α-Synuclein Regulates Neuronal Cholesterol Efflux
Abstract
:1. Introduction
2. Results
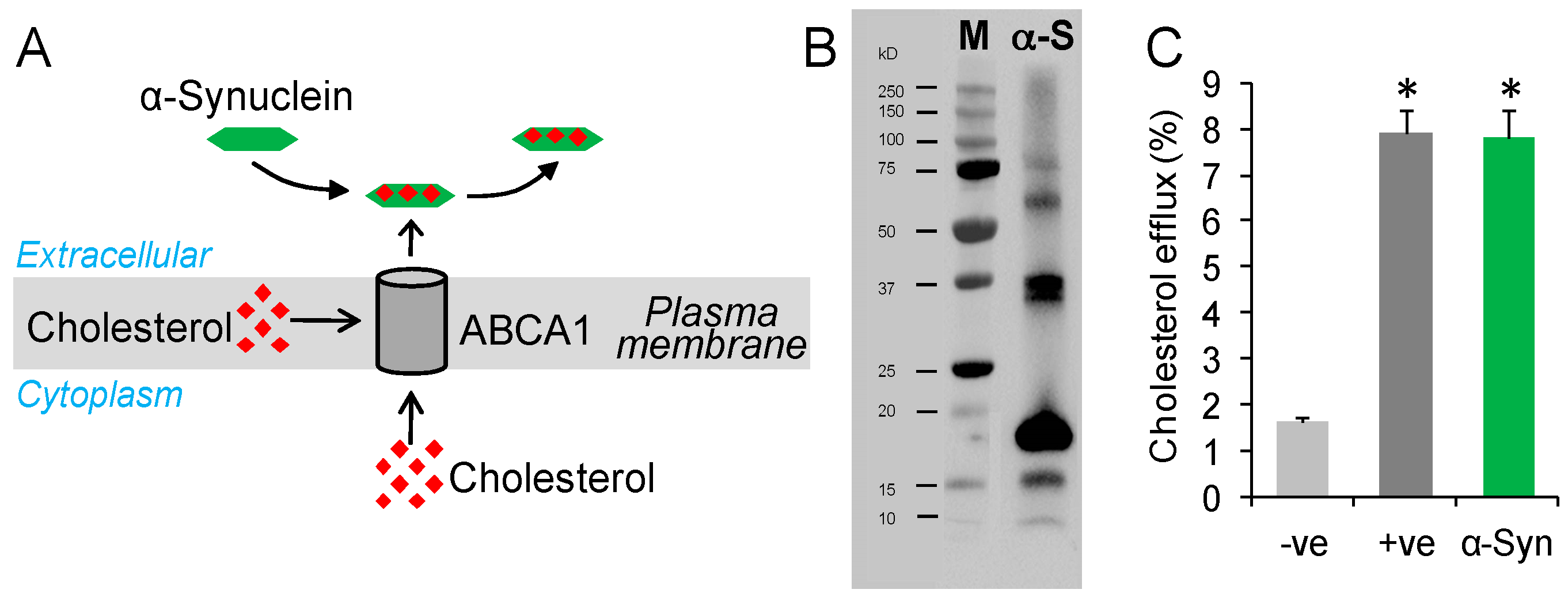
2.1. Testing Whether α-Synuclein Can Mediate Cholesterol Efflux
2.2. α-Synuclein Mediates Cholesterol Efflux in a Dose- and Time-Dependent Manner
2.3. α-Synuclein Mediates Cholesterol Efflux via ABCA1
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cholesterol Efflux Assay
4.3. Western Blotting
4.4. Transfection
4.5. RNA Extraction and Quantitative PCR
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.P.; Power, J.H.; Blumbergs, P.C.; Blessing, W.W. Multiple-system atrophy: A new alpha-synuclein disease? Lancet 1998, 352, 547–548. [Google Scholar] [CrossRef]
- Newell, K.L.; Boyer, P.; Gomez-Tortosa, E.; Hobbs, W.; Hedley-Whyte, E.T.; Vonsattel, J.P.; Hyman, B.T. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J. Neuropathol. Exp. Neurol. 1999, 58, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Pals, P.; Lincoln, S.; Manning, J.; Heckman, M.; Skipper, L.; Hulihan, M.; Van den Broeck, M.; De Pooter, T.; Cras, P.; Crook, J.; et al. Alpha-synuclein promoter confers susceptibility to parkinson’s disease. Ann. Neurol. 2004, 56, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Vilarino-Guell, C.; Rajput, M.L.; Ross, O.A.; Soto-Ortolaza, A.I.; Lincoln, S.J.; Cobb, S.A.; Heckman, M.G.; Farrer, M.J. Alpha-synuclein polymorphisms are associated with parkinson’s disease in a saskatchewan population. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 2411–2414. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, N.; Wilk, J.B.; Latourelle, J.C.; DeStefano, A.L.; Halter, C.; Pugh, E.W.; Doheny, K.F.; Gusella, J.F.; Nichols, W.C.; Foroud, T.; et al. Genomewide association study for susceptibility genes contributing to familial parkinson disease. Hum. Genet. 2009, 124, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Maraganore, D.M.; de Andrade, M.; Elbaz, A.; Farrer, M.J.; Ioannidis, J.P.; Kruger, R.; Rocca, W.A.; Schneider, N.K.; Lesnick, T.G.; Lincoln, S.J.; et al. Collaborative analysis of alpha-synuclein gene promoter variability and parkinson disease. J. Am. Med. Assoc. 2006, 296, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.; Kuhn, W.; Muller, T.; Woitalla, D.; Graeber, M.; Kosel, S.; Przuntek, H.; Epplen, J.T.; Schols, L.; Riess, O. Ala30pro mutation in the gene encoding alpha-synuclein in parkinson’s disease. Nat. Genet. 1998, 18, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Zarranz, J.J.; Alegre, J.; Gomez-Esteban, J.C.; Lezcano, E.; Ros, R.; Ampuero, I.; Vidal, L.; Hoenicka, J.; Rodriguez, O.; Atares, B.; et al. The new mutation, e46k, of alpha-synuclein causes Parkinson and lewy body dementia. Ann. Neurol. 2004, 55, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Proukakis, C.; Dudzik, C.G.; Brier, T.; MacKay, D.S.; Cooper, J.M.; Millhauser, G.L.; Houlden, H.; Schapira, A.H. A novel alpha-synuclein missense mutation in Parkinson disease. Neurology 2013, 80, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Lesage, S.; Anheim, M.; Letournel, F.; Bousset, L.; Honore, A.; Rozas, N.; Pieri, L.; Madiona, K.; Durr, A.; Melki, R.; et al. G51d alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 2013, 73, 459–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sailer, A.; Scholz, S.W.; Nalls, M.A.; Schulte, C.; Federoff, M.; Price, T.R.; Lees, A.; Ross, O.A.; Dickson, D.W.; Mok, K.; et al. A genome-wide association study in multiple system atrophy. Neurology 2016, 87, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.F.; George, J.M. The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998, 21, 249–254. [Google Scholar] [CrossRef]
- Fortin, D.L.; Troyer, M.D.; Nakamura, K.; Kubo, S.; Anthony, M.D.; Edwards, R.H. Lipid rafts mediate the synaptic localization of alpha-synuclein. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6715–6723. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Carlus, D.; Yahi, N. The fusogenic tilted peptide (67–78) of alpha-synuclein is a cholesterol binding domain. Biochim. Biophys. Acta 2011, 1808, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Varkey, J.; Mizuno, N.; Hegde, B.G.; Cheng, N.; Steven, A.C.; Langen, R. Alpha-synuclein oligomers with broken helical conformation form lipoprotein nanoparticles. J. Biol. Chem. 2013, 288, 17620–17630. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Wilson, A.; Marcil, M.; Clee, S.M.; Zhang, L.H.; Roomp, K.; van Dam, M.; Yu, L.; Brewer, C.; Collins, J.A.; Molhuizen, H.O.; et al. Mutations in abc1 in tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999, 22, 336–345. [Google Scholar] [PubMed]
- Bodzioch, M.; Orso, E.; Klucken, J.; Langmann, T.; Bottcher, A.; Diederich, W.; Drobnik, W.; Barlage, S.; Buchler, C.; Porsch-Ozcurumez, M.; et al. The gene encoding atp-binding cassette transporter 1 is mutated in tangier disease. Nat. Genet. 1999, 22, 347–351. [Google Scholar] [PubMed]
- Oram, J.F.; Lawn, R.M.; Garvin, M.R.; Wade, D.P. Abca1 is the camp-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000, 275, 34508–34511. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of apoa-i, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing abc1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef] [PubMed]
- McNeish, J.; Aiello, R.J.; Guyot, D.; Turi, T.; Gabel, C.; Aldinger, C.; Hoppe, K.L.; Roach, M.L.; Royer, L.J.; de Wet, J.; et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of atp-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA 2000, 97, 4245–4250. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Rahmanto, A.S.; Kamili, A.; Rye, K.A.; Guillemin, G.J.; Gelissen, I.C.; Jessup, W.; Hill, A.F.; Garner, B. Role of abcg1 and abca1 in regulation of neuronal cholesterol efflux to apolipoprotein e discs and suppression of amyloid-beta peptide generation. J. Biol. Chem. 2007, 282, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hsiao, J.H.; Paxinos, G.; Halliday, G.M.; Kim, W.S. Abca5 regulates amyloid-beta peptide production and is associated with Alzheimer’s disease neuropathology. J. Alzheimers Dis. 2015, 43, 857–869. [Google Scholar] [PubMed]
- Pitas, R.E.; Boyles, J.K.; Lee, S.H.; Hui, D.; Weisgraber, K.H. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein b,e(ldl) receptors in the brain. J. Biol. Chem. 1987, 262, 14352–14360. [Google Scholar] [PubMed]
- Fagan, A.M.; Holtzman, D.M.; Munson, G.; Mathur, T.; Schneider, D.; Chang, L.K.; Getz, G.S.; Reardon, C.A.; Lukens, J.; Shah, J.A.; et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoe (−/−), and human apoe transgenic mice. J. Biol. Chem. 1999, 274, 30001–30007. [Google Scholar] [CrossRef] [PubMed]
- LaDu, M.J.; Gilligan, S.M.; Lukens, J.R.; Cabana, V.G.; Reardon, C.A.; Van Eldik, L.J.; Holtzman, D.M. Nascent astrocyte particles differ from lipoproteins in csf. J. Neurochem. 1998, 70, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Ladu, M.J.; Reardon, C.; Van Eldik, L.; Fagan, A.M.; Bu, G.; Holtzman, D.; Getz, G.S. Lipoproteins in the central nervous system. Ann. N. Y. Acad. Sci. 2000, 903, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Weickert, C.S.; Garner, B. Role of atp-binding cassette transporters in brain lipid transport and neurological disease. J. Neurochem. 2008, 104, 1145–1166. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.L.; Morris, A.L.; Rhee, J.S.; Andersson, L.P.; Mendez, A.J.; Freeman, M.W. Naturally occurring mutations in the largest extracellular loops of abca1 can disrupt its direct interaction with apolipoprotein a-i. J. Biol. Chem. 2002, 277, 33178–33187. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.L.; Mendez, A.J.; Moore, K.J.; Andersson, L.P.; Panjeton, H.A.; Freeman, M.W. Atp-binding cassette transporter a1 contains an nh2-terminal signal anchor sequence that translocates the protein’s first hydrophilic domain to the exoplasmic space. J. Biol. Chem. 2001, 276, 15137–15145. [Google Scholar] [CrossRef] [PubMed]
- Chambenoit, O.; Hamon, Y.; Marguet, D.; Rigneault, H.; Rosseneu, M.; Chimini, G. Specific docking of apolipoprotein a-i at the cell surface requires a functional abca1 transporter. J. Biol. Chem. 2001, 276, 9955–9960. [Google Scholar] [CrossRef] [PubMed]
- Segrest, J.P.; Jones, M.K.; De Loof, H.; Brouillette, C.G.; Venkatachalapathi, Y.V.; Anantharamaiah, G.M. The amphipathic helix in the exchangeable apolipoproteins: A review of secondary structure and function. J. Lipid Res. 1992, 33, 141–166. [Google Scholar] [PubMed]
- Li, Z.; Mintzer, E.; Bittman, R. First synthesis of free cholesterol-bodipy conjugates. J. Org. Chem. 2006, 71, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.; Kellner-Weibel, G.; de la Llera-Moya, M.; Phillips, M.C.; Asztalos, B.F.; Bittman, R.; Rothblat, G.H. A sensitive assay for abca1-mediated cholesterol efflux using bodipy-cholesterol. J. Lipid Res. 2011, 52, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bittman, R. Synthesis and spectral properties of cholesterol- and fty720-containing boron dipyrromethene dyes. J. Org. Chem. 2007, 72, 8376–8382. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.; Ejsing, C.S.; Garcia-Saez, A.J.; Kaiser, H.J.; Sampaio, J.L.; Surma, M.A.; Shevchenko, A.; Schwille, P.; Simons, K. Yeast lipids can phase-separate into micrometer-scale membrane domains. J. Biol. Chem. 2010, 285, 30224–30232. [Google Scholar] [CrossRef] [PubMed]
- Holtta-Vuori, M.; Uronen, R.L.; Repakova, J.; Salonen, E.; Vattulainen, I.; Panula, P.; Li, Z.; Bittman, R.; Ikonen, E. Bodipy-cholesterol: A new tool to visualize sterol trafficking in living cells and organisms. Traffic 2008, 9, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are commercially available from AnaSpec Inc. (Fremont, CA, USA). |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, J.-H.T.; Halliday, G.M.; Kim, W.S. α-Synuclein Regulates Neuronal Cholesterol Efflux. Molecules 2017, 22, 1769. https://doi.org/10.3390/molecules22101769
Hsiao J-HT, Halliday GM, Kim WS. α-Synuclein Regulates Neuronal Cholesterol Efflux. Molecules. 2017; 22(10):1769. https://doi.org/10.3390/molecules22101769
Chicago/Turabian StyleHsiao, Jen-Hsiang T., Glenda M. Halliday, and Woojin Scott Kim. 2017. "α-Synuclein Regulates Neuronal Cholesterol Efflux" Molecules 22, no. 10: 1769. https://doi.org/10.3390/molecules22101769
APA StyleHsiao, J.-H. T., Halliday, G. M., & Kim, W. S. (2017). α-Synuclein Regulates Neuronal Cholesterol Efflux. Molecules, 22(10), 1769. https://doi.org/10.3390/molecules22101769



