Asiatic Acid Attenuates Myocardial Ischemia/Reperfusion Injury via Akt/GSK-3β/HIF-1α Signaling in Rat H9c2 Cardiomyocytes
Abstract
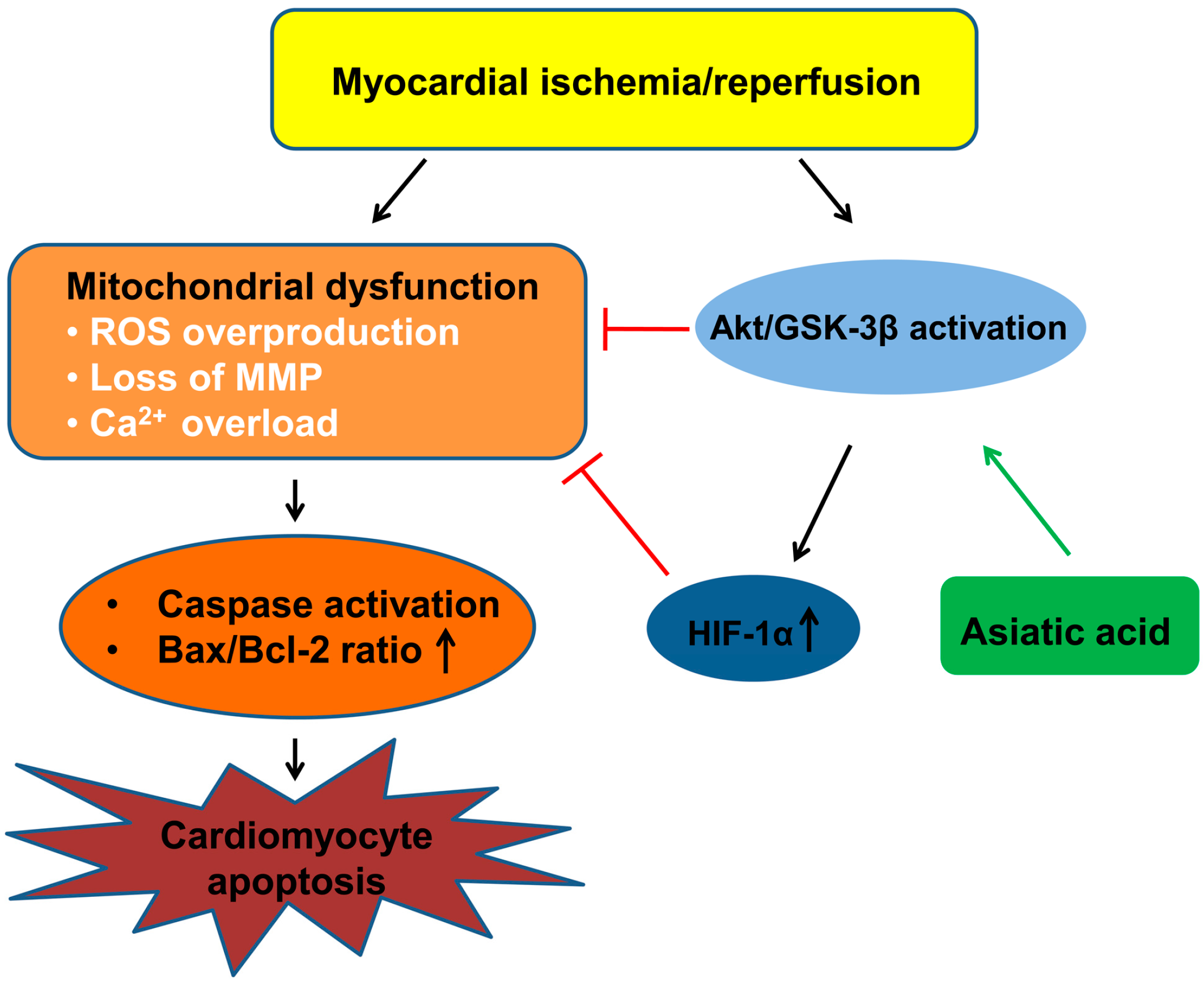
:1. Introduction
2. Results
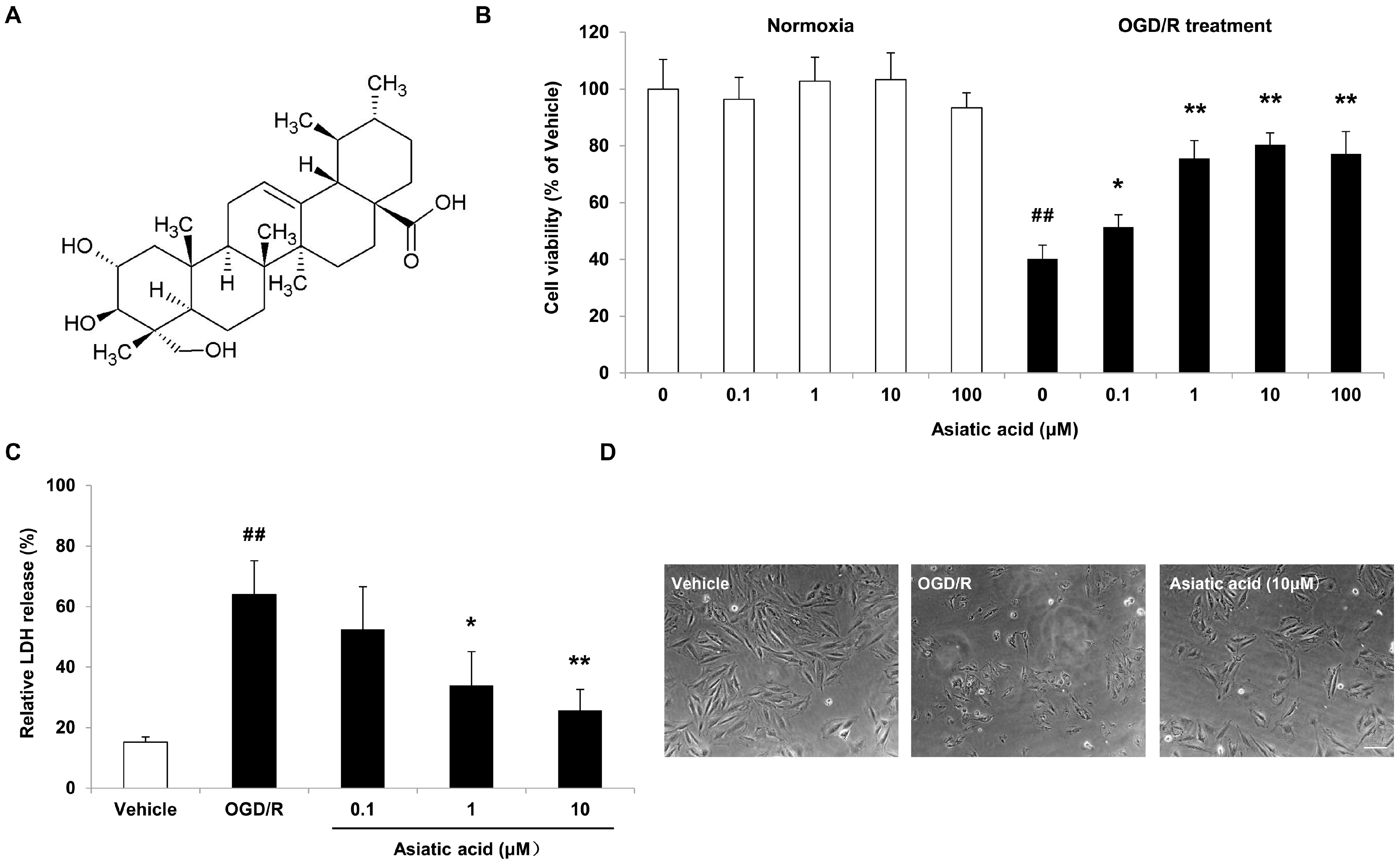
2.1. Asiatic Acid Attenuates OGD/R-Induced Cell Death in H9c2 Rat Cardiomyocytes
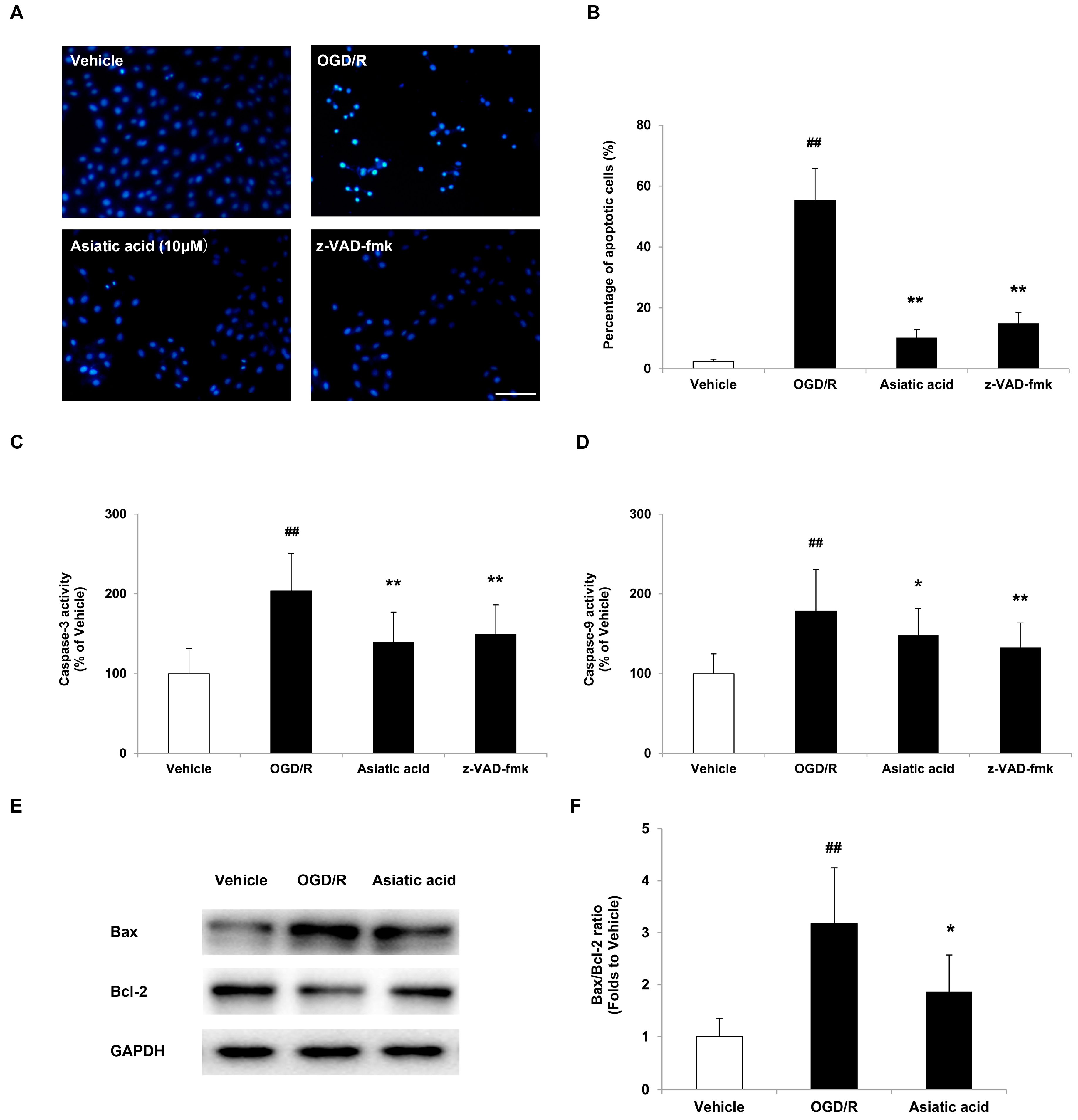
2.2. Asiatic Acid Prevents Apoptosis in H9c2 Cardiomyocytes after OGD/R
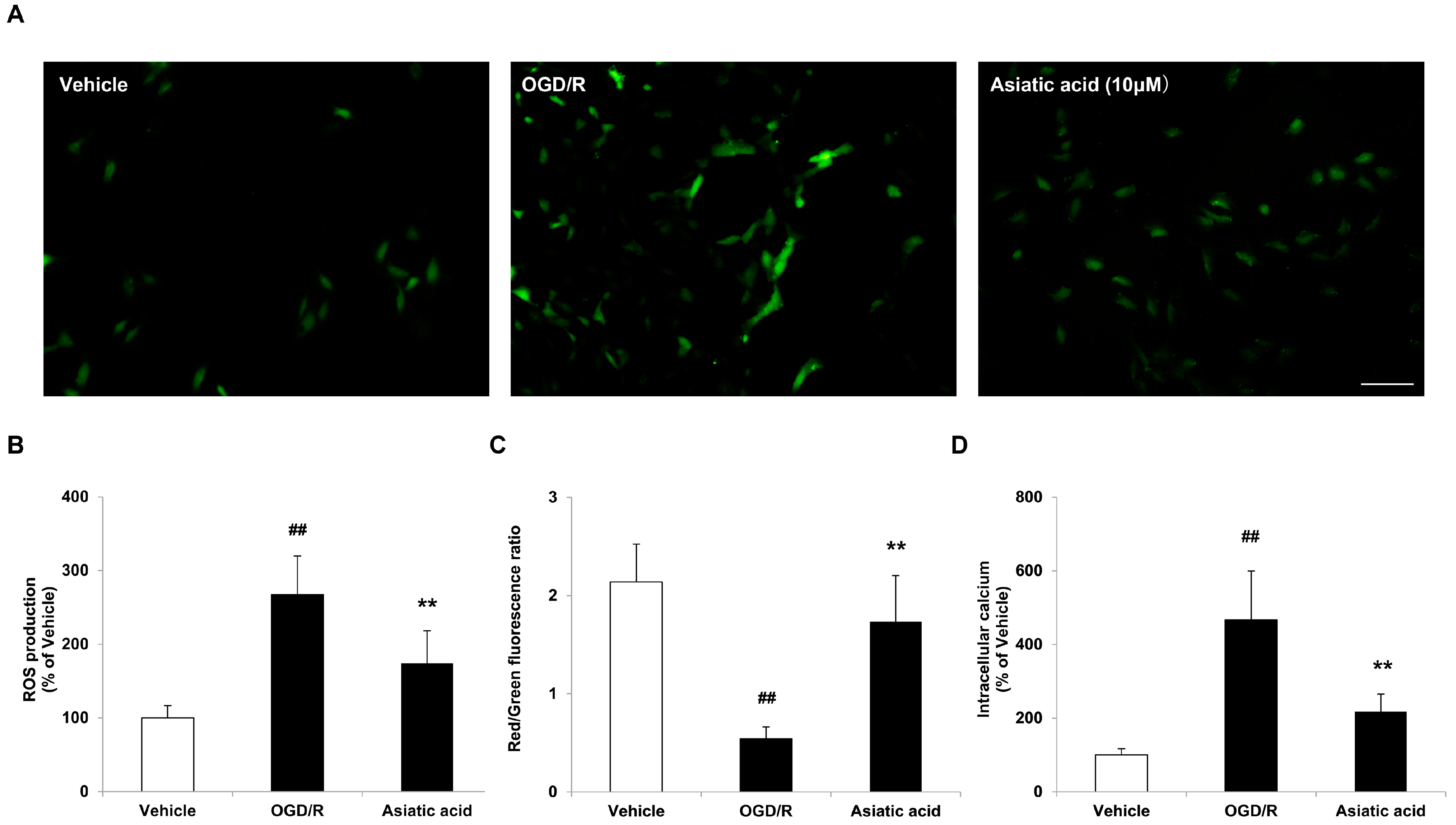
2.3. Asiatic Acid Prevents Mitochondrial Dysfunction Following OGD/R Injury
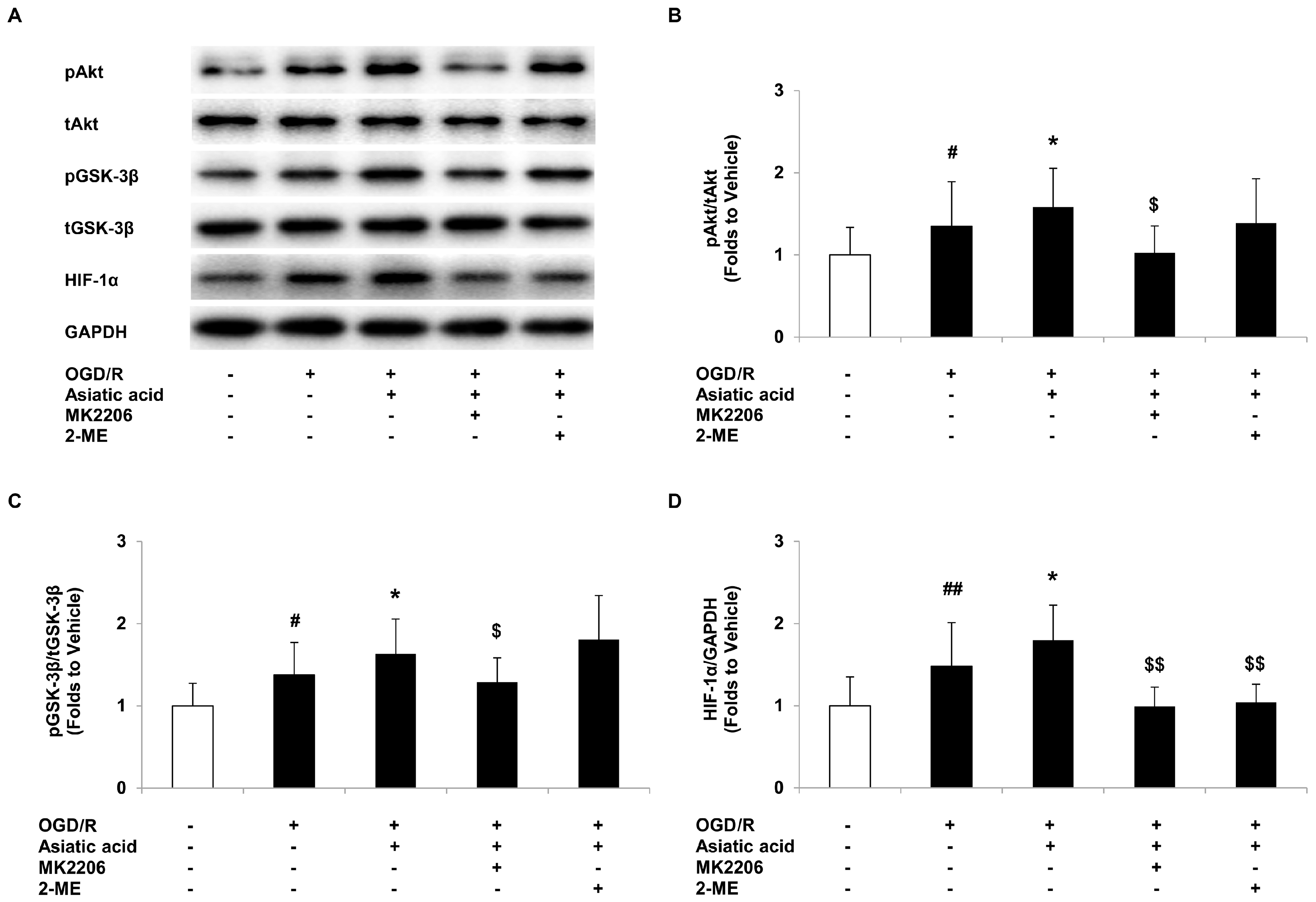
2.4. Asiatic Acid Regulates the Akt/GSK-3β Signal Pathway and Increases HIF-1α Levels in Hypoxic H9c2 Cells
2.5. Inhibition of Akt or HIF-1α Attenuates the Protective Effects of Asiatic Acid against OGD/R Injury
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Oxygen-Glucose Deprivation and Cell Culture Treatment
4.3. Hoechst 33258 Staining
4.4. Measurement of the Activities of Caspase-3 and Caspase-9
4.5. ROS Measurement
4.6. Determination of Intracellular Calcium Concentration
4.7. Measurement of Mitochondrial Membrane Potential
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buja, L.M. Myocardial ischemia and reperfusion injury. Cardiovasc. Pathol. 2005, 14, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.I.; Jou, M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, J.R.; Mongue-Din, H.; Eaton, P.; Shah, A.M. Redox signaling in cardiac physiology and pathology. Circ. Res. 2012, 111, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, J.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M. Neuroprotective effect of asiatic acid on rotenone-induced mitochondrial dysfunction and oxidative stress-mediated apoptosis in differentiated SH-SYS5Y cells. Nutr. Neurosci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.C.; Yin, M.C.; Mong, M.C. Anti-apoptotic and anti-glycative effects of asiatic acid in the brain of D-galactose treated mice. Food Funct. 2015, 6, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.M.; Yin, M.C. Antioxidative and antiinflammatory activities of asiatic acid, glycyrrhizic acid, and oleanolic acid in human bronchial epithelial cells. J. Agric. Food Chem. 2015, 63, 3196–3204. [Google Scholar] [CrossRef] [PubMed]
- Pakdeechote, P.; Bunbupha, S.; Kukongviriyapan, U.; Prachaney, P.; Khrisanapant, W.; Kukongviriyapan, V. Asiatic acid alleviates hemodynamic and metabolic alterations via restoring eNOS/iNOS expression, oxidative stress, and inflammation in diet-induced metabolic syndrome rats. Nutrients 2014, 6, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, S.H.; Yang, H.; Lim, D.Y.; Ryu, J.H.; Lee, E.S.; Jew, S.S.; Park, H.G.; Sung, S.H.; Kim, Y.C. Asiatic acid derivatives protect primary cultures of rat hepatocytes against carbon tetrachloride-induced injury via the cellular antioxidant system. Nat. Prod. Commun. 2009, 4, 765–768. [Google Scholar] [PubMed]
- Huo, L.; Shi, W.; Chong, L.; Wang, J.; Zhang, K.; Li, Y. Asiatic acid inhibits left ventricular remodeling and improves cardiac function in a rat model of myocardial infarction. Exp. Ther. Med. 2016, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Bae, O.N.; Serfozo, K.; Hejabian, S.; Moussa, A.; Reeves, M.; Rumbeiha, W.; Fitzgerald, S.D.; Stein, G.; Baek, S.H.; et al. Asiatic acid attenuates infarct volume, mitochondrial dysfunction, and matrix metalloproteinase-9 induction after focal cerebral ischemia. Stroke 2012, 43, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.G.; Senut, M.C.; Zemke, D.; Min, J.; Frenkel, M.B.; Greenberg, E.J.; Yu, S.W.; Ahn, N.; Goudreau, J.; Kassab, M.; et al. Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J. Neurosci. Res. 2009, 87, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.F.; Xiong, Y.Y.; Liu, J.K.; Qian, J.J.; Zhu, L.; Gao, J. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol. Sin. 2012, 33, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Dou, Y.; Xia, B.; Rong, W.; Rimbach, G.; Lou, Y. Asiatic acid protects primary neurons against C2-ceramide-induced apoptosis. Eur. J. Pharmacol. 2012, 679, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Perlman, H.; Zhang, X.; Chen, M.W.; Walsh, K.; Buttyan, R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999, 6, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Delgado-Esteban, M.; Bolanos, J.P.; Medina, J.M. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J. Neurochem. 2002, 81, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, X.; Zhao, S.; Wei, C.; Yin, Y.; Liu, T.; Jiang, S.; Xie, J.; Wan, X.; Mao, M.; et al. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem. Int. 2013, 63, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Rego, A.C.; Oliveira, C.R. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: Implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2003, 28, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Potier, L.; Waeckel, L.; Vincent, M.P.; Chollet, C.; Gobeil, F., Jr.; Marre, M.; Bruneval, P.; Richer, C.; Roussel, R.; Alhenc-Gelas, F.; et al. Selective kinin receptor agonists as cardioprotective agents in myocardial ischemia and diabetes. J. Pharmacol. Exp. Ther. 2013, 346, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Abdillahi, M.; Ananthakrishnan, R.; Vedantham, S.; Shang, L.; Zhu, Z.; Rosario, R.; Zirpoli, H.; Bohren, K.M.; Gabbay, K.H.; Ramasamy, R. Aldose reductase modulates cardiac glycogen synthase kinase-3beta phosphorylation during ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H297–H308. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, G.; Alasmari, A.; Mouli, S.; Eldoumani, H.; Quindry, J.; McGinnis, G.; Fu, X.; Berlin, A.; Peters, B.; Zhong, J.; et al. Cardioprotective HIF-1alpha-frataxin signaling against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H867–H879. [Google Scholar] [PubMed]
- Hwang, J.M.; Weng, Y.J.; Lin, J.A.; Bau, D.T.; Ko, F.Y.; Tsai, F.J.; Tsai, C.H.; Wu, C.H.; Lin, P.C.; Huang, C.Y.; et al. Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Mol. Cell. Biochem. 2008, 311, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cursio, R.; Gugenheim, J.; Ricci, J.E.; Crenesse, D.; Rostagno, P.; Maulon, L.; Saint-Paul, M.C.; Ferrua, B.; Auberger, A.P. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. FASEB J. 1999, 13, 253–261. [Google Scholar] [PubMed]
- Sarkey, J.P.; Chu, M.; McShane, M.; Bovo, E.; Ait Mou, Y.; Zima, A.V.; de Tombe, P.P.; Kartje, G.L.; Martin, J.L. Nogo-A knockdown inhibits hypoxia/reoxygenation-induced activation of mitochondrial-dependent apoptosis in cardiomyocytes. J. Mol. Cell. Cardiol. 2011, 50, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q. Oxidative stress-elicited myocardial apoptosis during reperfusion. Curr. Opin. Pharmacol. 2004, 4, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Saravanan, R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum. Exp. Toxicol. 2015, 34, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, T.; Lu, Q.; Shang, J.; Sun, H.; Zhang, L. Asiatic acid preserves beta cell mass and mitigates hyperglycemia in streptozocin-induced diabetic rats. Diabetes/Metab. Res. Rev. 2010, 26, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Fujio, Y.; Nguyen, T.; Wencker, D.; Kitsis, R.N.; Walsh, K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 2000, 101, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Paillard, M.; Thibault, H.; Derumeaux, G.; Ovize, M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation 2008, 117, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Zorov, D.B.; Yaniv, Y.; Nuss, H.B.; Wang, S.; Sollott, S.J. Role of glycogen synthase kinase-3beta in cardioprotection. Circ. Res. 2009, 104, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Ko, S.Y.; Fong, J.H.; Chang, K.W.; Liu, T.Y.; Kao, S.Y. Expression of phosphorylated Akt in oral carcinogenesis and its induction by nicotine and alkaline stimulation. J. Oral Pathol. Med. 2009, 38, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Inhibition of GSK-3beta as a target for cardioprotection: The importance of timing, location, duration and degree of inhibition. Exp. Opin. Ther. Targets 2005, 9, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Pap, M.; Cooper, G.M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J. Biol. Chem. 1998, 273, 19929–19932. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.H.; Chen, J.; Xie, L.; Cai, X.M.; Yang, R.H.; Wang, X.; Gong, J.B. Hydroxytyrosol Protects against Myocardial Ischemia/Reperfusion Injury through a PI3K/Akt-Dependent Mechanism. Mediat. Inflamm. 2016, 2016, 1232103. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Imahashi, K.; Steenbergen, C.; Murphy, E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ. Res. 2002, 90, 377–379. [Google Scholar] [CrossRef]
- Mayerhofer, M.; Valent, P.; Sperr, W.R.; Griffin, J.D.; Sillaber, C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood 2002, 100, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Mottet, D.; Dumont, V.; Deccache, Y.; Demazy, C.; Ninane, N.; Raes, M.; Michiels, C. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J. Biol. Chem. 2003, 278, 31277–31285. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Y.; Yin, H.; Shen, B.; Smith, R.S., Jr.; Liu, Y.; Gao, L.; Chao, L.; Chao, J. Tissue kallikrein promotes neovascularization and improves cardiac function by the Akt-glycogen synthase kinase-3beta pathway. Cardiovasc. Res. 2008, 80, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.; Hausenloy, D.J. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol. Therap. 2012, 136, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, P.; Li, Y.; Ardell, C.; Der, T.; Shohet, R.; Chen, M.; Wright, G.L. HIF-1alpha in heart: Protective mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H821–H828. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Liu, Y.; Zhao, J.; Cai, L.; Li, X.; Feng, W. Activation of HIF-1 by metallothionein contributes to cardiac protection in the diabetic heart. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2528–H2835. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.M.; Roy, M.; Robitaille, G.A.; Richard, D.E.; Bonnet, S. HIF-1 inhibition decreases systemic vascular remodelling diseases by promoting apoptosis through a hexokinase 2-dependent mechanism. Cardiovasc. Res. 2010, 88, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.; Lee, W.H.; Theodorou, L.; Kodo, K.; Lim, S.Y.; Shukla, D.H.; Briston, T.; Kiriakidis, S.; Ashcroft, M.; Davidson, S.M.; et al. HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovasc. Res. 2014, 104, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.G.; Zhu, X.M.; Chu, X.P.; Minami, M.; Hey, J.; Wei, W.L.; MacDonald, J.F.; Wemmie, J.A.; Price, M.P.; Welsh, M.J.; et al. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell 2004, 118, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zuo, L.; Lv, Y.; Chen, C.; Yang, Y.; Xin, H.; Li, Y.; Qian, Y. Asiatic Acid Attenuates Myocardial Ischemia/Reperfusion Injury via Akt/GSK-3β/HIF-1α Signaling in Rat H9c2 Cardiomyocytes. Molecules 2016, 21, 1248. https://doi.org/10.3390/molecules21091248
Huang X, Zuo L, Lv Y, Chen C, Yang Y, Xin H, Li Y, Qian Y. Asiatic Acid Attenuates Myocardial Ischemia/Reperfusion Injury via Akt/GSK-3β/HIF-1α Signaling in Rat H9c2 Cardiomyocytes. Molecules. 2016; 21(9):1248. https://doi.org/10.3390/molecules21091248
Chicago/Turabian StyleHuang, Xiang, Li Zuo, Yanni Lv, Chuqiao Chen, Yaqin Yang, Hongbo Xin, Yunman Li, and Yisong Qian. 2016. "Asiatic Acid Attenuates Myocardial Ischemia/Reperfusion Injury via Akt/GSK-3β/HIF-1α Signaling in Rat H9c2 Cardiomyocytes" Molecules 21, no. 9: 1248. https://doi.org/10.3390/molecules21091248
APA StyleHuang, X., Zuo, L., Lv, Y., Chen, C., Yang, Y., Xin, H., Li, Y., & Qian, Y. (2016). Asiatic Acid Attenuates Myocardial Ischemia/Reperfusion Injury via Akt/GSK-3β/HIF-1α Signaling in Rat H9c2 Cardiomyocytes. Molecules, 21(9), 1248. https://doi.org/10.3390/molecules21091248





