Two New Clerodane Diterpenes from Tinospora sagittata
Abstract
:1. Introduction
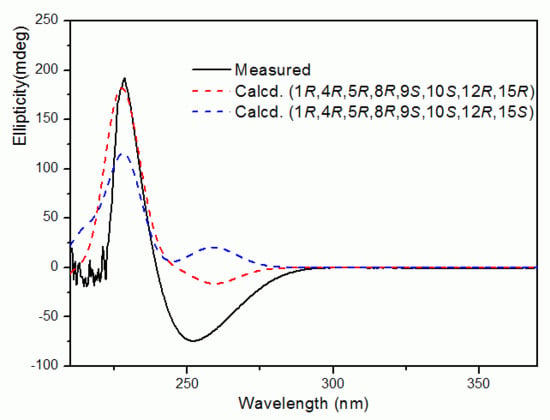
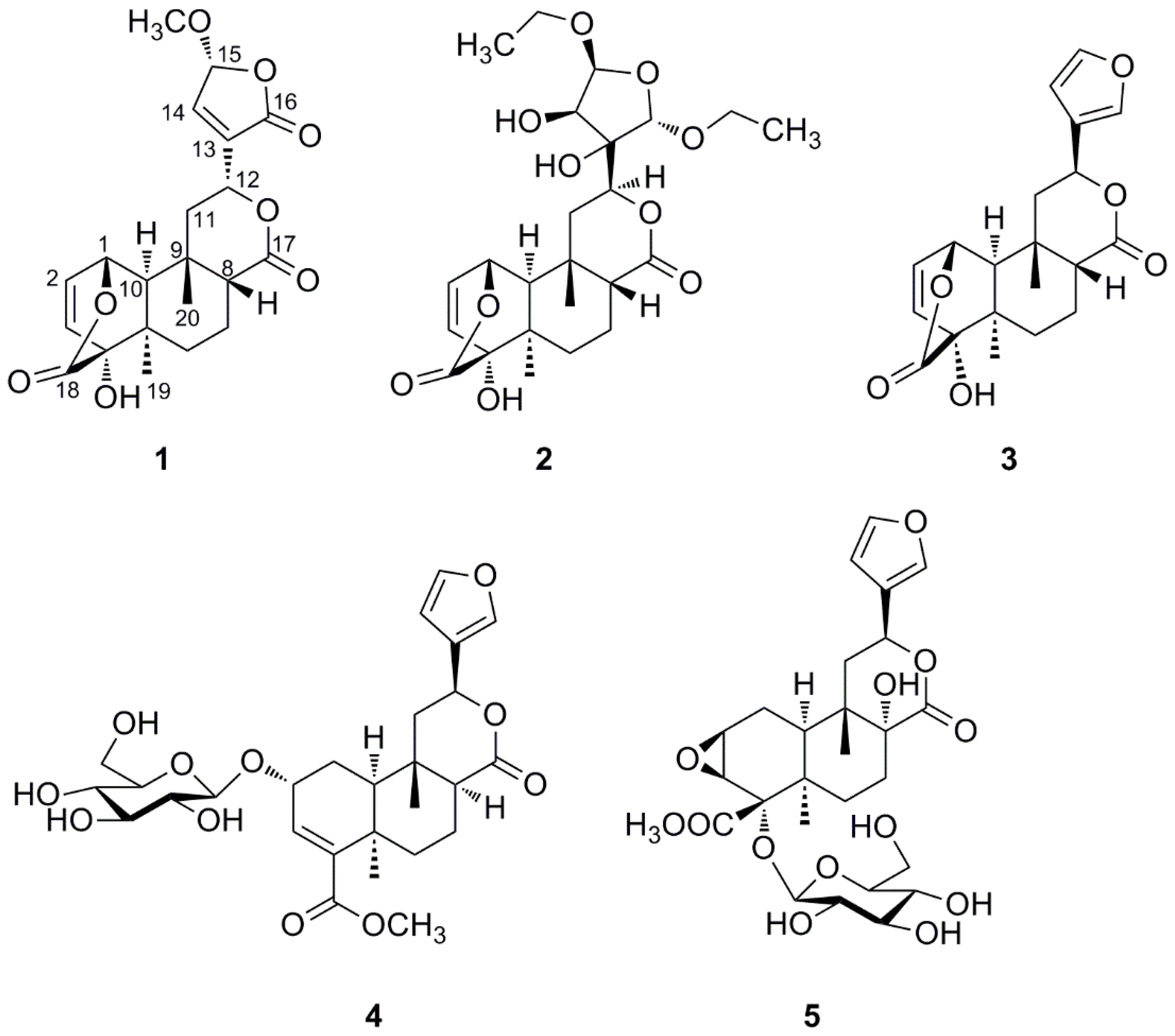
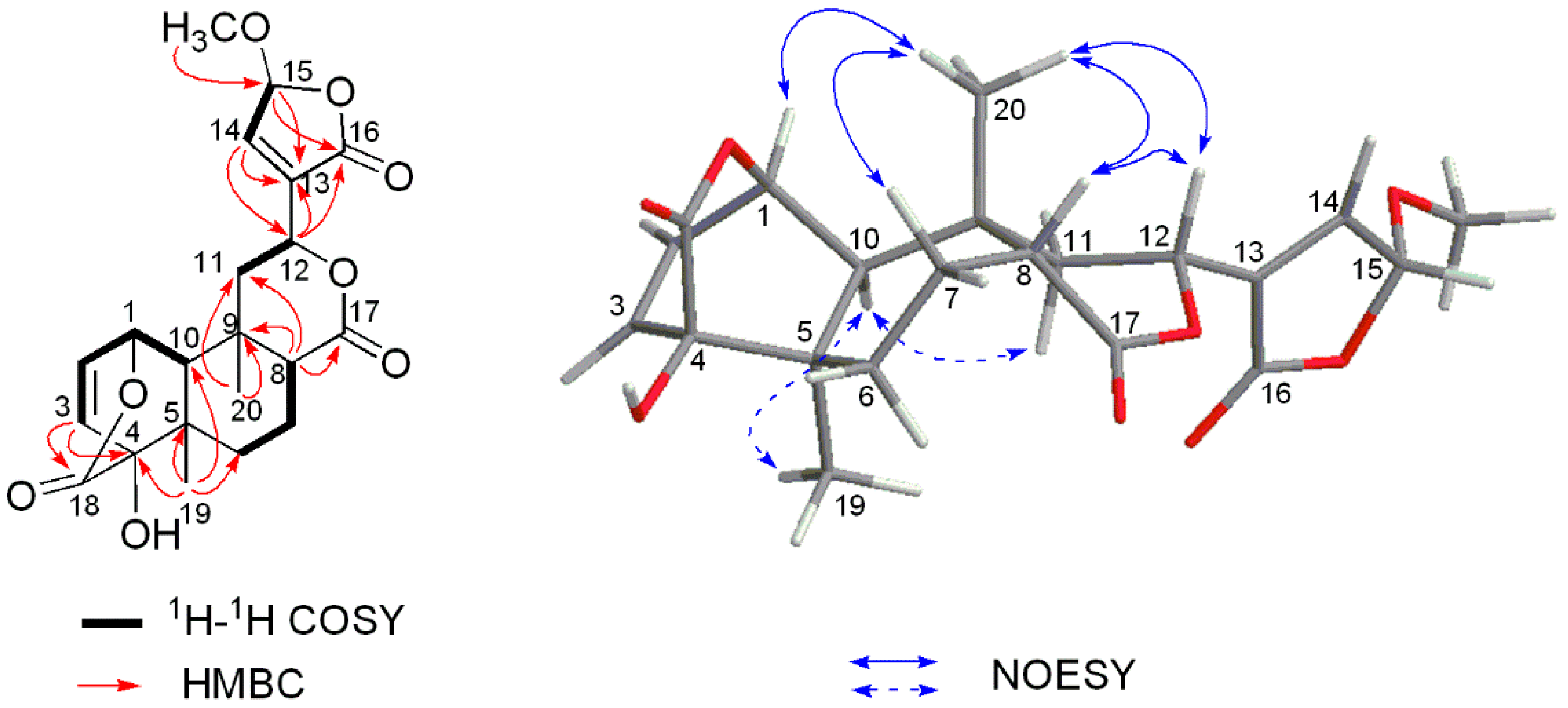
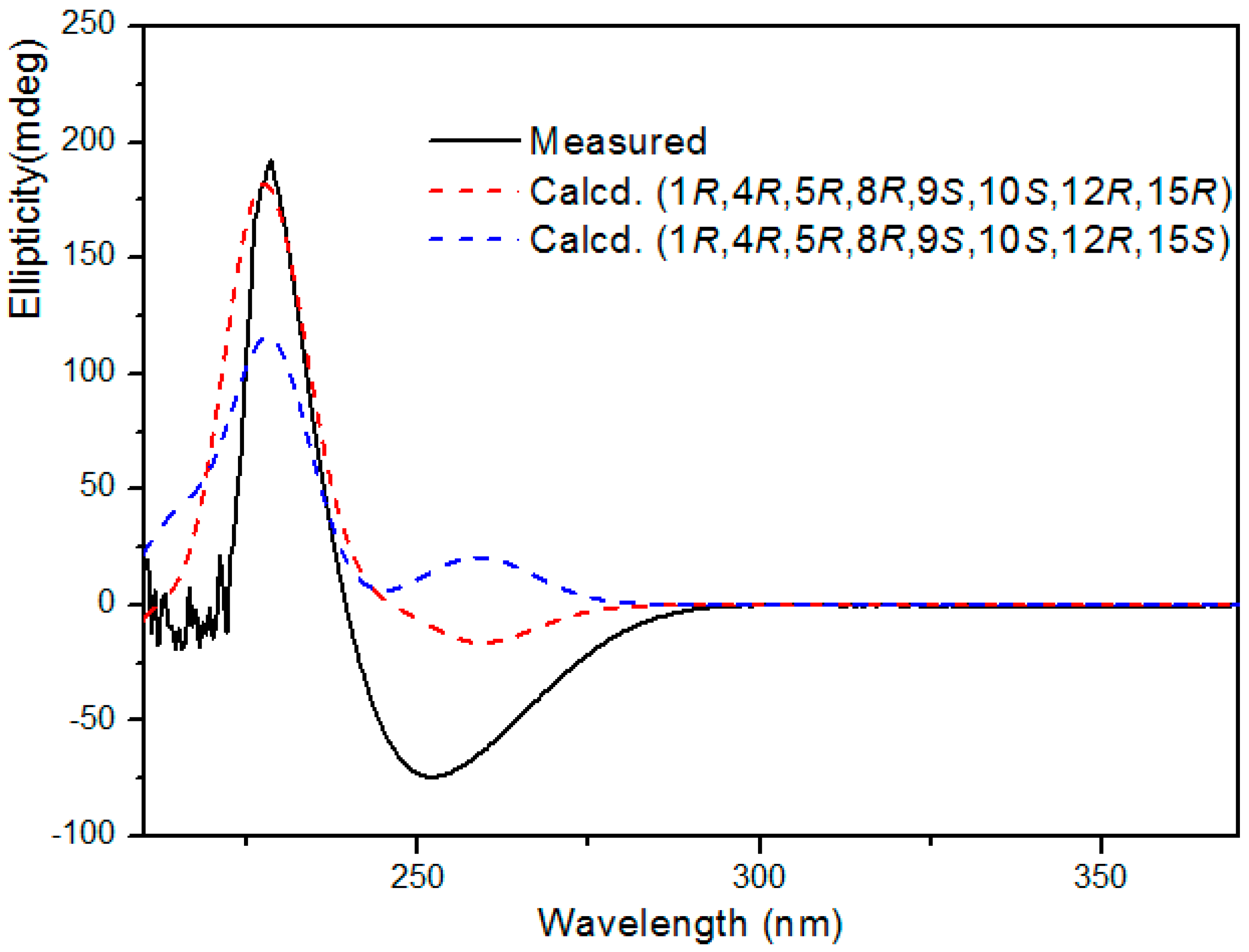
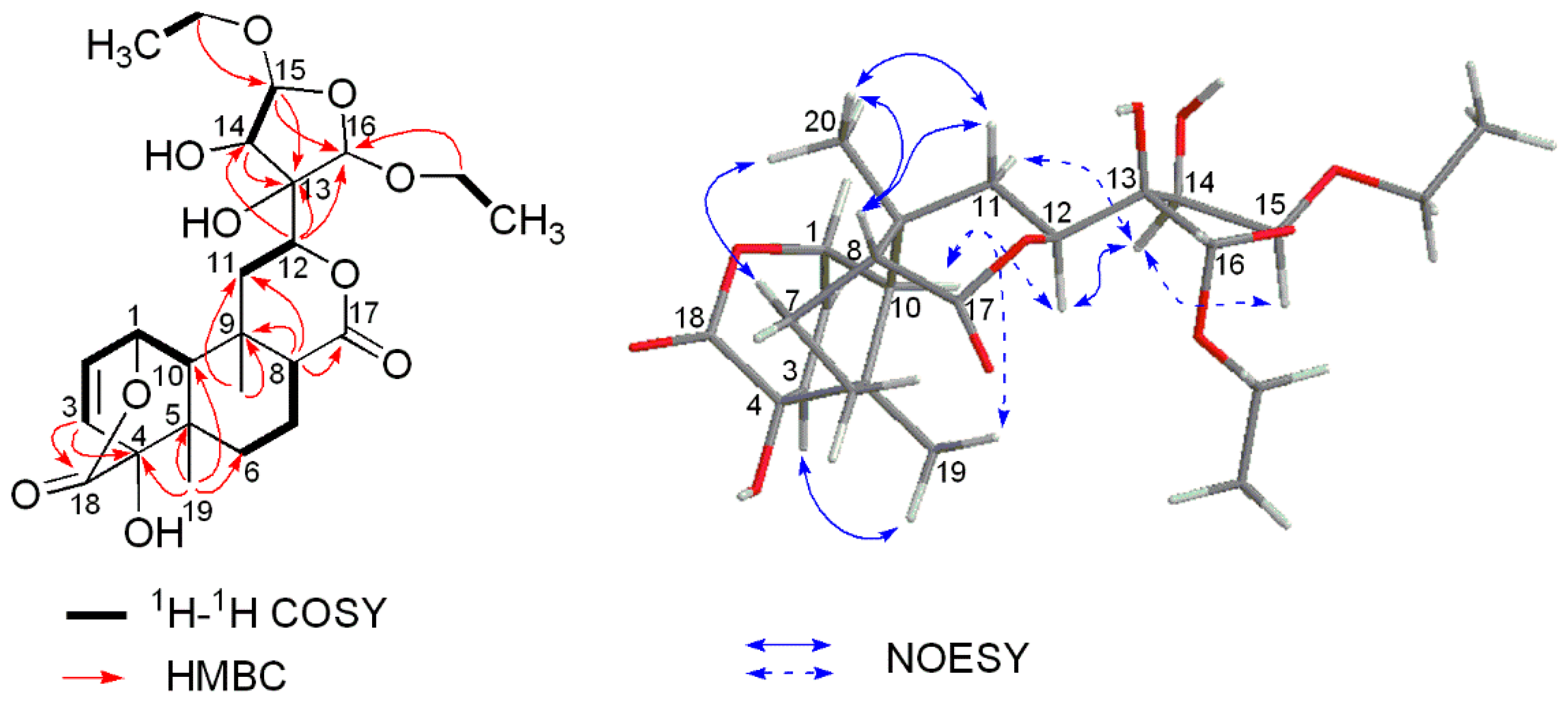
2. Results
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of 1 and 2
3.5. Computational methods for ECD of Compound 1
3.6. α-Glucosidase Inhibition Assay
3.7. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China, 1996; Volume 30, p. 23. [Google Scholar]
- Zhao, T.F.; Wang, X.K.; Rimando, A.M.; Che, C.T. Folkloric medicinal plants: Tinospora sagittata var. cravaniana and mahonia bealei. Planta Med. 1991, 57, 505. [Google Scholar] [CrossRef] [PubMed]
- State of Administration of Traditional Chinese Medicine of People’s Republic of China. Zhong Hua Ben Cao; Shanghai Scientific &Technical Publisher: Shanghai, China, 1999; pp. 1988–1990. [Google Scholar]
- Shi, L.M.; Li, R.Q.; Liu, W.H. Two new furanoid diterpenoids from Tinospora sagittata. Helv. Chim. Acta 2008, 91, 978–982. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Z.; Shi, Q.; Zeng, H.; Shen, Y.; Jin, H.; Zhang, W. Anti-inflammatory and anti-nociceptive activities of compounds from Tinospora sagittata (Oliv.) Gagnep. Arch. Pharm. Res. 2010, 33, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, C.; Li, S.; Ma, F.; Li, Q.; Asada, Y.; Koik, K. Clerodane diterpenoids from Tinospora sagittata (Oliv.) Gagnep. Planta Med. 2012, 78, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, W.; Ma, F.; Li, Q.; Asada, Y.; Koike, K. Cheminform abstract: Tinospinosides D, E, and tinospin E, further clerodane diterpenoids from Tinospora sagittata. Chem. Pharm. Bull. 2012, 60, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.B.; Wang, A.L.; Li, D.H.; Wang, K.B.; Lin, B.; Li, Z.L.; Hua, H.M. Cytotoxic clerodane furanoditerpenoids from the root of Tinospora sagittata. Phytochem Lett. 2015, 27, 173–176. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, H.; Hu, S.; Xu, H.; Yang, B.; Yang, Q.; Xue, Y.X.; Cheng, L.; Jiang, J.; Zhang, J.; et al. Clerodane-type diterpenoids from tuberous roots of Tinospora sagittata (Oliv.) Gagnep. Fitoterapia 2016, 110, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Maeda, M.; Tanine, M.; Tsukio, Y.; Veda, N.; Wada, K.; Sugie, S.; Mori, H.; Tanaka, T. A bitter diterpenoid furanolactone, columbin, from Calumbae Radix inhibits azoxymethane-induced rat colon carcinogenesis. Cancer Lett. 2002, 183, 131–139. [Google Scholar] [CrossRef]
- Beg, M.; Shankar, K.; Varshney, S.; Rajan, S.; Singh, S.P.; Jagdale, P.; Puri, A.; Chaudhari, B.P.; Sashidhara, K.V.; Gaikwad, A.N. A clerodane diterpene inhibit adipogenesis by cell cycle arrest and ameliorate obesity in C57BL/6 mice. Mol. Cell. Endocrinol. 2015, 399, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.R.; Shen, Y.H.; Zhang, C.; Liu, R.H.; Zhang, W.D. Chemical constituents of two original plants used as radix tinosporae. Chin. J. Nat. Med. 2008, 6, 186–190. [Google Scholar] [CrossRef]
- Huang, X.Z.; Cheng, C.M.; Yun, D.; Fu, G.M.; Guo, J.M.; Yin, Y.; Liang, H. A novel 18-norclerodane diterpenoid from the roots of Tinospora sagittata var. yunnanensis. Molecules 2010, 15, 8360–8365. [Google Scholar] [CrossRef] [PubMed]
- Akhila, A.; Rani, K.; Thakur, R.S. Biosynthesis of the clerodane furano-diterpene lactone skeleton in Tinospora cordifolia. Phytochemistry 1991, 30, 2573–2576. [Google Scholar] [CrossRef]
- Guo, D.X.; Wang, X.N.; Zhu, R.X.; Liu, N.; Zhou, J.C.; Yu, W.T. Cis-clerodane diterpenoids from the chinese liverwort Scapania parva, steph. Phytochem. Lett. 2012, 5, 535–540. [Google Scholar] [CrossRef]
- “The dried plant powder (100 g) were extracted three times with Me2CO (85%, v/v) at room temperature and concentrated under reduced pressure to obtain a crude residue (9.5 g). The residue was further fractionated by an ODS-C18 column eluting with a gradient solvent system consisting of 30%, 50% and 100% acetonitrile. The fraction eluted with 50% acetonitrile was chromatographed on a Sephadex LH-20 column (Me2CO) to obtained 30 fractions. Then, each fraction was successively detected by TLC and HPLC based on the corresponding retention time of compound 1 or 2. Finally, compound 1 was detected in the twenty-second fraction and 2 in the twentieth, which further demonstrate by their pseudo-molecular ions in HR-ESIMS spectra”.
- Gotō, H.; Ōsawa, E. Corner flapping: A simple and fast algorithm for exhaustive generation of ring conformations. J. Am. Chem. Soc. 1989, 111, 8950–8951. [Google Scholar] [CrossRef]
- Gotō, H.; Ōsawa, E. An efficient algorithm for searching low-energy conformers of cyclic and acyclic molecules. J. Chem. Soc., Perkin Trans. 2 1993, 187–198. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010.
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Q.L.; Zheng, M.F.; Ren, H.; Lei, T.; Wu, P.; Zhou, Z.Y.; Wei, X.Y.; Tan, J.W. Bioactive 30-noroleanane triterpenes from the pericarps of Akebia trifoliata. Molecules 2014, 19, 4301–4312. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Tan, N.H.; Zeng, G.Z.; Zhang, Y.M. Two new cinnamyl isovalerate derivatives from Sabina gaussenii. Molecules 2016, 21, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 3–5 are available from the authors.
| No. | 1 a | No. | 2 b | ||
|---|---|---|---|---|---|
| δH | δC | δH | δC | ||
| 1 | 5.10 br t, 3.4 | 74.8 | 1 | 5.30 dd, J = 5.2, 1.7 | 73.2 |
| 2 | 6.34 overlapped | 128.3 | 2 | 6.23 dd, J = 7.9, 5.2 | 129.3 |
| 3 | 6.33 overlapped | 137.3 | 3 | 6.42 dd, J = 7.9, 1.7 | 136.9 |
| 4 | 80.7 | 4 | 80.8 | ||
| 5 | 37.8 | 5 | 37.1 | ||
| 6 (β-H) | 1.35 m | 26.0 | 6 (β-H) | 1.79 m | 26.0 |
| 6 (α-H) | 1.76 ddd, J = 14.2, 6.6, 2.0 | 6 (α-H) | 2.03 m | ||
| 7 (α-H) | 1.94 m | 17.1 | 7 (α-H) | 2.01 m | 17.5 |
| 7 (β-H) | 2.53 m | 7 (β-H) | 2.85 m | ||
| 8 | 2.55 m | 43.6 | 8 | 2.52 m | 44.1 |
| 9 | 36.3 | 9 | 34.1 | ||
| 10 | 1.30 br s | 55.3 | 10 | 2.00 br s | 46.8 |
| 11 (β-H) | 2.48 dd, J = 14.0, 2.6 | 45.6 | 11 (α-H) | 2.60 dd, J = 14.8, 5.6 | 34.0 |
| 11 (α-H) | 1.64 m | 11 (β-H) | 2.54 m | ||
| 12 | 5.22 br d, J = 11.5 | 70.2 | 12 | 5.42 dd, J = 14.8, 5.6 | 76.8 |
| 13 | 136.1 | 13 | 80.6 | ||
| 14 | 7.22 br s | 144.7 | 14 | 4.76 d, J = 4.2 | 76.4 |
| 15 | 5.83 br s | 103.2 | 15 | 5.59 d, J = 4.2 | 110.2 |
| 16 | 168.6 | 16 | 5.57, s | 106.9 | |
| 17 | 173.7 | 17 | 173.7 | ||
| 18 | 175.6 | 18 | 174.8 | ||
| 19 | 1.10 s | 24.1 | 19 | 1.23 s | 23.9 |
| 20 | 1.41 s | 28.3 | 20 | 1.22 s | 27.2 |
| 15-OCH3 | 3.58 s | 57.4 | 15-OCH2CH3 | 3.54 m; 3.91 m | 63.7 |
| 15-OCH2CH3 | 1.17 t, J = 7.1 | 14.7 | |||
| 16-OCH2CH3 | 3.57 m; 3.89 m | 62.4 | |||
| 16-OCH2CH3 | 1.14 t, J = 7.1 | 14.4 | |||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Ding, W.; Wan, F.; Li, Y. Two New Clerodane Diterpenes from Tinospora sagittata. Molecules 2016, 21, 1250. https://doi.org/10.3390/molecules21091250
Li G, Ding W, Wan F, Li Y. Two New Clerodane Diterpenes from Tinospora sagittata. Molecules. 2016; 21(9):1250. https://doi.org/10.3390/molecules21091250
Chicago/Turabian StyleLi, Guanhua, Wenbing Ding, Fanghao Wan, and Youzhi Li. 2016. "Two New Clerodane Diterpenes from Tinospora sagittata" Molecules 21, no. 9: 1250. https://doi.org/10.3390/molecules21091250
APA StyleLi, G., Ding, W., Wan, F., & Li, Y. (2016). Two New Clerodane Diterpenes from Tinospora sagittata. Molecules, 21(9), 1250. https://doi.org/10.3390/molecules21091250