Recent Update on the Role of Chinese Material Medica and Formulations in Diabetic Retinopathy
Abstract
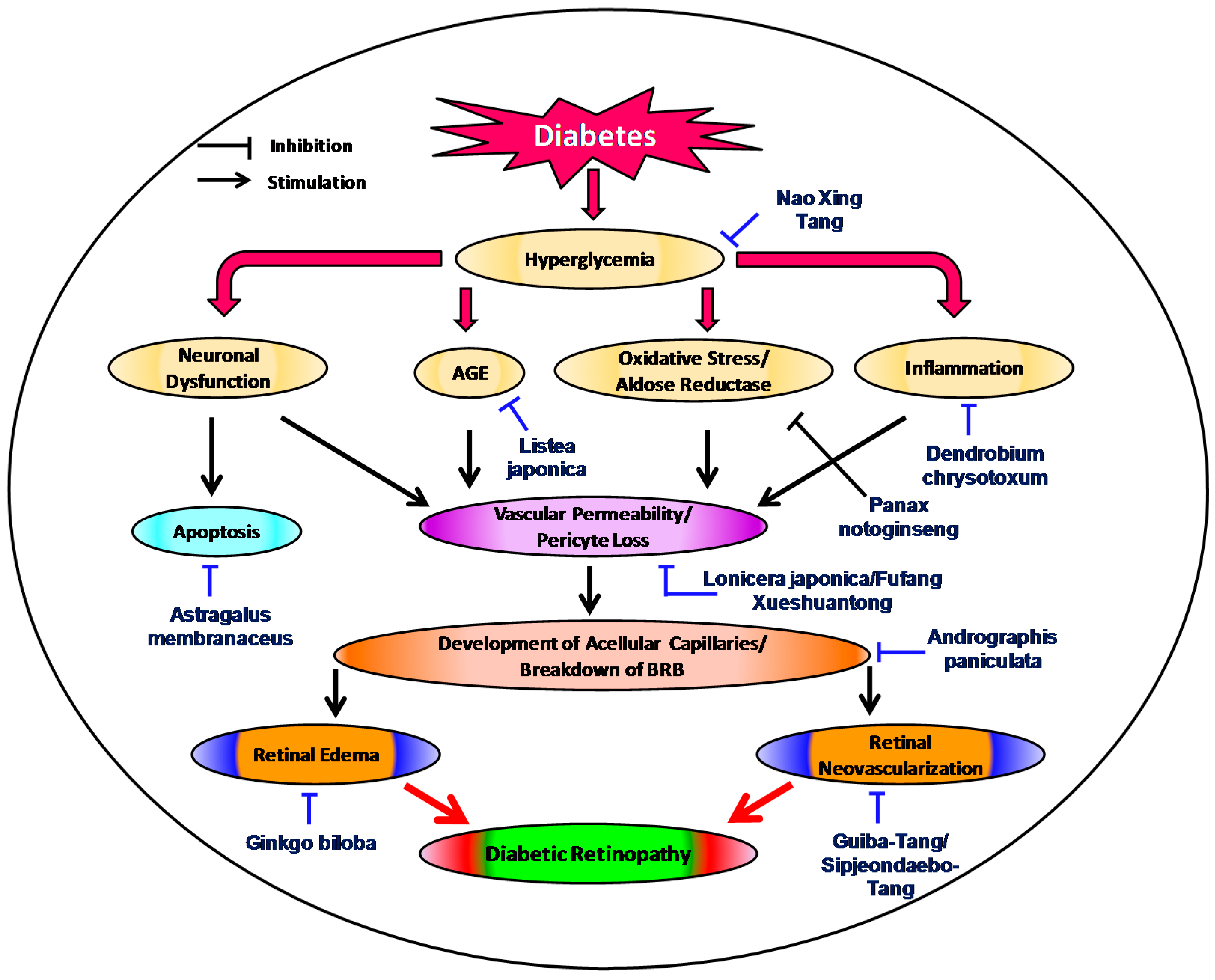
:1. Introduction
2. Status of Traditional Herbal Medicine in Diabetic Retinopathy
3. Recent Evidences of Traditional Medicinal Plants in Diabetic Retinopathy
3.1. Litsea japonica
3.2. Ginkgo biloba
3.3. Pueraria lobata
3.4. Lonicera japonica
3.5. Andrographis paniculata
3.6. Astragalus membranaceus
3.7. Salvia miltiorrhiza
3.8. Dendrobium chrysotoxum
3.9. Vaccinium myrtillus
3.10. Zingiber zerumbet
3.11. Trigonella foenum-graecum
3.12. Guibi-Tang
3.13. Samul-Tang
3.14. Fufang Xueshuantong
3.15. Ligusticum chuanxiong Hort
3.16. Sipjeondaebo-Tang
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A. Diabetic retinopathy: Pathogenic mechanisms and current treatments. Diabetes Metab. Syndr. Clin. Res. Rev. 2011, 5, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.H. Diabetic retinopathy. Medicine 2010, 38, 656–660. [Google Scholar] [CrossRef]
- Shin, E.S.; Sorenson, C.M.; Sheibani, N. Diabetes and retinal vascular dysfunction. J. Ophthalmic Vis. Res. 2014, 9, 362. [Google Scholar] [PubMed]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar] [PubMed]
- Control, D.; Group, C.T.R. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Stratton, I.; Kohner, E.; Aldington, S.; Turner, R.; Holman, R.; Manley, S.; Matthews, D. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001, 44, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E.; Cruickshanks, K.J. The Wisconsin epidemiologic study of diabetic retinopathy: XIV. Ten-year incidence and progression of diabetic retinopathy. Arch. Ophthalmol. 1994, 112, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Klein, B.E. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII: The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008, 115, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L., 3rd; Klein, R. Diabetic retinopathy. Diabetes Care 1998, 21, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Chen, H.; Tinkham, N.H.; Zhang, K. Genetic susceptibility of diabetic retinopathy. Curr. Diabetes Rep. 2008, 8, 257–262. [Google Scholar] [CrossRef]
- Warpeha, K.; Chakravarthy, U. Molecular genetics of microvascular disease in diabetic retinopathy. Eye 2003, 17, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.T.; Herman, I.M. Microvascular modifications in diabetic retinopathy. Curr. Diabetes Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C. The clinical features and classification of diabetic retinopathy. Ophthalmic Physiol. Opt. 1996, 16, S43–S48. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.I.; Siddiquei, M.M.; Al-Amro, S.; El-Asrar, A.M.A. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes Complicat. 2012, 26, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Patel, A.; Group, A.C. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet 2007, 370, 829–840. [Google Scholar] [CrossRef]
- Lang, G. Laser treatment of diabetic retinopathy. Dev. Ophthalmol. 2007, 39, 48–68. [Google Scholar] [PubMed]
- Lewis, H. The role of vitrectomy in the treatment of diabetic macular edema. Am. J. Ophthalmol. 2001, 131, 123–125. [Google Scholar] [CrossRef]
- Li, D.-D.; Chen, J.-H.; Chen, Q.; Li, G.-W.; Chen, J.; Yue, J.-M.; Chen, M.-L.; Wang, X.-P.; Shen, J.-H.; Shen, X. Swietenia mahagony extract shows agonistic activity to PPAR gamma and gives ameliorative effects on diabetic db/db mice. Acta Pharmacol. Sin. 2005, 26, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Al-Rowais, N.A. Herbal medicine in the treatment of diabetes mellitus. Saudi Med. J. 2002, 23, 1327–1331. [Google Scholar] [PubMed]
- Bailey, C.J.; Day, C. Traditional plant medicines as treatments for diabetes. Diabetes Care 1989, 12, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Dey, L.; Attele, A. Alternative therapies for type 2 Diabetes. Altern. Med. Rev. 2002, 7, 45–58. [Google Scholar] [PubMed]
- Head, K. Natural therapies for ocular disorders, part one: Diseases of the retina. Altern. Med. Rev. J. Clin. Ther. 1999, 4, 342–359. [Google Scholar]
- Ceylan-Isik, A.F.; Fliethman, R.M.; Wold, L.E.; Ren, J. Herbal and traditional Chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Curr. Diabetes Rev. 2008, 4, 320–328. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Traditional Medicine Strategy 2014–2023. Available online: http://apps.who.int/iris/handle/10665/92455 (accessed on 15 May 2016).
- World Health Organization. Legal Status of Traditional Medicine and Complementary/Alternative Medicine: A Worldwide Review. Available online: http://apps.who.int/medicinedocs/en/d/Jh2943e/ (accessed on 15 May 2016).
- Patwardhan, B.; Mashelkar, R.A. Traditional medicine-inspired approaches to drug discovery: Can Ayurveda show the way forward? Drug Discov. Today 2009, 14, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B. 2008 FDA drug approvals. Nat. Rev. Drug Discov. 2009, 8, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-X.; Li, X.-J.; Zhang, H.-Y. Where is the hope for drug discovery? Let history tell the future. Drug Discov. Today 2009, 14, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Chan, P. Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evid.-Based Complement. Altern. Med. 2013, 2013, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.-S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Litsea japonica extract inhibits neuronal apoptosis and the accumulation of advanced glycation end products in the diabetic mouse retina. Mol. Med. Rep. 2015, 12, 1075–1081. [Google Scholar] [PubMed]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lu, B.; Sheng, Y.; Zhou, L.; Ji, L.; Wang, Z. Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yuan, S.; Liu, X.; Mao, P.; Zhao, C.; Huang, Q.; Zhang, R.; Fang, Y.; Song, Q.; Yuan, D. Protective Effects of Astragaloside IV on db/db Mice with Diabetic Retinopathy. PLoS ONE 2014, 9, e112207. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, Y.M.; Ahn, E.M.; Kim, K.W.; Yu, Y.S. Decursin inhibits retinal neovascularization via suppression of VEGFR-2 activation. Mol. Vis. 2009, 15, 1868–1875. [Google Scholar] [PubMed]
- Gong, C.-Y.; Yu, Z.-Y.; Lu, B.; Yang, L.; Sheng, Y.-C.; Fan, Y.-M.; Ji, L.-L.; Wang, Z.-T. Ethanol extract of Dendrobium chrysotoxum Lindl ameliorates diabetic retinopathy and its mechanism. Vasc. Pharmacol. 2014, 62, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Min, B.-S.; Kim, J.-H.; Lee, J.-K.; Kim, T.-J.; Kim, C.-S.; Kim, Y.-H.; Lee, H.-K. Flavonoids from the leaves of Litsea japonica and their anti-complement activity. Phytother. Res. 2005, 19, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Lee, S.Y.; Kim, J.H.; Kwon, O.K.; Park, B.Y.; An, R.B.; Lee, J.K.; Moon, H.I.; Kim, T.J.; Kim, Y.H. Lactones from the Leaves of Litsea j aponica and Their Anti-complement Activity. J. Nat. Prod. 2003, 66, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.-S.; Lee, I.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.H.; Kim, J.S. Extract of Litsea japonica ameliorates blood-retinal barrier breakdown in db/db mice. Endocrine 2014, 46, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.-J.R.; Perry, N.S.; Houghton, P.J. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother. Res. 2003, 17. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, K.; Flora, S. Oral co-administration of α-lipoic acid, quercetin and captopril prevents gallium arsenide toxicity in rats. Environ. Toxicol. Pharmacol. 2009, 28, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Bronner, C.; Landry, Y. Kinetics of the inhibitory effect of flavonoids on histamine secretion from mast cells. Agents Actions 1985, 16, 147–151. [Google Scholar] [CrossRef]
- Fiorani, M.; de Sanctis, R.; Menghinello, P.; Cucchiarini, L.; Cellini, B.; Dachà, M. Quercetin prevents glutathione depletion induced by dehydroascorbic acid in rabbit red blood cells. Free Radic. Res. 2009, 34, 639–648. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.-X.; Xing, N.-Z.; Cao, X.-G. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 246, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, M.; Tuo, J.; Shen, D.; Chan, C.-C. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp. Eye Res. 2010, 91, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. 2012, 47, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar Gupta, S.; Kumar, B.; Srinivasan, B.; Nag, T.C.; Srivastava, S.; Saxena, R.; Aggarwal, A. Retinoprotective effects of Moringa oleifera via antioxidant, anti-inflammatory, and anti-angiogenic mechanisms in streptozotocin-induced diabetic rats. J. Ocul. Pharmacol. Ther. 2013, 29, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.M.; Kim, C.-S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free Radic. Biol. Med. 2012, 53, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Cui, H.; Yang, M.; Song, H.; Zhang, Q.; Su, Y.; Zheng, J. Protective effect of puerarin on diabetic retinopathy in rats. Mol. Biol. Rep. 2009, 36, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-L.; Liu, I.-M.; Kuo, D.-H.; Chen, W.-C.; Su, H.-C.; Cheng, J.-T. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J. Nat. Prod. 2003, 66, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xiao, S.; Lu, G.; Liang, Y.; Bi, X. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur. J. Pharmacol. 2006, 529, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, Y.M.; Lee, G.Y.; Jang, D.S.; Bae, K.H.; Kim, J.S. Constituents of the roots of Pueraria lobata inhibit formation of advanced glycation end products (AGEs). Arch. Pharm. Res. 2006, 29, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, W.; Guo, L.; Gu, Y.; Cai, P.; Xie, N.; Yang, X.; Shu, Y.; Wu, X.; Sun, Y. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int. Immunopharmacol. 2014, 20, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.-N.; Wang, M.; Ma, J.-L.; Yang, T. Puerarin decreases apoptosis of retinal pigment epithelial cells in diabetic rats by reducing peroxynitrite level and iNOS expression. Sheng Li Xue Bao 2012, 64, 199–206. [Google Scholar] [PubMed]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.M.; Zhang, X.G.; Zhu, J.J.; Gao, H.M.; Wang, Z.M.; Wang, W.H. Two new triterpenoid saponins from the flowers and buds of Lonicera japonica. J. Asian Nat. Prod. Res. 2008, 10, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; De Souza, G.E.P. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Puupponen Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dong, L.; Bai, Y.; Zhao, J.; Zhang, Y.; Zhang, L. Chlorogenic acid against carbon tetrachloride-induced liver fibrosis in rats. Eur. J. Pharmacol. 2009, 623, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Arion, W.J.; Canfield, W.K.; Ramos, F.C.; Schindler, P.W.; Burger, H.-J.; Hemmerle, H.; Schubert, G.; Below, P.; Herling, A.W. Chlorogenic acid and hydroxynitrobenzaldehyde: New inhibitors of hepatic glucose 6-phosphatase. Arch. Biochem. Biophys. 1997, 339, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Herling, A.W.; Burger, H.-J.; Schubert, G.; Hemmerle, H.; Schaefer, H.-L.; Kramer, W. Alterations of carbohydrate and lipid intermediary metabolism during inhibition of glucose-6-phosphatase in rats. Eur. J. Pharmacol. 1999, 386, 75–82. [Google Scholar] [CrossRef]
- De Sotillo, D.V.R.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef]
- Lim, J.C.W.; Chan, T.K.; Ng, D.S.W.; Sagineedu, S.R.; Stanslas, J.; Wong, W.S.F. Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer. Clin. Exp. Pharmacol. Physiol. 2012, 39, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, L.; Wang, X.; Liao, B.; Li, G. Inhibition of NF-κB expression and allergen-induced airway inflammation in a mouse allergic asthma model by andrographolide. Cell. Mol. Immunol. 2009, 6, 381. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Tee, W.; Ng, D.; Chan, T.; Peh, H.; Ho, W.; Cheng, C.; Mak, J.; Wong, W. Andrographolide protects against cigarette smoke-induced oxidative lung injury via augmentation of Nrf2 activity. Br. J. Pharmacol. 2013, 168, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, W.; Xiao, M.; Chen, H.; Jin, H. Protective role of andrographolide in bleomycin-induced pulmonary fibrosis in mice. Int. J. Mol. Sci. 2013, 14, 23581–23596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Hufnagl, P.; Binder, B.R.; Wojta, J. Anti-inflammatory activity of astragaloside IV is mediated by inhibition of NF-κB activation and adhesion molecule expression. Thromb. Haemost. 2003, 90, 904–914. [Google Scholar] [PubMed]
- Zheng, Z.; Liu, D.; Song, C.; Cheng, C.; Hu, Z. Studies on chemical constituents and immunological function activity of hairy root of Astragalus membranaceus. Chin. J. Biotechnol. 1997, 14, 93–97. [Google Scholar]
- Gui, D.; Guo, Y.; Wang, F.; Liu, W.; Chen, J.; Chen, Y.; Huang, J.; Wang, N. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS ONE 2012, 7, e39824. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, W.; Sun, W.-Y.; Li, X. Protective effects of astragaloside IV on porcine-serum-induced hepatic fibrosis in rats and in vitro effects on hepatic stellate cells. J. Ethnopharmacol. 2009, 122, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Wu, S.-Y.; Wang, G.-F.; Zhang, J.-J.; Pang, J.-X.; Liu, Z.-Q.; Xu, W.; Wu, S.-G.; Rao, J.-J. Effect of astragaloside IV on hepatic glucose-regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Phytother. Res. 2010, 24, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Fujiwara, Y.; Kiyota, N.; Tsurushima, K.; Takeya, M.; Nohara, T.; Nagai, R.; Ikeda, T. Astragalosides isolated from the root of astragalus radix inhibit the formation of advanced glycation end products. J. Agric. Food Chem. 2009, 57, 7666–7672. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Sun, S.; Shen, J.; Qiu, J.; Yin, X.; Yin, H.; Jiang, S. Inhibitory effects of astragaloside IV on diabetic peripheral neuropathy in rats. Can. J. Physiol. Pharmacol. 2006, 84, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhang, L.; Wang, X.-Y.; Guo, J.-H.; Guo, Z.-X.; Ma, X.-H. The effect of Compound Danshen Dripping Pills, a Chinese herb medicine, on the pharmacokinetics and pharmacodynamics of warfarin in rats. J. Ethnopharmacol. 2011, 137, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.J.; Li, S.M.; Lv, Y.P.; Huang, Z.Y.; Huang, H. Effect of compound danshen dripping pills on vascular endothelial function in early diabetic retinopathy patients. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 340–343. [Google Scholar]
- Lian, F.; Wu, L.; Tian, J.; Jin, M.; Zhou, S.; Zhao, M.; Wei, L.; Zheng, Y.; Wang, Y.; Zhang, M. The effectiveness and safety of a danshen-containing Chinese herbal medicine for diabetic retinopathy: A randomized, double-blind, placebo-controlled multicenter clinical trial. J. Ethnopharmacol. 2015, 164, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Xu, G.; Xu, L.; Wang, Z. Studies on Chemical Constituents of Dendrobium chrysotoxum Lindl. Acta Pharm. Sin. 1994, 29, 766. [Google Scholar]
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Sze, S.C.W.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Son, Y.-O.; Kim, S.-S.; Jang, Y.-S.; Lee, J.-C. Antioxidant and anti-hyperglycemic activity of polysaccharide isolated from Dendrobium chrysotoxum Lindl. BMB Rep. 2007, 40, 670–677. [Google Scholar] [CrossRef]
- Yu, Z.; Gong, C.; Lu, B.; Yang, L.; Sheng, Y.; Ji, L.; Wang, Z. Dendrobium chrysotoxum Lindl. alleviates diabetic retinopathy by preventing retinal inflammation and tight junction protein decrease. J. Diabetes Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, J.-I.; Tanaka, I.; Seo, S.; Yamazaki, M.; Saito, K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. BioMed Res. Int. 2004, 2004, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Colantuoni, A.; Bertuglia, S.; Magistretti, M.; Donato, L. Effects of Vaccinium Myrtillus anthocyanosides on arterial vasomotion. Arzneim.-Forsch. 1991, 41, 905–909. [Google Scholar]
- Lietti, A.; Cristoni, A.; Picci, M. Studies on Vaccinium myrtillus anthocyanosides. I. Vasoprotective and antiinflammatory activity. Arzneim.-Forsch. 1975, 26, 829–832. [Google Scholar]
- Matsunaga, N.; Chikaraishi, Y.; Shimazawa, M.; Yokota, S.; Hara, H. Vaccinium myrtillus (Bilberry) extracts reduce angiogenesis in vitro and in vivo. Evid.-Based Complement. Altern. Med. 2010, 7, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.-S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes—Induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, S.M.; Sagare, A.P.; Lee, C.Y.; Kao, C.L.; Tsay, H.S. Studies on tissue culture of Chinese medicinal plant resources in Taiwan and their sustainable utilization. Bot. Bull. Acad. Sin. 2003, 44, 79–98. [Google Scholar]
- Prakash, R.O.; Rabinarayan, A.; Kumar, M.S. Zingiber zerumbet (L.) Sm., a reservoir plant for therapeutic uses: A review. Int. J. Res. Ayurveda Pharm. 2011, 2, 1–22. [Google Scholar]
- Yob, N.; Jofrry, S.M.; Affandi, M.; Teh, L.; Salleh, M.; Zakaria, Z. Zingiber zerumbet (L.) Smith: A review of its ethnomedicinal, chemical, and pharmacological uses. Evid.-Based Complement. Altern. Med. 2011, 2011, 543216. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.-Y.; Tzeng, T.-F.; Liou, S.-S.; Liu, I.-M. The ethanol extract of Zingiber zerumbet rhizomes mitigates vascular lesions in the diabetic retina. Vasc. Pharmacol. 2016, 76, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, T.; Sharma, R.; Sivakumar, B.; Sahay, B. Effect of fenugreek seeds on intravenous glucose disposition in non-insulin dependent diabetic patients. Phytother. Res. 1994, 8, 83–86. [Google Scholar] [CrossRef]
- Marzouk, M.; Soliman, A.; Omar, T. Hypoglycemic and antioxidative effects of fenugreek and termis seeds powder in streptozotocin-diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 559–565. [Google Scholar] [PubMed]
- Chaturvedi, U.; Shrivastava, A.; Bhadauria, S.; Saxena, J.K.; Bhatia, G. A mechanism-based pharmacological evaluation of efficacy of trigonella foenum graecum (fenugreek) seeds in regulation of dyslipidemia and oxidative stress in hyperlipidemic rats. J. Cardiovasc. Pharmacol. 2013, 61, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, G.; Ratheesh, M.; Shyni, G.; Nambisan, B.; Helen, A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int. Immunopharmacol. 2012, 12, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Middha, S.; Bhattacharjee, B.; Saini, D.; Baliga, M.; Nagaveni, M.; Usha, T. Protective role of Trigonella foenum graceum extract against oxidative stress in hyperglycemic rats. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 427–435. [Google Scholar] [PubMed]
- Kumar, P.; Kale, R.; McLean, P.; Baquer, N. Antidiabetic and neuroprotective effects of Trigonella foenum-graecum seed powder in diabetic rat brain. Prague Med. Rep. 2012, 113, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Hur, J. Donguibogam; Namsandang: Seoul, Korea, 2007; p. 98. [Google Scholar]
- Rosenfarb, A. Healing Your Eyes with Chinese Medicine: Acupuncture, Acupressure Chinese Herbs; North Atlantic Books: Berkeley, CA, USA, 2007. [Google Scholar]
- Lim, J.; Kim, J.; Chung, S.; Cho, S.; Oh, M.; Hwang, W. The Antioxidative and Neuroprotective Effect of Guibi-tang (Guipitang) and Guibi-tang gamibang (Guipitang jiaweijang) on PC12 cells. J. Orient. Neuropsychiatry 2009, 20, 1–19. [Google Scholar]
- Eun, J.; Song, J. Effects of Kwibi-tang on serum levels of hormone and the non-specific immune response after immobilization stress in mice. Korean J. Orient. Med. Physiol. Pathol. 2004, 18, 172–178. [Google Scholar]
- Kim, H.; Choi, J.; Lim, S. The defensive effect of Keuibi-tang on the gastric mucous membrane of mouse injured by stress and ethanol. J. Orient. Med. 2003, 24, 155–168. [Google Scholar]
- Busta, I.; Xie, H.; Kim, M.-S. The use of Gui-Pi-Tang in small animals with immune-mediated blood disorders. Korea Soc. Vet. J. 2009, 26, 181–184. [Google Scholar]
- Lee, Y.M.; Lee, Y.-R.; Kim, C.-S.; Jo, K.; Sohn, E.; Kim, J.S.; Kim, J. Effect of Guibi-Tang, a Traditional Herbal Formula, on Retinal Neovascularization in a Mouse Model of Proliferative Retinopathy. Int. J. Mol. Sci. 2015, 16, 29900–29910. [Google Scholar] [CrossRef] [PubMed]
- So, H.S.; Oh, J.; Chung, Y.T.; Moon, Y.J.; Kim, D.H.; Moon, B.S.; Lee, H.S.; Baek, S.W.; Park, C.; Lim, Y.S.; et al. The water extract of Samultang protects the lipopolysaccharide (LPS)/phorbol 12-myristate 13-acetate (PMA)-induced damage and nitric oxide production of C6 glial cells via down-regulation of NF-kappaB. Gen. Pharmacol. 2000, 34, 303–310. [Google Scholar] [CrossRef]
- Xie, M. Modern Study of the Medical Formulae in Traditional Chinese Medicine; Xue Yue Press: Beijing, China, 1997. [Google Scholar]
- Lee, Y.M.; Kim, C.-S.; Jo, K.; Sohn, E.J.; Kim, J.S.; Kim, J. Inhibitory effect of Samul-tang on retinal neovascularization in oxygen-induced retinopathy. BMC Complement. Altern. Med. 2015, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Dai, X.; Jiu, Y. Modern Study of Medical Formulae in Traditional Chinese Medicine; Beijing Academy Press: Beijing, China, 1997. [Google Scholar]
- Seo, C.-S.; Ha, H.; Jung, D.-Y.; Lee, H.Y.; Shin, H.-K. Evaluation of the immune-stimulating activity of Samul-tang, a traditional Korean herbal medicine, standardized by HPLC-PDA. Korean J. Orient. Med. 2011, 32, 25–34. [Google Scholar]
- Kojima, S.; Inaba, K.; Kobayashi, S.; Kimura, M. Inhibitory effects of traditional Chinese medicine Shimotsu-to and its included crude fractions on adjuvant-induced chronic inflammation of mice. Biol. Pharm. Bull. 1996, 19, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yby, J.Y.M.; Hye Kyung, H.; Dae Sun, H.; Hyun Kyoo, S. Subacute toxicity study on Samul-tang in SD rats. Korean J. Orient. Physiol. Pathol. 2008, 22, 137–141. [Google Scholar]
- State Medical License Number: Z20030017; China Food Drug Administration: Beijing, China, 2003.
- Sheng, S.; Wang, Y.; Long, C.; Su, W.; Rong, X. Chinese medicinal formula Fufang Xueshuantong capsule could inhibit the activity of angiotensin converting enzyme. Biotechnol. Biotechnol. Equip. 2014, 28, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Z.; Yuan, F.; Xu, Q.Y.; Yu, J.; Li, L.; Zhang, J.L. Effect of Fufang Xueshuantong Capsule on a rat model of retinal vein occlusion. Chin. J. Integr. Med. 2011, 17, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.X. Clinical effect observation of compound xueshuantong capsule in the treatment of diabetic retinopathy. GuideChinaMed 2013, 11, 215–216. [Google Scholar]
- Duan, H.; Huang, J.; Li, W.; Tang, M. Protective effects of fufang xueshuantong on diabetic retinopathy in rats. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Yu, S.; Tang, M.; Duan, H.; Huang, J. A Combination of the Main Constituents of Fufang Xueshuantong Capsules Shows Protective Effects against Streptozotocin-induced Retinal Lesions in Rats. J. Ethnopharmacol. 2015, 182, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Xiong, X.; Fang, Z.; Lu, H.; Wang, Z. Protective effect of tetramethylpyrazine on myocardial ischemia-reperfusion injury. Evid.-Based Complement. Altern. Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z. Neural protection by naturopathic compounds—An example of tetramethylpyrazine from retina to brain. J. Ocul. Biol. Dis. Inform. 2009, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wei, T.; Hou, J.; Li, G.; Yu, S.; Xin, W. Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes. Life Sci. 2003, 72, 2465–2472. [Google Scholar] [CrossRef]
- Kao, T.-K.; Chang, C.-Y.; Ou, Y.-C.; Chen, W.-Y.; Kuan, Y.-H.; Pan, H.-C.; Liao, S.-L.; Li, G.-Z.; Chen, C.-J. Tetramethylpyrazine reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. Exp. Neurol. 2013, 247, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ma, J.; Zhang, P.; Luo, A.; Zhang, S.; Kong, L.; Qian, C. The effect of ligustrazine on l-type calcium current, calcium transient and contractility in rabbit ventricular myocytes. J. Ethnopharmacol. 2012, 144, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-L.; Kao, T.-K.; Chen, W.-Y.; Lin, Y.-S.; Chen, S.-Y.; Raung, S.-L.; Wu, C.-W.; Lu, H.-C.; Chen, C.-J. Tetramethylpyrazine reduces ischemic brain injury in rats. Neurosci. Lett. 2004, 372, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Ivanov, V.N.; Davidson, M.M.; Hei, T.K. Tetramethylpyrazine (TMP) protects against sodium arsenite-induced nephrotoxicity by suppressing ROS production, mitochondrial dysfunction, pro-inflammatory signaling pathways and programed cell death. Arch. Toxicol. 2015, 89, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhou, H.; Ding, Y.; Li, J.; Yang, C.; Luo, Y.; Li, S.; Sun, G.; Liao, X.; Min, W. TMP Prevents Retinal Neovascularization and Imparts Neuroprotection in an Oxygen-Induced Retinopathy ModelTMP Blocks Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Dong, X.; Liu, X.-Y.; Cheng, X.-C.; Cheng, Y.-N.; Yu, L.-G.; Guo, X.-L. Mechanism of tetramethylpyrazine analogue CXC195 inhibition of hydrogen peroxide-induced apoptosis in human endothelial cells. Biol. Pharm. Bull. 2010, 33, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.-I.; Tomi, M.; Ohtsuki, S.; Takanaga, H.; Ueda, M.; Yanai, N.; Obinata, M.; Terasaki, T. Conditionally immortalized retinal capillary endothelial cell lines (TR-iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp. Eye Res. 2001, 72, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, K.; Zhang, K.; Tan, X.; Wu, Z.; Sun, S.; Zhou, F.; Zhu, L. Tetramethylpyrazine Protects Retinal Capillary Endothelial Cells (TR-iBRB2) against IL-1β-Induced Nitrative/Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 21775–21790. [Google Scholar] [CrossRef] [PubMed]
- Kogure, T.; Hoshino, A.; Ito, K.; Sato, H.; Tatsumi, T.; Ohyama, Y.; Kawata, E.; Fujita, K.I.; Tamura, J.I. Beneficial effect of complementary alternative medicine on lymphedema with rheumatoid arthritis. Mod. Rheumatol. 2005, 15, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Yu, Y.B.; Seo, C.S.; Ha, H.K.; Lee, M.Y.; Huang, D.S.; Kim, J.H.; Shin, H.K. Subchronic toxicity of Sipjeondaebo-tang (SDT) in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2011, 59, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Tagami, K.; Niwa, K.; Lian, Z.; Gao, J.; Mori, H.; Tamaya, T. Preventive effect of Juzen-taiho-to on endometrial carcinogenesis in mice is based on Shimotsu-to constituent. Biol. Pharm. Bull. 2004, 27, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, C.-S.; Sohn, E.; Jo, K.; Lim, H.R.; Kim, S.K.; Kim, J.S.; Kim, J. Sipjeondaebo-tang, a traditional herbal formula, inhibits retinal neovascularization in a mouse model of oxygen-induced retinopathy. Tohoku J. Exp. Med. 2014, 234, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Altunkaya, A.; Gökmen, V. Effect of various anti-browning agents on phenolic compounds profile of fresh lettuce (L. sativa). Food Chem. 2009, 117, 122–126. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Li, B.-Y.; Li, X.-L.; Cheng, M.; Cai, Q.; Yu, F.; Wang, W.-D.; Tan, M.; Yan, G.; Hu, S.-L. Effects of phlorizin on diabetic retinopathy according to isobaric tags for relative and absolute quantification-based proteomics in db/db mice. Mol. Vis. 2013, 19, 812–821. [Google Scholar] [PubMed]
- Bateman, H.R.; Liang, Q.; Fan, D.; Rodriguez, V.; Lessner, S.M. Sparstolonin B inhibits pro-angiogenic functions and blocks cell cycle progression in endothelial cells. PLoS ONE 2013, 8, e70500. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Kumar, B.; Nag, T.C.; Agrawal, S.S.; Agrawal, R.; Agrawal, P.; Saxena, R.; Srivastava, S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 2011, 27, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Subramoniam, A. Plants with Anti-Diabetes Mellitus Properties; CRC Press: Boca Raton, FL, USA, 2016; pp. 18–432. [Google Scholar]
- Ibrahim, A.S.; El-Shishtawy, M.M.; Peña, A.; Liou, G.I. Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Mol. Vis. 2010, 16, 2033–2042. [Google Scholar] [PubMed]
- Yang, L.-P.; Sun, H.-L.; Wu, L.-M.; Guo, X.-J.; Dou, H.-L.; Tso, M.O.; Zhao, L.; Li, S.-M. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liao, S.; Mi, H.; Guo, C.; Qi, D.; Li, F.; Zhang, C.; Yang, Z. Hesperidin prevents retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Molecules 2012, 17, 12868–12881. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-D.; Zhu, H.-Z.; Li, S.-W.; Yang, J.-M.; Xiao, Y.; Kang, Q.-R.; Li, C.-Y.; Zhao, Y.-S.; Zeng, Y.; Li, Y. Crude Saponins of Panax notoginseng Have Neuroprotective Effects to Inhibit Palmitate-Triggered Endoplasmic Reticulum Stress-Associated Apoptosis and Loss of Postsynaptic Proteins in Staurosporine Differentiated RGC-5 Retinal Ganglion Cells. J. Agric. Food Chem. 2016, 64, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shi, X.; Wei, W.; Wang, N. Effect of the regimen of Gaoshan Hongjingtian on the mechanism of poly (ADP-ribose) polymerase regulation of nuclear factor kappa B in the experimental diabetic retinopathy. Chin. Med. J. 2012, 126, 1693–1699. [Google Scholar]
- Gao, D.; Guo, Y.; Li, X.; Li, X.; Li, Z.; Xue, M.; Ou, Z.; Liu, M.; Yang, M.; Liu, S. An aqueous extract of Radix Astragali, Angelica sinensis, and Panax notoginseng is effective in preventing diabetic retinopathy. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.-S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. KIOM-79 protects AGE-induced retinal pericyte apoptosis via inhibition of NF-kappaB activation in vitro and in vivo. PLoS ONE 2012, 7, e43591. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.S.; Kim, J.; Kim, C.-S.; Kim, N.H.; Kim, J.S. KIOM-79 prevents methyglyoxal-induced retinal pericyte apoptosis in vitro and in vivo. J. Ethnopharmacol. 2010, 129, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pan, Q.; Chen, Y.; Yang, X.; Zhao, B.; Jia, L.; Zhu, Y.; Zhang, B.; Gao, X.; Li, X. Administration of Danhong Injection to diabetic db/db mice inhibits the development of diabetic retinopathy and nephropathy. Sci. Rep. 2015, 5, 11219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Meng, X.-B.; Lu, S.; Wang, T.-T.; Liu, Y.; Sun, G.-B.; Sun, X.-B. Evaluation of hypoglycemic efficacy of Tangningtongluo formula, a traditional Chinese Miao medicine, in two rodent animal models. J. Diabetes Res. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Tan, H.-Y.; Zhang, Y.; Feng, Y. Protective effect of a Chinese Medicine formula He-Ying-Qing-Re Formula on diabetic retinopathy. J. Ethnopharmacol. 2015, 169, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, B.; Zhao, Y.; Tang, S.; Xu, H.; Wang, L.; Liang, R.; Yang, H. BNC protects H9c2 cardiomyoblasts from H2O2-induced oxidative injury through ERK1/2 signaling pathway. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
| Plant/Formulation | Active Ingredient/Plant Name | Experimental Evidence | References |
|---|---|---|---|
| Lactuca sativa | Phlorizin | Phlorizin noticeably diminishes formation of AGE, retina cell apoptosis and glial fibrillary acidic protein expression in the retinas of db/db mice. | [136,137] |
| Sparganium stoloniferum | Sparstolonin B (SsnB) | SsnB blocks the function of endothelial cells associated with angiogenesis in several ex vivo and cellular assays. SsnB restricts endothelial cell division in the G1 phase and negatively regulates the cell cycle proteins cdc6 and cyclin E2. Furthermore, SsnB causes a considerable decrease in blood vessel length and number of branches in the chick chorioallantoic membrane assay. | [138] |
| Eriodictyon californicum | Eriodictyol | Eriodictyol decreases ICAM-1, VEGF, TNF-α, and endothelial nitric oxide synthase in the retina of diabetic rats. Eriodictyol diminishes 40% breakdown of BRB in diabetic rats. | [139] |
| Curcuma longa | Curcumin | Curcumin ameliorates experimental DR in rats via its anti-inflammatory, hypoglycemic, and antioxidant effects. | [140] |
| Cyamopsis tetragonoloba | Genistein | Genistein mitigates retinal inflammation related to diabetes by targeting activation of microglial cells. | [141,142] |
| Scutellaria baicalensis | Baicalein | Baicalein decreases inflammation in a rodent model of DR. | [143] |
| Citrus. reticulata | Hesperidin | Treatment with hesperidin decreases BRB breakdown and augmented retina thickness. Hisperidin decreases blood glucose, aldose reductase and retinal VEGF activity, IL-1β, TNF-α, ICAM-1, and AGEs levels. | [144] |
| Crude saponin fraction of P. notoginseng (CSPN) | Ginsenosides Rg1, Rh1, Rd, and Re | CSPN represses the abnormally increased apoptosis and loss of postsynaptic scaffolding protein PSD-95 by palmitate in staurosporine-differentiated RGC-5 cells. Furthermore, CSPN decreases palmitate-induced endoplasmic reticulum stress-associated eIF2α/ATF4/CHOP, generation of ROS and caspase 12 pathways. | [145] |
| Green Tea (GT) (Camellia sinensis) | Extract of GT | Extract of GT restores retinal anti-oxidant enzymes and decreased proinflammatory factors as compared to a diabetic group. | [48] |
| Gaoshan Hongjingtian (RG) | Salvia miltiorrhiza, Ligusticum chuanxiong Hort and Radix Et Rhizoma Glycyrrhizae | RG inhibits the diabetes-mediated changes in levels of ICAM-1 and NF-κB transcripts. RG attenuates capillary degeneration in diabetic rats. RG diminishes the expression of ICAM-1 and NF-κB in DR. RG inhibits the thickening of basement membrane in retinal capillaries. | [146] |
| Dang Gui Bu Xue Tang (DBT) | Radix Astragali, Radix Angelica sinensis and Panax notoginseng (RRP) | RRP suppresses leukostasis, acellular capillaries, and vascular leakage in diabetic rats. RRP reduces the expression of inflammatory factors including MCP-1, ICAM-1, VCAM-1, IL-1β, IL-6, TNF-α, NF-κB, in the retinas of diabetic rats. | [147] |
| KIOM-79 | Magnolia officinalis, Pueraria lobata, Glycyrrhiza uralensis and Euphorbia pekinensis | KIOM-79 mitigates AGE-stimulated apoptosis of retinal pericytes by inhibiting NF-κB activation. KIOM-79 exerts its effect via an antioxidant mechanism to ameliorate oxidative stress-induced apoptosis in retinal pericytes. | [148,149] |
| Danhong Injection (DHI) | Mixture of Carthamus tinctorius and Salvia miltiorrhiza with main components of tanshinone, tanshinol acid and safflor yellow. | Administration of DHI blocks retinal and retinal sub-layer shrinkage, together with a reduction in AGE. DHI stimulates the expression of fibroblast growth factor 21, peroxisome proliferator-activated γ, and activates the expression of genes responsible for energy expenditure. | [150] |
| Tangningtongluo formula (TNTL) | Herba Plantaginis, Flos Lonicerae and herba Agrimoniae | Administration of TNTL to C57BL/KsJ-db/db mice markedly alleviates the layer thickness of the optic nerve and decreases the density of vascular calibers in fundus oculi. | [151] |
| He-Ying-Qing-Re Formula (HF) | Rehmannia glutinosa, Lycium barbarum Polygonatum sibiricum, Lonicera japonica, Angelica sinensis, Scrophularia ningpoensis and Glycyrrhiza uralensis. | Experimental studies found HR to inhibit AGE, degeneration of retinal vasculature and BRB permeability damage. | [152] |
| Buchang NaoXinTong (NXT) | Semen Persicae, Carthamus tinctorius L., Frankincense, myrrh, Spatholobus suberectus, Achyranthes Root, Cassia Twig, Mulberry Twig, earthworms, scorpions, Astragalus membranaceus, Salvia miltiorrhiza, Ligusticum, Radix Paeoniae Rubra, Szechwan Lovage Rhizome, and Hirudo | NXT inhibits the diabetes-induced shrinkage of multiple layers, such as the outer nuclear/plexiform layers and photoreceptor layer in the retina of db/db mice. | [153] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasant More, S.; Kim, I.-S.; Choi, D.-K. Recent Update on the Role of Chinese Material Medica and Formulations in Diabetic Retinopathy. Molecules 2017, 22, 76. https://doi.org/10.3390/molecules22010076
Vasant More S, Kim I-S, Choi D-K. Recent Update on the Role of Chinese Material Medica and Formulations in Diabetic Retinopathy. Molecules. 2017; 22(1):76. https://doi.org/10.3390/molecules22010076
Chicago/Turabian StyleVasant More, Sandeep, In-Su Kim, and Dong-Kug Choi. 2017. "Recent Update on the Role of Chinese Material Medica and Formulations in Diabetic Retinopathy" Molecules 22, no. 1: 76. https://doi.org/10.3390/molecules22010076
APA StyleVasant More, S., Kim, I.-S., & Choi, D.-K. (2017). Recent Update on the Role of Chinese Material Medica and Formulations in Diabetic Retinopathy. Molecules, 22(1), 76. https://doi.org/10.3390/molecules22010076







