Crosstalk Influence between P38MAPK and Autophagy on Mitochondria-Mediated Apoptosis Induced by Anti-Fas Antibody/Actinomycin D in Human Hepatoma Bel-7402 Cells
Abstract
:1. Introduction
2. Results
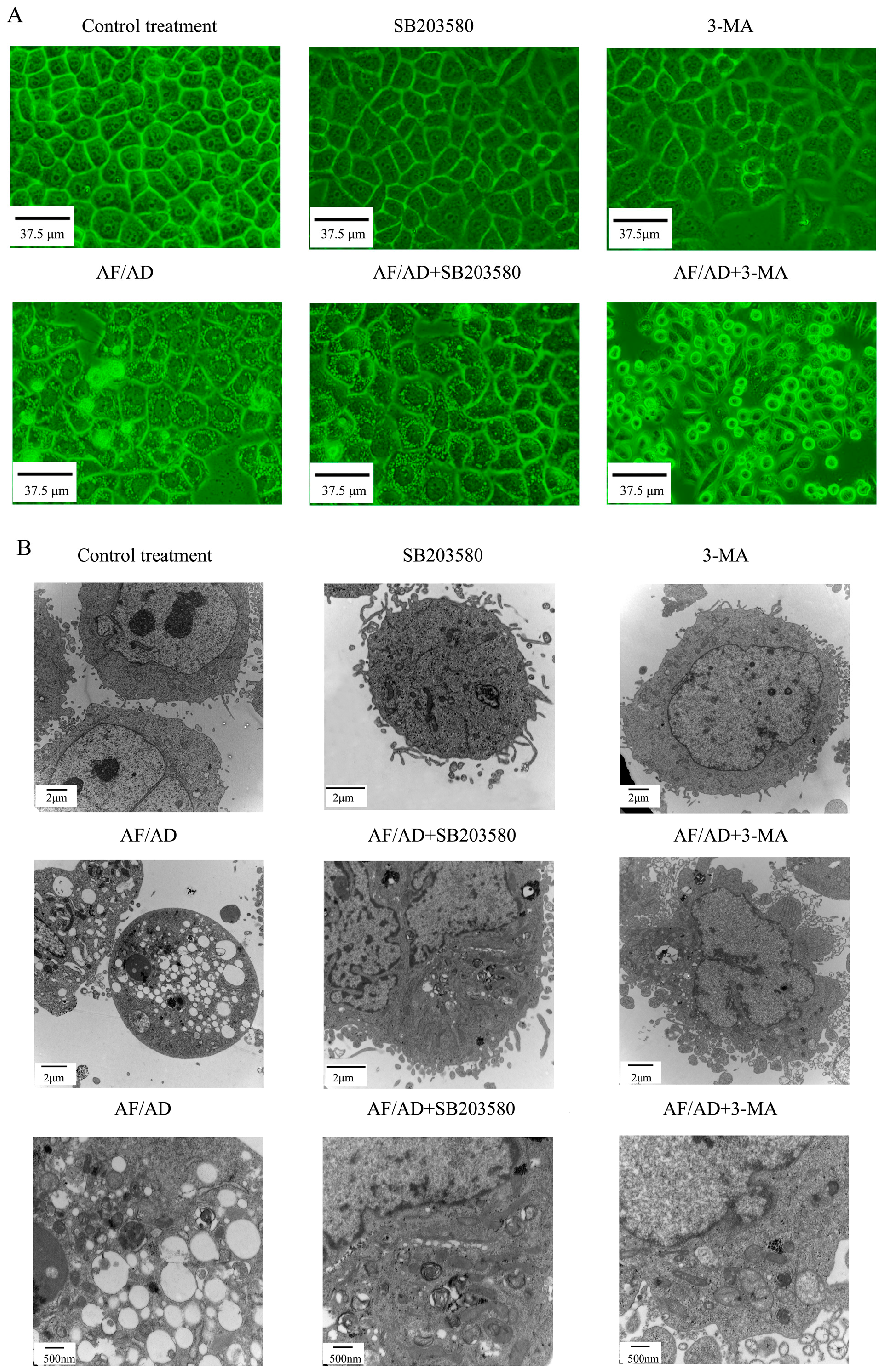
2.1. AF/AD Induces Apoptosis Concomitant with Autophagy in Bel-7402 Cells
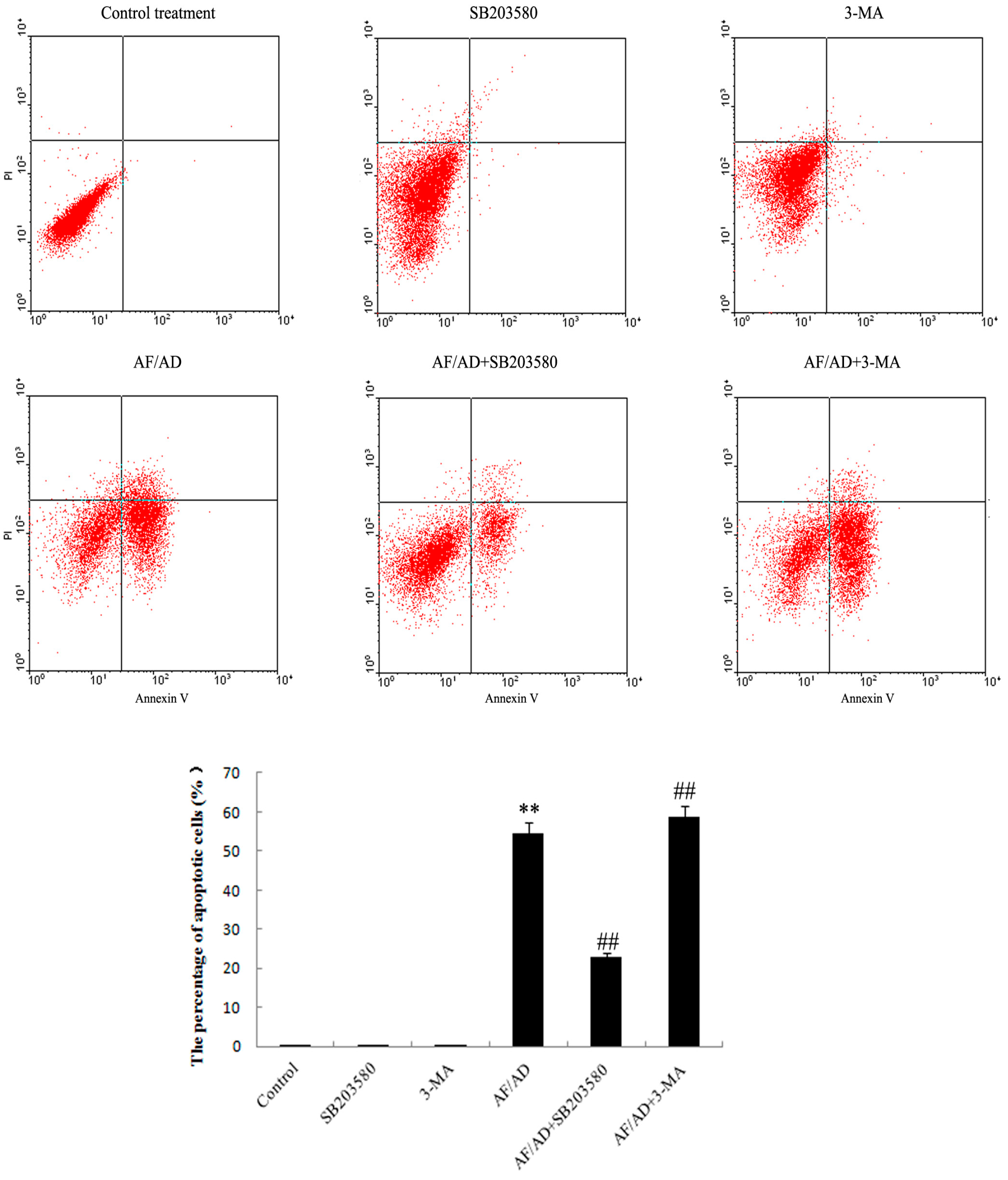
2.2. Autophagy Regulates AF/AD-Induced Apoptosis of Bel-7402 Cells
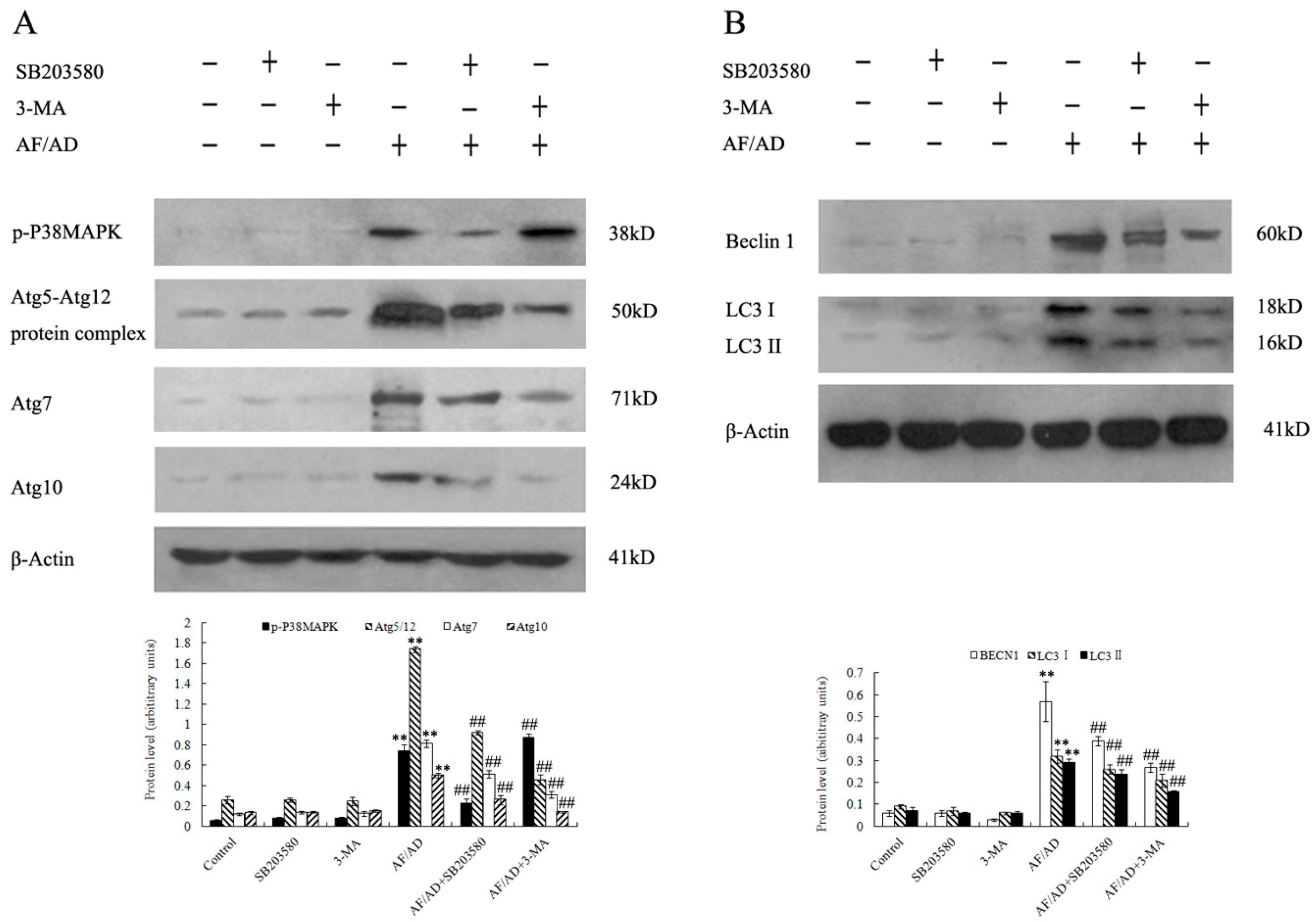
2.3. P38MAPK Regulates AF/AD-Induced Apoptosis Concomitant with Autophagy in Bel-7402 Cells
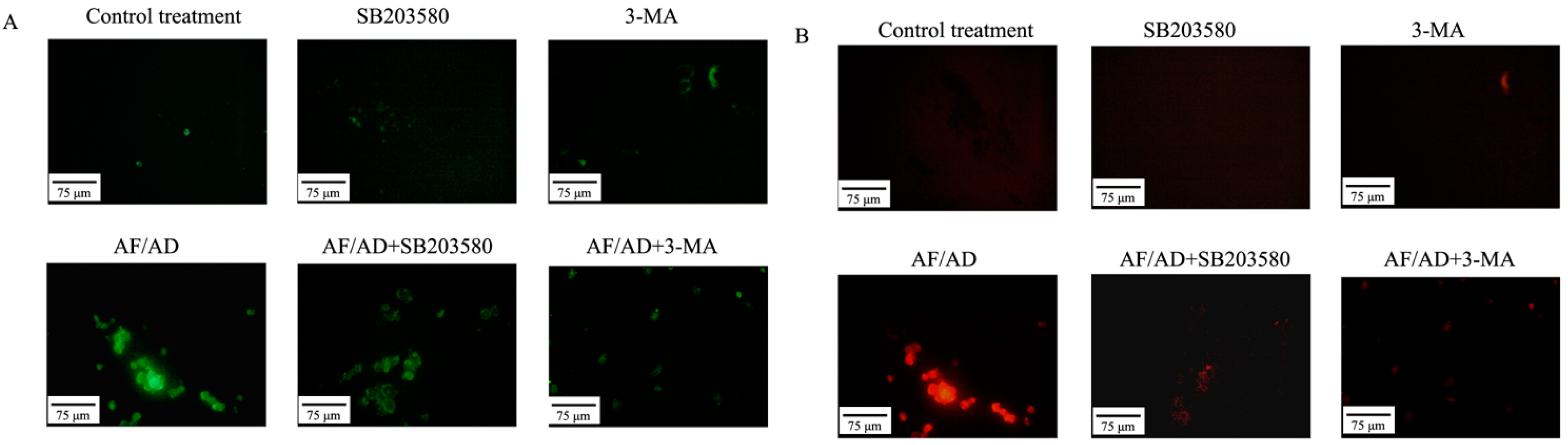
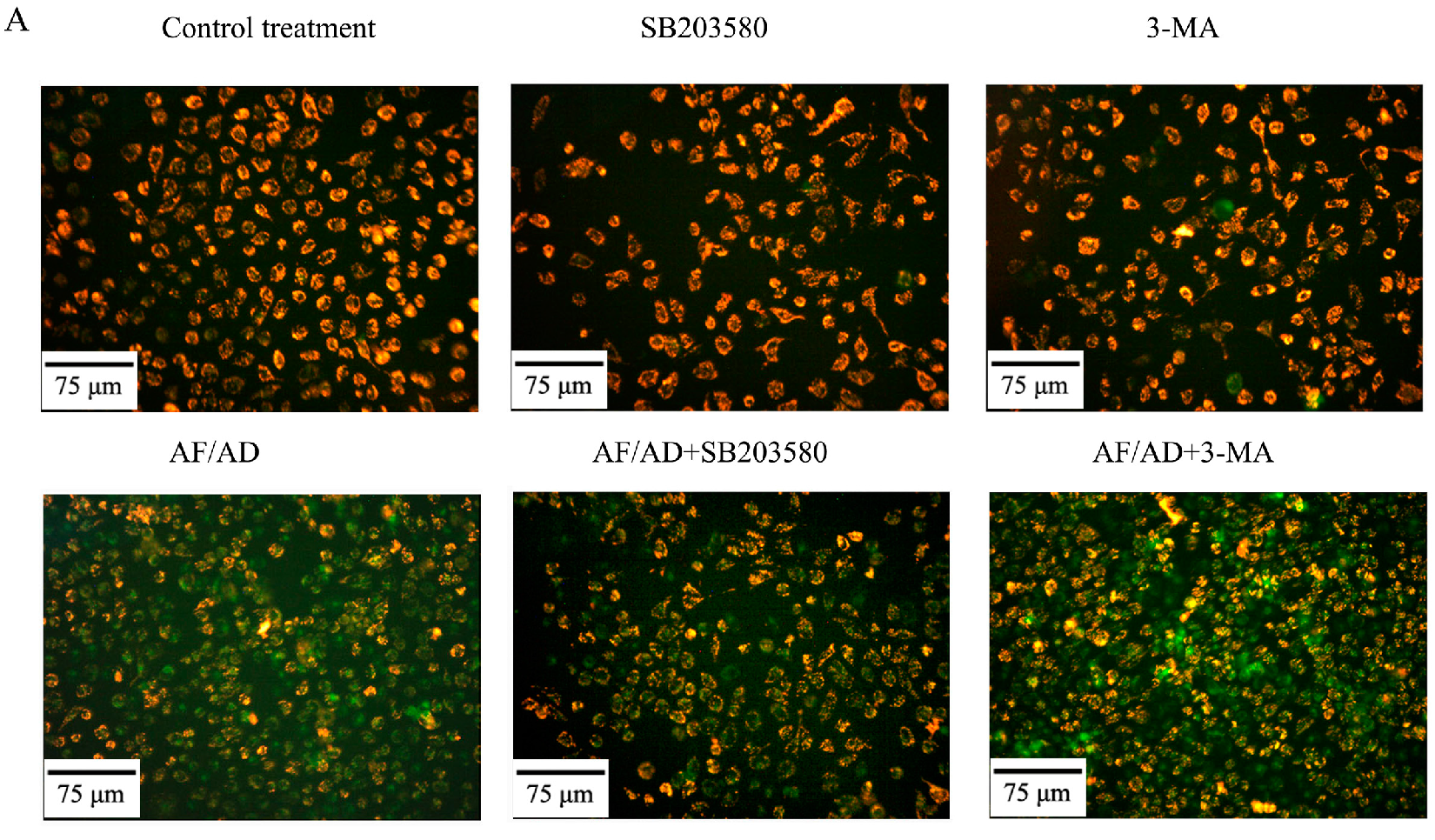
2.4.Crosstalk between P38MAPK and Autophagy Regulates AF/AD-Induced Apoptosis of Bel-7402 Cells
2.5. Crosstalk between P38MAPK and Autophagy Regulates Mitochondria in AF/AD-Induced Apoptosis of Bel-7402 Cells
3. Discussion
4. Exprimental Section
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Cell Morphology Assay
4.4. Annexin V-FLUOS/Propidium Iodide (PI) Double-Staining Analysis of Apoptosis
4.5. Immunoblot Assay
4.6. Immunofluorescence Assay of Beclin-1 and LC3
4.7. JC-1 Assay for ΔΨm
4.8. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, N.; Liu, L.; Liu, W.W.; Li, F.; Hayashi, T.; Tashiro, S.I.; Onodera, S.; Ikejima, T. Crosstalk of ROS/RNS and autophagy in silibinin-induced apoptosis of MCF-7 human breast cancer cells in vitro. Acta Pharmacol. Sin. 2017, 38, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Bastola, T.; An, R.B.; Kim, Y.C.; Kim, J.; Seo, J. Cearoin Induces Autophagy, ERK Activation and Apoptosis via ROS Generation in SH-SY5Y Neuroblastoma Cells. Molecules 2017, 22, 242. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, G.; Peng, F.; Jie, X.; Dongye, G.; Cai, K.; Feng, R.; Li, B.; Zeng, Q.; Lun, K.; et al. The induction of autophagy against mitochondria-mediated apoptosis in lung cancer cells by a ruthenium (II) imidazole complex. Oncotarget 2016, 7, 80716–80734. [Google Scholar]
- Wang, X.; Wei, S.; Zhao, Y.; Shi, C.; Liu, P.; Zhang, C.; Lei, Y.; Zhang, B.; Bai, B.; Huang, Y.; et al. Anti-proliferation of breast cancer cells with itraconazole: Hedgehog pathway inhibition induces apoptosis and autophagic cell death. Cancer Lett. 2017, 385, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.Y.; Du, Z.Y.; Ma, D.L.; Chen, W.B.; Fu, W.Y.; Ruan, B.B.; Rui, W.; Zhang, J.X.; Wang, S.; Wong, N.S.; et al. B5, a thioredoxin reductase inhibitor, induces apoptosis in human cervical cancer cells by suppressing the thioredoxin system, disrupting mitochondrion-dependent pathways and triggeringautophagy. Oncotarget 2015, 6, 30939–30956. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Gong, K.; Chen, C.; Wang, H.; Li, W. P38 MAP kinase functions as a switch in MS-275-induced reactive oxygen species-dependent autophagy and apoptosis in human colon cancer cells. Free Radic. Biol. Med. 2012, 53, 532–543. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Morcillo, M.A.; Valero, M.L.; Callejas-Valera, J.L.; Arias-González, L.; Melgar-Rojas, P.; Galán-Moya, E.M.; García-Gil, E.; García-Cano, J.; Sánchez-Prieto, R. P38MAPK is a major determinant of the balance between apoptosis and autophagy triggered by 5-fluorouracil: Implication in resistance. Oncogene 2012, 31, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Schmich, K.; Schlatter, R.; Corazza, N.; Sá Ferreira, K.; Ederer, M.; Brunner, T.; Borner, C.; Merfort, I. Tumor necrosis factor α sensitizes primary murine hepatocytes to Fas/CD95-induced apoptosis in a Bim- and Bid-dependent manner. Hepatology 2011, 53, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Auh, S.; Blokh, L.; Long, C.; Gagnon, I.; Hamann, K.J. TNF-alpha induces transient resistance to Fas-induced apoptosis in eosinophilic acute myeloid leukemia cells. Cell. Mol. Immunol. 2007, 4, 43–52. [Google Scholar] [PubMed]
- Nagata, S.; Golstein, P. The Fas death factor. Science 1995, 267, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Zajkowicz, A.; Gdowicz-Kłosok, A.; Krześniak, M.; Ścieglińska, D.; Rusin, M. Actinomycin D and nutlin-3a synergistically promote phosphorylation of p53 on serine 46 in cancer cell lines of different origin. Cell Signal. 2015, 27, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Choong, M.L.; Yang, H.; Lee, M.A.; Lane, D.P. Specific activation of the p53 pathway by low dose actinomycin D: A new route to p53 based cyclotherapy. Cell Cycle 2009, 8, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Sobell, H. Actinomycin and DNA transcription. Proc. Natl. Acad. Sci. USA 1985, 82, 5328–5331. [Google Scholar] [CrossRef] [PubMed]
- Förster, A.; Falcone, F.H.; Gibbs, B.F.; Preussner, L.M.; Fiebig, B.S.; Altunok, H.; Seeger, J.M.; Cerny-Reiterer, S.; Rabenhorst, A.; et al. Anti-Fas/CD95 and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) differentially regulate apoptosis in normal and neoplastic human basophils. Leuk. Lymphoma 2013, 54, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Park, M.A.; Pejovic, V.; Kerisit, K.G.; Junius, S.; Thoene, J.G. Increased apoptosis in cystinotic fibroblasts and renal proximal tubule epithelial cells results from cysteinylation of protein kinase Cdelta. J. Am. Soc. Nephrol. 2006, 17, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Takemura, G.; Maruyama, R.; Kosai, K.; Takahashi, T.; Koda, M.; Hayakawa, K.; Kawase, Y.; Minatoguchi, S.; Fujiwara, H. Molecular mechanisms of non-apoptosis by Fas stimulation alone versus apoptosis with an additional actinomycin D in cultured cardiomyocytes. Cardiovasc. Res. 2002, 55, 787–798. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Xia, C.; Ye, L.; Wang, B. P38MAPK Regulates Caspase-3 by Binding to Caspase-3 in Nucleus of Human Hepatoma Bel-7402 Cells during Anti-Fas Antibody- and Actinomycin D-Induced Apoptosis. Biomed. Pharmacother. 2009, 63, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The Role of Atg Proteins in Autophagosome Formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.H.; Li, J.P.; Liu, S.L.; Zou, X.; Fang, L.H.; Zhou, J.Y.; Wu, J.; Xi, S.Y.; Chen, Y.; Zhang, Y.Y.; et al. Autophagy Protects from Raddeanin A-Induced Apoptosis in SGC-7901 HumanGastric Cancer Cells. Evid. Based Complement. Alternat. Med. 2016, 2016, 9406758. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wei, S.; Wang, J.; Liu, X. Isoorientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38 signaling pathways in HepG2 cancer cells. J. Agric. Food Chem. 2014, 62, 5390–5400. [Google Scholar] [CrossRef] [PubMed]
- Utaipan, T.; Athipornchai, A.; Suksamrarn, A.; Chunsrivirot, S.; Chunglok, W. Isomahanine induces endoplasmic reticulum stress and simultaneously triggers p38 MAPK-mediated apoptosis and autophagy in multidrug-resistant human oral squamous cell carcinomacells. Oncol. Rep. 2017, 37, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Yu, Y.; Guan, R.; Xu, Z.; Liang, H.; Hong, L. Vitamin K2 Induces Mitochondria-Related Apoptosis in Human Bladder Cancer Cells via ROS and JNK/p38 MAPK Signal Pathways. PLoS ONE 2016, 11, e0161886. [Google Scholar] [CrossRef] [PubMed]
- Beccafico, S.; Morozzi, G.; Marchetti, M.C.; Riccardi, C.; Sidoni, A.; Donato, R.; Sorci, G. Artesunate induces ROS-and p38 MAPK-mediated apoptosis and counteracts tumor growth in vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis 2015, 36, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Lee, S.Y.; Kang, S.S.; Kim, Y.S. Antitumor activity of jujuboside B and the underlying mechanism via induction of apoptosis and autophagy. J. Nat. Prod. 2014, 77, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Lee, D.H.; Joo, E.J.; Son, K.H.; Kim, Y.S. Akebia saponin PA induces autophagic and apoptotic cell death in AGS human gastric cancer cells. Food Chem. Toxicol. 2013, 59, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, K.; Liu, P.; Zhang, X.; Dong, X.; Gao, J.; Liu, Q.; Barr, M.P.; Zhang, Q.; Hou, X.; et al. Bafilomycin A1 induces caspase-independent cell death in hepatocellular carcinoma cells via targeting of autophagy and MAPK pathways. Sci. Rep. 2016, 6, 37052. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhu, H.; Sheng, F.; Tian, Y.; Zhou, J.; Chen, Y.; Li, S.; Lin, J. Activation of the MAPK11/12/13/14 (p38 MAPK) pathway regulates the transcription of autophagy genes in response to oxidative stress induced by a novel copper complex in HeLa cells. Autophagy 2014, 10, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, M.; Jiang, Z.; Zhao, F.; Xi, B.; Zhang, X.; Fu, H.; Zhou, K. Platycodin-D Induced Autophagy in Non-Small Cell Lung Cancer Cells via PI3K/Akt/mTOR and MAPK Signaling Pathways. J. Cancer 2015, 6, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Wang, M.M.; Wang, Y.H.; Zhang, Z.N.; Cao, H.R.; Lv, Y.H.; Yang, Y.; Fan, P.H.; Qiu, F.; Gao, X.M. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem. Toxicol. 2014, 67, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Luo, X.; Nie, P.; Wu, B.; Xu, W.; Shi, X.; Chang, H.; Li, B.; Yu, X.; Zou, Z. CQ synergistically sensitizes human colorectal cancer cells to SN-38/CPT-11 through lysosomal and mitochondrial apoptotic pathway via p53-ROS cross-talk. Free Radic. Biol. Med. 2017, 104, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Meng, F.; Li, S.; Liu, L.; Zhao, L.; Liu, Y.; Hu, Y.; Li, Z.; Yao, Y.; Xi, Z.; et al. Autophagy Induction by Endothelial-Monocyte Activating Polypeptide II Contributes to the Inhibition of Malignant Biological Behaviors by the Combination of EMAP II with Rapamycin in Human Glioblastoma. Front. Mol. Neurosci. 2015, 8, 74. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xia, C.; Lv, Y.; Li, C.; Mei, Q.; Li, H.; Wang, H.; Li, S. Crosstalk Influence between P38MAPK and Autophagy on Mitochondria-Mediated Apoptosis Induced by Anti-Fas Antibody/Actinomycin D in Human Hepatoma Bel-7402 Cells. Molecules 2017, 22, 1705. https://doi.org/10.3390/molecules22101705
Wang Y, Xia C, Lv Y, Li C, Mei Q, Li H, Wang H, Li S. Crosstalk Influence between P38MAPK and Autophagy on Mitochondria-Mediated Apoptosis Induced by Anti-Fas Antibody/Actinomycin D in Human Hepatoma Bel-7402 Cells. Molecules. 2017; 22(10):1705. https://doi.org/10.3390/molecules22101705
Chicago/Turabian StyleWang, Yu, Chunhui Xia, Yanxin Lv, Chengjun Li, Qingbu Mei, Hongmei Li, Haijun Wang, and Shuang Li. 2017. "Crosstalk Influence between P38MAPK and Autophagy on Mitochondria-Mediated Apoptosis Induced by Anti-Fas Antibody/Actinomycin D in Human Hepatoma Bel-7402 Cells" Molecules 22, no. 10: 1705. https://doi.org/10.3390/molecules22101705
APA StyleWang, Y., Xia, C., Lv, Y., Li, C., Mei, Q., Li, H., Wang, H., & Li, S. (2017). Crosstalk Influence between P38MAPK and Autophagy on Mitochondria-Mediated Apoptosis Induced by Anti-Fas Antibody/Actinomycin D in Human Hepatoma Bel-7402 Cells. Molecules, 22(10), 1705. https://doi.org/10.3390/molecules22101705



