Bioassay-Guided Isolated Compounds from Morinda officinalis Inhibit Alzheimer’s Disease Pathologies
Abstract
:1. Introduction
2. Results
2.1. Identification of Compounds 1–10 Isolated from M. officinalis
2.2. AChE, BChE, BACE1, and AGE Formation Inhibitory Activities of the Extracts and Fractions from M. officinalis
2.3. AChE, BChE, BACE1, and AGE Formation Inhibitory Activities of Compounds 1–10 Isolated from M. officinalis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Instruments and Reagents
4.3. Extraction, Fractionation, and Isolation of M. officinalis
4.4. Identification of Compounds Isolated from M. officinalis
4.4.1. NMR
4.4.2. UHPLC-ESI/LTQ-Orbitrap-HRMS Conditions
4.5. HPLC Analysis
4.6. Bioactivities Assay
4.6.1. Measurement of ChE Inhibitory Activities
4.6.2. Measurement of BACE1 Inhibition
4.6.3. Measurement of Inhibition of AGE Formation
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Potterat, O.; Hamburger, M. Morinda citrifolia (noni) fruit-phytochemistry, pharmacology, safety. Planta Med. 2007, 73, 191–199. [Google Scholar] [CrossRef]
- Wang, M.Y.; West, B.J.; Jensen, C.J.; Nowichi, D.; Su, C.; Palu, A.; Anderson, G. Morinda citrifolia (Noni): A literature review and recent advances in Noni research. Acta Pharmacol. Sin. 2002, 23, 1127–1141. [Google Scholar]
- Zhang, H.L.; Zhang, Q.W.; Zhang, X.Q.; Ye, W.C.; Wang, Y.T. Chemical constituents from the roots of Morinda officinalis. Chin. J. Nat. Med. 2010, 8, 192–195. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Wang, C.J.; Wang, X.; Chen, S.H.; Zhu, H.; Zhu, H.M. Extraction of polysaccharides from Morinda officinalis by response surface methodology and effect of the polysaccharides on bone-related genes. Carbohydr. Polym. 2011, 85, 23–28. [Google Scholar] [CrossRef]
- Wu, Y.B.; Zheng, C.J.; Qin, L.P.; Sun, L.N.; Han, T.; Jiao, L.; Zhang, Q.Y.; Wu, J.Z. Antiosteoporotic activity of anthraquinones from Morinda officinalis on osteoblasts and osteoclasts. Molecules 2009, 14, 573–583. [Google Scholar] [CrossRef]
- Bao, L.; Qin, L.; Liu, L.; Wu, Y.; Han, T.; Xue, L.; Zhang, Q. Anthraquinone compounds from Morinda officinalis inhibit osteoclastic bone resorption in vitro. Chem. Biol. Interact. 2011, 194, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Lu, Q.Y.; Lu, H.Y.; Liao, W.M.; Wu, Z.P.; Kuang, G.Z.; Feng, H.J. Protective effect of Morinda officinalis polysaccharides on bone degeneration in the aged rats. Int. J. Phys. Sci. 2011, 6, 112–115. [Google Scholar]
- Zhu, M.Y.; Wang, C.J.; Gu, Y.; He, C.S.; Teng, X.; Zhang, P.; Lin, N. Extraction, characterization of polysaccharides from Morinda officinalis and its antioxidant activities. Carbohydr. Polym. 2009, 78, 497–501. [Google Scholar]
- Zhang, H.L.; Li, J.; Li, G.; Wang, D.M.; Zhu, L.P.; Yang, D.P. Structural characterization and anti-fatigue activity of polysaccharides from the roots of Morinda officinalis. Int. J. Biol. Macromol. 2009, 44, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, K.T.; Choi, M.Y.; Nam, J.W.; Jung, H.J.; Park, S.K.; Park, H.J. Antinociceptive anti-inflammatory effect of monotropein isolated from the root of Morinda officinalis. Biol. Pharm. Bull. 2005, 28, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kim, S.B.; Ahn, J.H.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Anthraquinones from Morinda officinalis roots enhance adipocyte differentiation in 3T3-L1 cells. Nat. Prod. Res. 2012, 26, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Yamaguchi, S.; Nishisaka, H.; Yamahara, J.; Murakami, N. Chemical constituents of Chinese natural medicine, Morindae Radix, the dried roots of Morinda officinalis How: Structures of morindolide and morofficinaloside. Chem. Pharm. Bull. 1995, 43, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.L.; Crai, P.L.; Parsons, O.A. Neuropsychology of dementia. Neurol. Clin. 1984, 4, 387–405. [Google Scholar]
- Aisen, P.S.; Davis, K.L. The search for disease-modifying treatment for Alzheimer’s disease. Neurology 1997, 48, 35–41. [Google Scholar] [CrossRef]
- Jann, M.W. Preclinical pharmacology of metrifonate. Pharmacotherapy 1998, 18, 55–67. [Google Scholar] [PubMed]
- Ali, M.Y.; Jannat, S.; Jung, H.A.; Choi, R.J.; Roy, A.; Choi, J.S. Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis. Asian Pac. J. Trop. Med. 2016, 9, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Massoulie, J.; Pezzementi, L.; Bom, S.; Krejci, E.; Vallette, F.M. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 1993, 41, 31–91. [Google Scholar] [CrossRef]
- Liang, Y.; Rihui, C.; Wei, Y.; Qin, Y.; Zhiyong, C.; Lin, M.; Wenile, P.; Huacan, S. Synthesis of 4-[(diethylamino)methyl]-phenol derivatives as novel cholinesterase inhibitors with selectivity towards butyrylcholinesterase. Bioorg. Med. Chem. Lett. 2010, 20, 3254–3258. [Google Scholar]
- Querfurth, H.W.; LaFerla, F.M. Mechanisms of disease: Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Hong, H.S.; Nam, D.W.; Baik, S.H.; Song, H.; Kook, S.Y.; Kim, Y.S.; Lee, J.; Mook, J.I. Intracellular amyloid-b accumulation in calcium-binding protein-deficient neurons leads to amyloid-β plaque formation in animal model of Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 1–4. [Google Scholar]
- Pereira, C.; Agostinho, P.; Moreira, P.I.; Cardoso, S.M.; Oliveira, C.R. Alzheimer’s disease-associated neurotoxic mechanisms and neuroprotective strategies. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 383–403. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Bienkowski, M.J.; Shuck, M.E.; Miao, H.; Tory, M.C.; Pauley, A.M.; Brashler, J.R.; Stratman, N.C.; Mathews, W.R.; Buhl, A.E. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature 1999, 402, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R. β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Sciences 1999, 286, 735–741. [Google Scholar] [CrossRef]
- Kuk, E.B.; Jo, A.R.; Oh, S.I.; Sohn, H.S.; Seong, S.H.; Roy, A.; Choi, J.S.; Jung, H.A. Anti-Alzheimer’s disease activity of compounds from the root bark of Morus alba L. Arch. Pharm. Res. 2017, 40, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Jung, H.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against β-secretase and cholinesterases. J. Ethnopharmacol. 2016, 191, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Fukatsu, R.; Tsuzuki, K.; Hayashi, Y.; Yoshida, T.; Fujii, N.; Koike, T.; Wakayama, I.; Yanagihara, R.; Garruto, R.; et al. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am. J. Pathol. 1998, 153, 1149–1155. [Google Scholar] [CrossRef]
- Schulz, V. Ginkgo extract or cholinesterase inhibitors in patients with dementia: What clinical trials and guidelines fail to consider. Phytomedicine 2003, 10, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Robins, R.V.; Barry, P.P.; Buckholts, N.S.; Dekosky, S.T.; Ferris, S.H.; Finkel, S.I.; Gwyther, L.P.; Khachaturian, Z.S.; Lebowitz, B.D.; et al. Diagnosis and treatment of Alzheimer’s disease and related disorder. JAMA 1997, 278, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D. New drug treatment for Alzheimer’s disease: Lesson for healthcare policy. BMJ 1998, 316, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Park, S.Y.; Choo, B.K.; Chun, J.M.; Lee, A.Y.; Kim, H.K. Standardization of Morinda officinalis How. Kor. J. Pharmacogn. 2006, 37, 241–245. [Google Scholar]
- Sun, P.; Huo, J.; Kurtan, T.; Mandi, A.; Antus, S.; Tang, H.; Draeger, S.; Schulz, B.; Hussain, H.; Krohn, K.; et al. Structural and stereochemical studies of hydroxyanthraquinone derivatives from the endophytic fungus Coniothyrium sp. Chirality 2013, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Hong, E.Y.; Whang, W.K. Inhibitory effect of chemical constituents isolated from Artemisia iwayomogi on polyol pathway and simultaneous quantification of major bioactive compounds. Biomed. Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and acethyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res. 2004, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Katzman, R. Early detection of senile dementia. Hosp. Pract. 1981, 16, 61–76. [Google Scholar]
- Heuvel, C.V.D.; Thornton, E.; Vink, R. Traumatic brain injury and Alzheimer’s disease: A review. Prog. Brain Res. 2007, 161, 303–316. [Google Scholar]
- Orhan, I.; Senol, F.S.; Kartal, M.; Dborska, M.; Zemlicka, M.; Smejkal, K.; Mokry, P. Cholinesterase inhibitory effects of the extracts and compounds of Maclura pomifera (Rafin.) Schneider. Food Chem. Toxicol. 2009, 47, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, P.; Sabphone, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Park, J.J.; Islam, M.N.; Jin, S.E.; Min, B.S.; Lee, J.H.; Sohn, H.S.; Choi, J.S. Inhibitory activity of coumarins from Artemisia capillaris against advanced glycation endproduct formation. Arch. Pharm. Res. 2012, 35, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
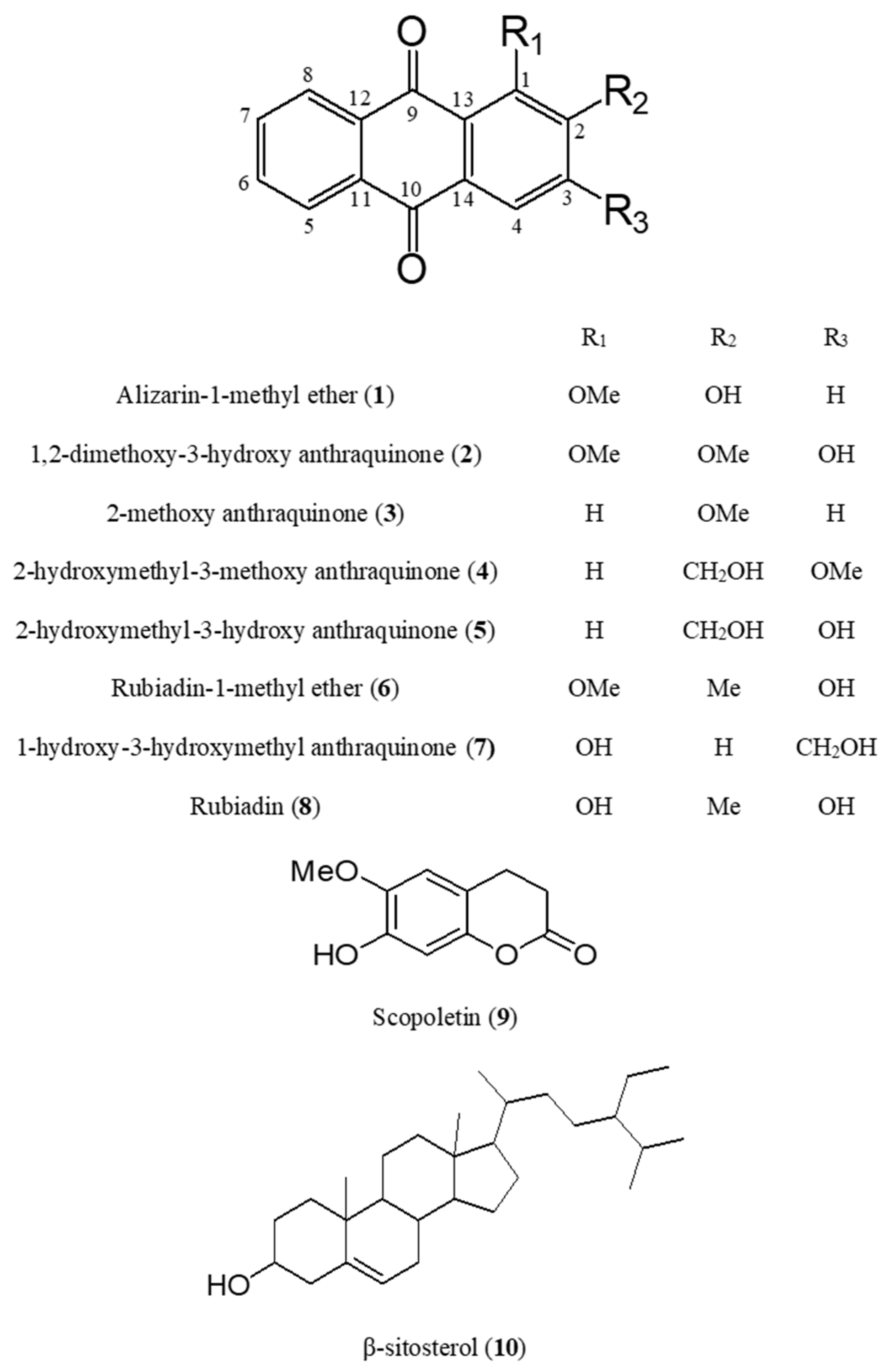
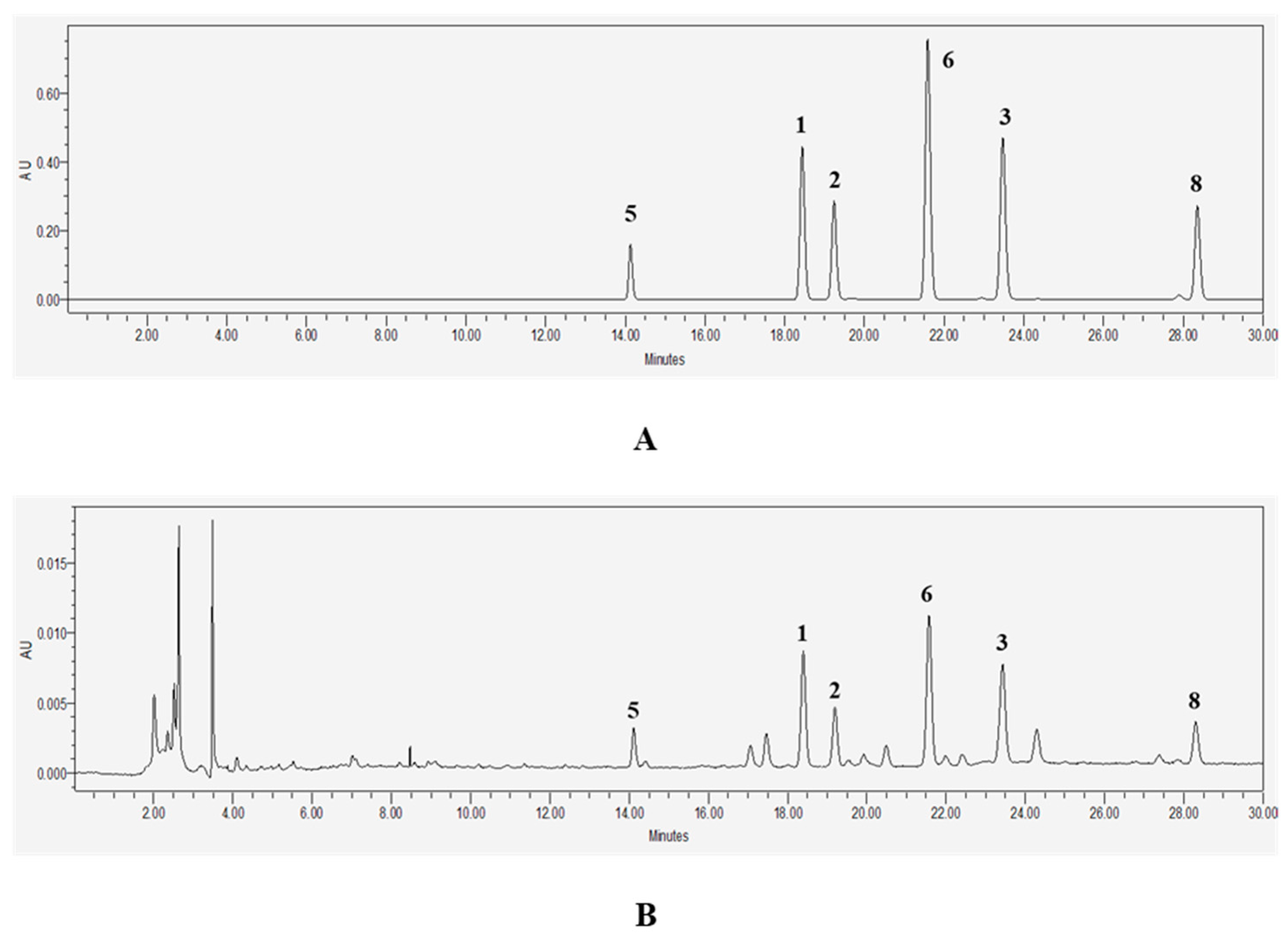
| No. | Compound | Rt (min) | Formula | Mass Mode | Theoretical Mass | Observed Mass | Mass Error (Da) | Mass Accuracy (ppm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Alizarin-1-methyl ether | 7.84 | C15H10O4 | Positive | 255.0652 | 255.0652 | 0.0000 | 0.0 |
| 2 | 1,2-dimethoxy-3-hydroxy anthraquinone | 7.95 | C16H12O5 | Positive | 285.0757 | 285.0758 | 0.0001 | 0.4 |
| 3 | 2-methoxy anthraquinone | 8.61 | C15H10O3 | Positive | 239.0703 | 239.0706 | 0.0003 | 1.3 |
| 4 | 2-hydroxymethyl-3-methoxy anthraquinone | 7.15 | C16H12O4 | Negative | 267.0653 | 267.0655 | 0.0002 | 0.7 |
| 5 | 2-hydroxymethyl-3-hydroxy anthraquinone | 7.16 | C15H10O4 | Positive | 253.0573 | 253.0574 | 0.0001 | 0.4 |
| 6 | Rubiadin-1-methyl ether | 8.29 | C15H10O4 | Positive | 269.0808 | 269.0808 | 0.0000 | 0.0 |
| 7 | 1-hydroxy-3-hydroxymethyl anthraquinone | 8.70 | C16H12O4 | Negative | 253.0452 | 253.0455 | 0.0003 | 1.2 |
| 8 | Rubiadin | 9.26 | C15H10O4 | Positive | 255.0652 | 255.0654 | 0.0002 | 0.8 |
| 9 | Scopoletin | 5.65 | C10H8O4 | Positive | 193.0495 | 193.0497 | 0.0002 | 1.0 |
| 10 | β-sitosterol | 13.42 | C29H50O | Positive | 437.3754 | 437.3768 | 0.0014 | 3.2 |
| Sample | IC50 a (μg/mL) | |||
|---|---|---|---|---|
| AChE | BChE | BACE1 | AGE Formation | |
| Ext. | 58.82 ± 9.13 ** | 445.55 ± 32.05 ** | 24.40 ± 2.84 *** | ND e |
| Hx fr. | 33.66 ± 4.73 ** | 105.99 ± 0.69 *** | 42.36 ± 3.94 ** | 166.03 ± 7.76 *** |
| EA fr. | 80.14 ± 16.65 * | >500 | 64.45 ± 4.22 ** | 417.92 ± 14..29 *** |
| BuOH fr. | 188.83 ± 2.44 *** | >500 | ND e | ND e |
| Water fr. | >500 | ND e | ND e | ND e |
| Berberine b | 0.14 ± 0.01 *** | 1.70 ± 0.07 ** | - | - |
| AG c | - | - | - | 104.87 ± 6.94 *** |
| Quercetin d | - | - | 6.87 ± 0.36 ** | - |
| Compound | IC50 a (μM) | |||
|---|---|---|---|---|
| AChE | BChE | BACE1 | AGEs Formation | |
| 1 | 174.83 ± 10.71 ** | 450.47 ± 8.82 *** | 192.41 ± 7.32 *** | 292.37 ± 2.28 ** |
| 2 | 147.00 ± 13.33 ** | 441.53 ± 10.58 ** | 114.63 ± 21.62 * | 437.86 ± 23.94 ** |
| 3 | 187.20 ± 20.12 * | 230.18 ± 5.97 ** | 9.29 ± 1.92 ** | 88.40 ± 3.28 ** |
| 4 | 27.05 ± 1.49 ** | >500 | >200 | 529.79 ± 15.53 ** |
| 5 | 19.06 ± 3.58 * | 459.02 ± 13.11 ** | >200 | 355.03 ± 12.00 ** |
| 6 | 87.19 ± 6.56 ** | >500 | 25.89 ± 2.11 ** | >1000 |
| 7 | 96.38 ± 17.23 ** | >500 | 178.43 ± 12.15 *** | 178.43 ± 12.15 *** |
| 8 | 44.31 ± 12.20 * | >500 | 19.82 ± 3.05 * | 522.42 ± 10.11 ** |
| 9 | 235.70 ± 21.17 ** | 50.43 ± 1.61 *** | >200 | 5.43 ± 0.11 *** |
| 10 | >500 | >500 | ND e | ND e |
| Berberine b | 0.42 ± 0.03 * | 5.05 ± 0.21 ** | - | - |
| AG c | - | - | - | 762.05 ± 69.10 *** |
| Quercetin d | - | - | 22.75 ± 1.20 *** | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.K.; Bang, H.J.; Oh, J.B.; Whang, W.K. Bioassay-Guided Isolated Compounds from Morinda officinalis Inhibit Alzheimer’s Disease Pathologies. Molecules 2017, 22, 1638. https://doi.org/10.3390/molecules22101638
Lee YK, Bang HJ, Oh JB, Whang WK. Bioassay-Guided Isolated Compounds from Morinda officinalis Inhibit Alzheimer’s Disease Pathologies. Molecules. 2017; 22(10):1638. https://doi.org/10.3390/molecules22101638
Chicago/Turabian StyleLee, Yoon Kyoung, Hyo Jeong Bang, Jeong Bin Oh, and Wan Kyunn Whang. 2017. "Bioassay-Guided Isolated Compounds from Morinda officinalis Inhibit Alzheimer’s Disease Pathologies" Molecules 22, no. 10: 1638. https://doi.org/10.3390/molecules22101638
APA StyleLee, Y. K., Bang, H. J., Oh, J. B., & Whang, W. K. (2017). Bioassay-Guided Isolated Compounds from Morinda officinalis Inhibit Alzheimer’s Disease Pathologies. Molecules, 22(10), 1638. https://doi.org/10.3390/molecules22101638





