.; Wheatley, A.E.H. Simple trivalent organoaluminum species: perspectives on structure, bonding, and reactivity. Top. Organomet. Chem. 2013, 41, 1–58. [Google Scholar] [CrossRef]
- Apblett, A.W.; Warren, A.C.; Barren, A.R. Synthesis and characterization of triethylsiloxy-substituted alumoxanes: Their structural relationship to the minerals boehmite and diaspore. Chem. Mater. 1992, 4, 167–182. [Google Scholar] [CrossRef]
- Landry, C.C.; Davis, J.A.; Apblett, A.W.; Barron, A.R. Siloxy-substituted alumoxanes: Their synthesis from polydialkylsiloxanes and trimethylaluminium, and application as aluminosilicate precursors. J. Mater. Chem. 1993, 3, 597–602. [Google Scholar] [CrossRef]
- Gonzalez-Gallardo, S.; Jancik, V.; Delgado-Robles, A.A.; Moya-Cabrera, M. Cyclic Alumosiloxanes and alumosilicates: Exemplifying the loewenstein rule at the molecular level. Inorg. Chem. 2011, 50, 4226–4228. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, Y.K.; Reilly, R.; Kessler, V.G. A convenient route to anionic and cyclic aluminosiloxanes: Crystal structures of [PyH] [Al{OSiPh2(OSiPh2)2O}2] and the first twelve-membered organic aluminosilicate Al2Si4O6 ring. New J. Chem. 2001, 25, 528–530. [Google Scholar] [CrossRef]
- McMahon, C.N.; Obrey, S.J.; Keys, A.; Bott, S.G.; Barron, A.R. Reaction of 1,3-diols with Al(tBu)3 and Ga(tBu)3: Aluminium- and gallium-based bifunctional tetradentate ligands. J. Chem. Soc. Dalton Trans. 2000, 2151–2161. [Google Scholar] [CrossRef]
- Veith, M. Molecular Alumo-Siloxanes and base adducts. Adv. Organomet. Chem. 2006, 54, 49–72. [Google Scholar] [CrossRef]
- Veith, M.; Rammo, A.; Huch, V.; Biegler, J. The reaction behaviour of the polycylic oligoalumosiloxane [Ph2SiO]8[AlO(OH)]4 towards hexamethyldisilazane. Z. Anorg. Allg. Chem. 2007, 633, 246–250. [Google Scholar] [CrossRef]
- Veith, M.; Kolano, D.; Kirs, T.; Huch, V. Condensation reaction through base assistance within (Ph2SiO)8[AlO(OH)]4. J. Organomet. Chem. 2010, 695, 1074–1079. [Google Scholar] [CrossRef]
- Veith, M.; Kolano, D.; Huch, V.; Sutter, J.P. Reactions of the alumopolysiloxane (Ph2SiO)8[AlO(OH)]4 with 4,4-Bipyridine and azobipyridines. Z. Anorg. Allg. Chem. 2011, 637, 1922–1930. [Google Scholar] [CrossRef]
- Veith, M.; Hreleva-Caparrotti, H.; Sahin, F.; Huch, V. [Al2(OH)8]2—building blocks incorporated in macromolecular alumopolysiloxane rings of the type [O-SiPh2-O-SiPh2-O-Al+]n. Z. Anorg. Allg. Chem. 2014, 640, 863–867. [Google Scholar] [CrossRef]
- Otto, M. World of Chemistry. Modern Methods of Analytic Chemistry; Translation from German under the editorship of A.V. Garmash; Tekhnosfera: Moscow, Russia, 2006. [Google Scholar]
- Galiulina, R.F.; Pankratova, V.N.; Stepovik, L.P.; Dodonov, V.A. Production and thermal decomposition reactions of zinc, cadmium and aluminium tribenzylsiloxy derivatives. Zhurnal Obshchei Khimii 1981, 51, 74–79. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

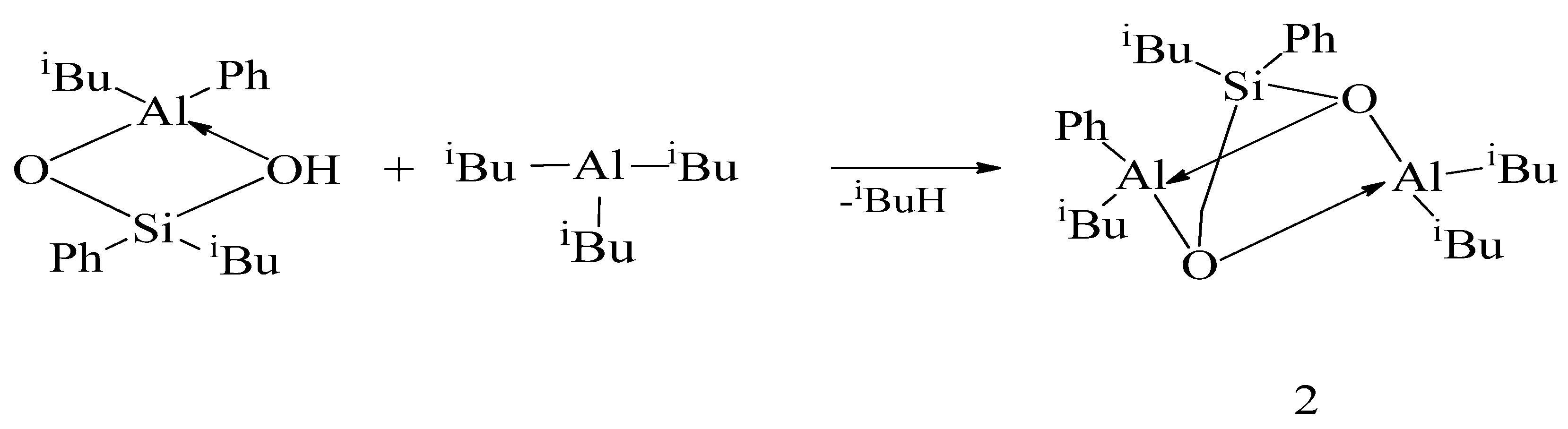
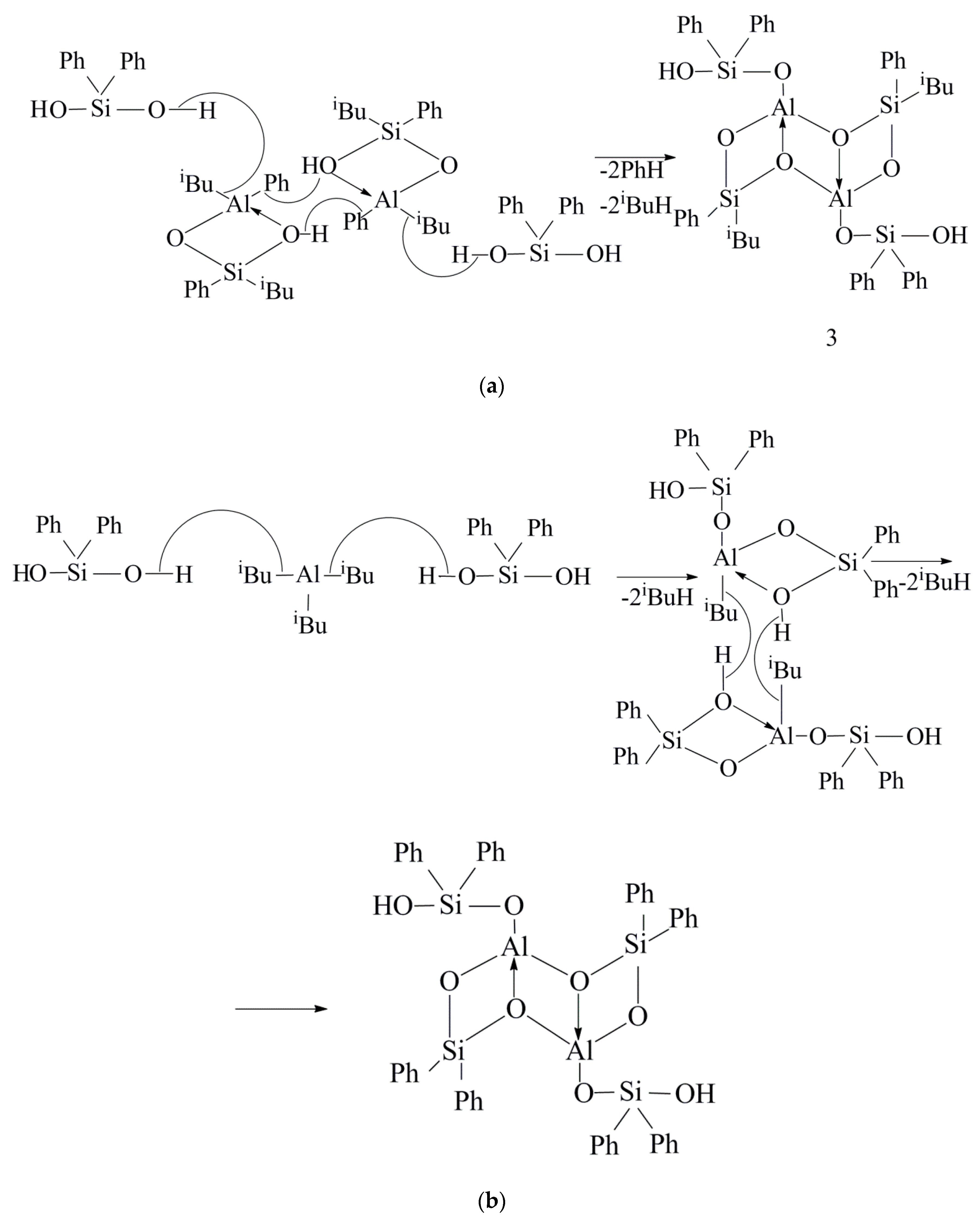







| No. | OAC | Al:Si Molar Ratio in the Reaction Mixture | Amount of Gas Released in the Reaction, mL | Product Yield, wt % | |
|---|---|---|---|---|---|
| Calculated | Experiment | ||||
| 1 | Al(iBu)3 | 1:1 | 223.28 | 210 | 94.0 |
| 2 | Al(iBu)3 | 2:1 | 77.75 | 80 | 99.3 |
| 3 | Al(iBu)3 | 1:2 | 299.04 | 258 | 86.3 |
| 4 | tetraisobutylalumoxane | 1:1 | 279.11 | 280 | 99.6 |
| 5 | tetraisobutylalumoxane | 2:1 | 93.70 | 93 | 99.3 |
| No. | OAC | Organosilicon compounds (OSC) | Molar Ratio | Amount of Gas Released in the Reaction, mL | Product Yield, wt % | ||
|---|---|---|---|---|---|---|---|
| OAC:OSC | Al:Si | Calculated | Experiment | ||||
| 6 | Al(iBu)3 | α-diol | 1:1 | 1:2 | 155.50 | 115 | 74.0 |
| 7 | Al(iBu)3 | α-diol | 2:1 | 1:1 | 75.75 | 76 | 95.5 |
| 8 | Al(iBu)3 | γ-diol | 1:1 | 1:2 | 239.23 | 203 | 85.0 |
| 9 | Al(iBu)3 | γ-diol | 2:1 | 1:1 | 207.30 | 194 | 93.4 |
| 10 | tetraisobutylalumoxane | α-diol | 1:1 | 1:1 | 149.52 | 150 | 99.9 |
| 11 | tetraisobutylalumoxane | γ-diol | 1:1 | 1:1 | 193.70 | 200 | 99.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbakova, G.; Storozhenko, P.; Kisin, A. Synthesis of Siloxyalumoxanes and Alumosiloxanes Based on Organosilicon Diols. Molecules 2017, 22, 1776. https://doi.org/10.3390/molecules22101776
Shcherbakova G, Storozhenko P, Kisin A. Synthesis of Siloxyalumoxanes and Alumosiloxanes Based on Organosilicon Diols. Molecules. 2017; 22(10):1776. https://doi.org/10.3390/molecules22101776
Chicago/Turabian StyleShcherbakova, Galina, Pavel Storozhenko, and Alexander Kisin. 2017. "Synthesis of Siloxyalumoxanes and Alumosiloxanes Based on Organosilicon Diols" Molecules 22, no. 10: 1776. https://doi.org/10.3390/molecules22101776
APA StyleShcherbakova, G., Storozhenko, P., & Kisin, A. (2017). Synthesis of Siloxyalumoxanes and Alumosiloxanes Based on Organosilicon Diols. Molecules, 22(10), 1776. https://doi.org/10.3390/molecules22101776