A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-κB and p38 MAPK Pathways in BV2 Microglia
Abstract
:1. Introduction
2. Results
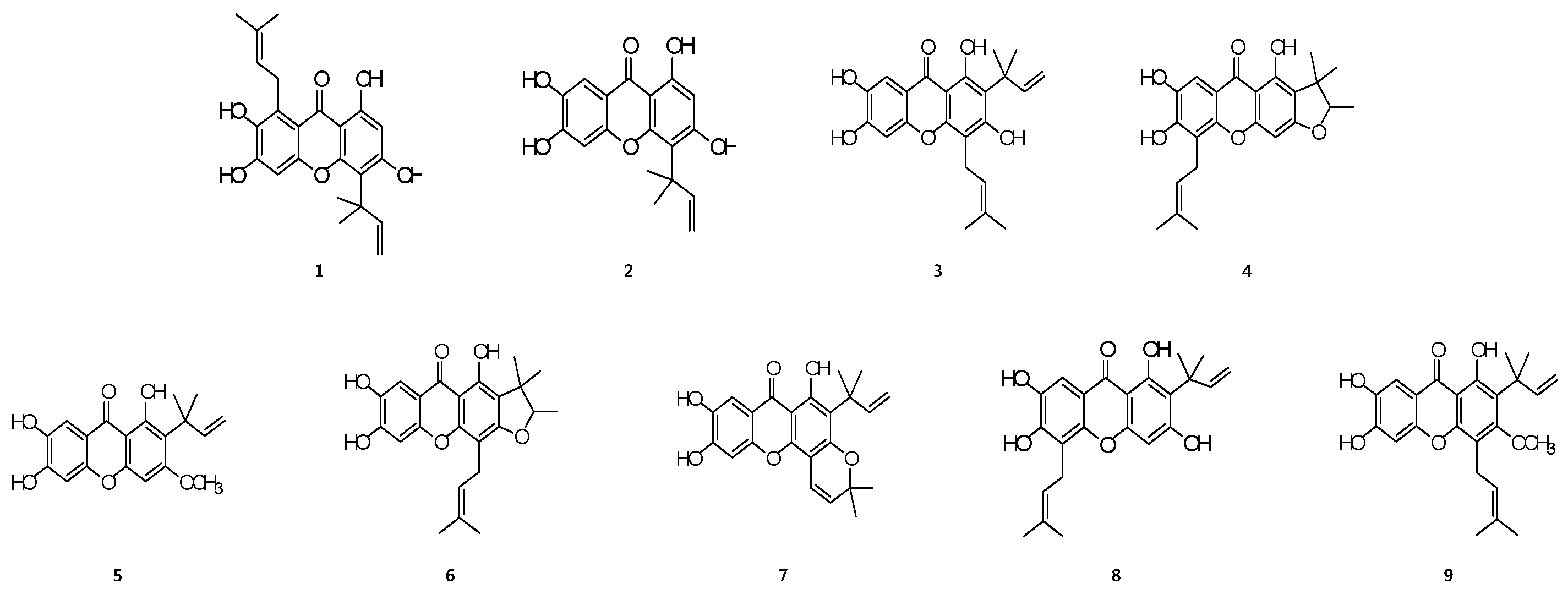
2.1. Structures of Prenylated Xanthones 1–9
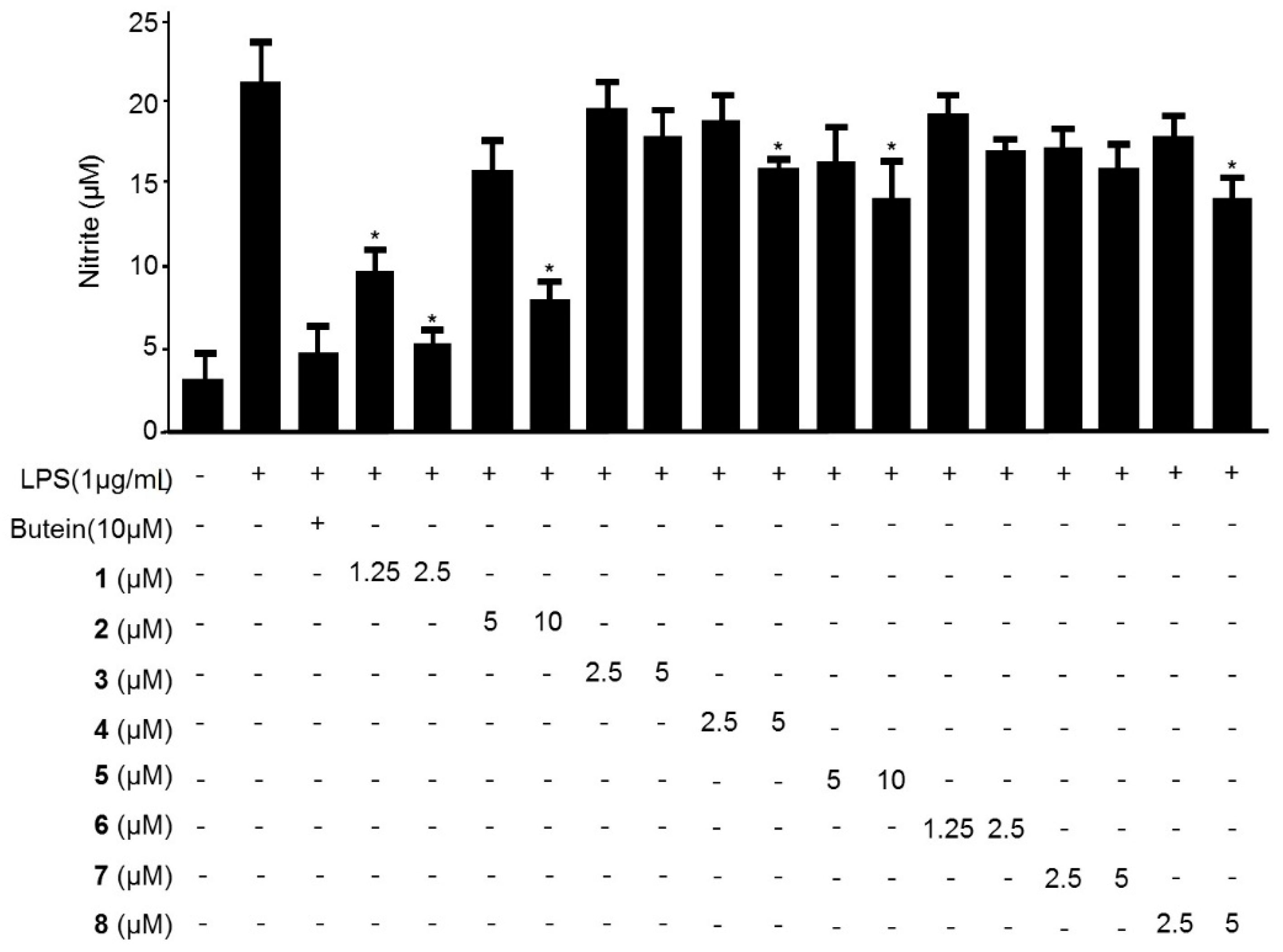
2.2. Effects of Compounds 1–9 on the Production of Nitric Oxide in LPS-Stimulated BV2 Microglia
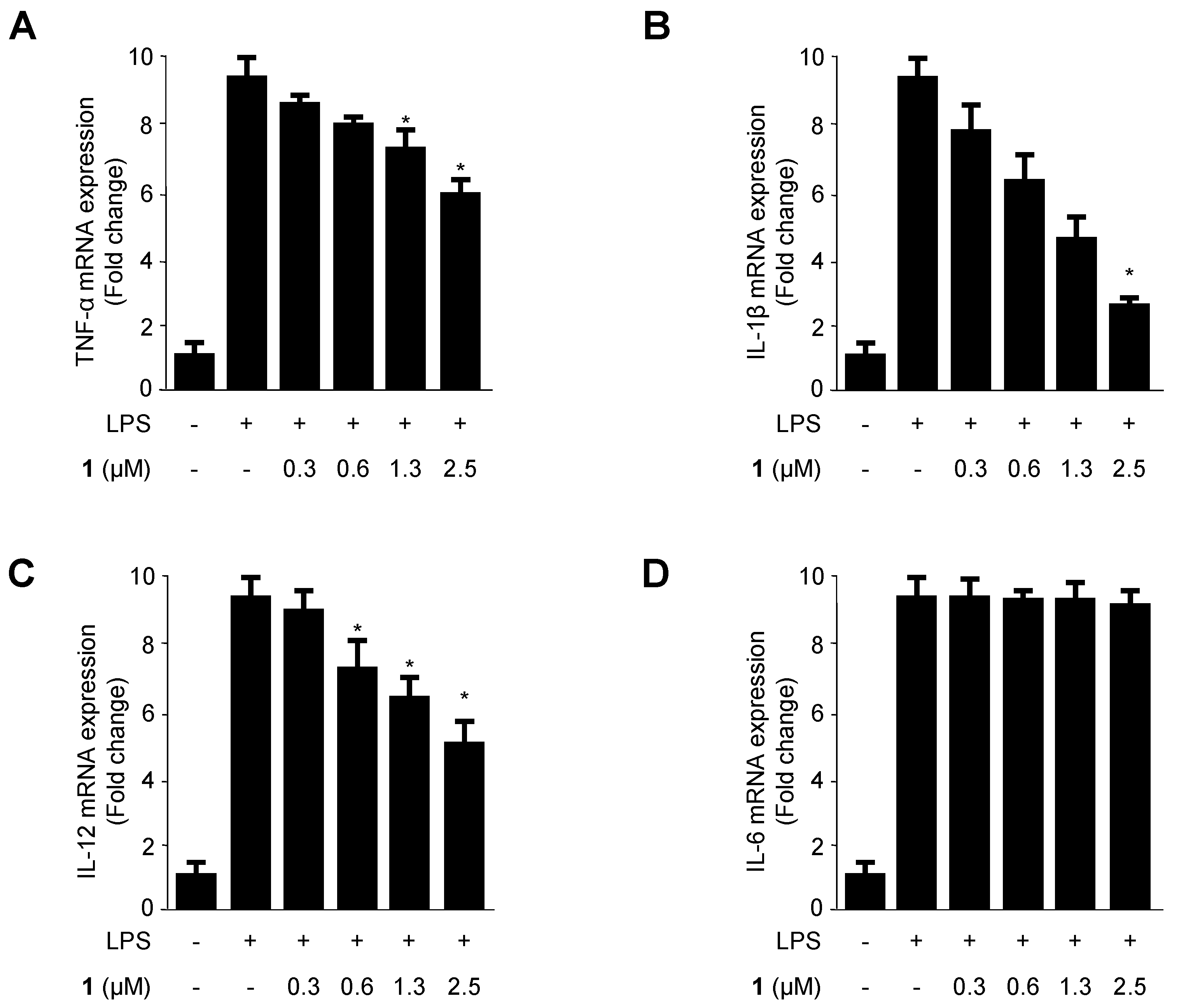
2.3. Effects of Cudratricusxanthone A (1) on the mRNA Expression of the Pro-Inflammatory Cytokines TNF-α, IL-1β, IL-12, and IL-6 in LPS-Stimulated BV2 Microglia
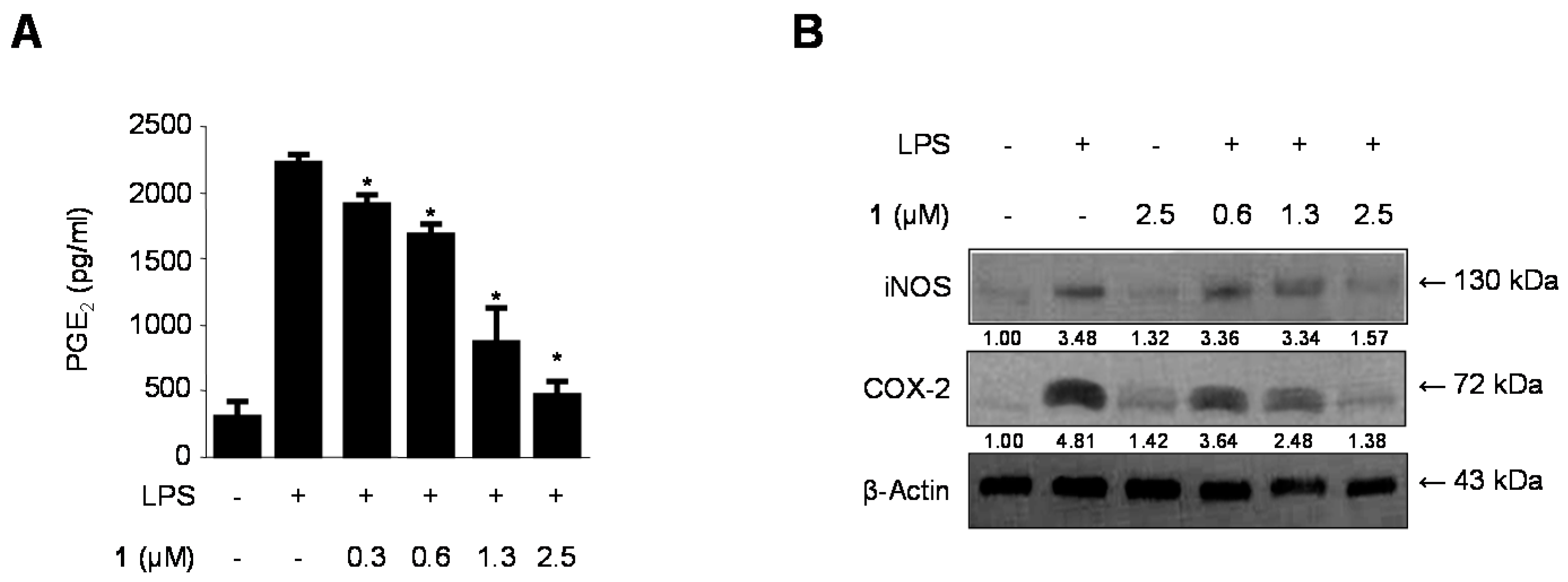
2.4. Effects of Cudratricusxanthone A (1) on PGE2 Production and iNOS and COX-2 Protein Expression in LPS-Stimulated BV2 Microglia
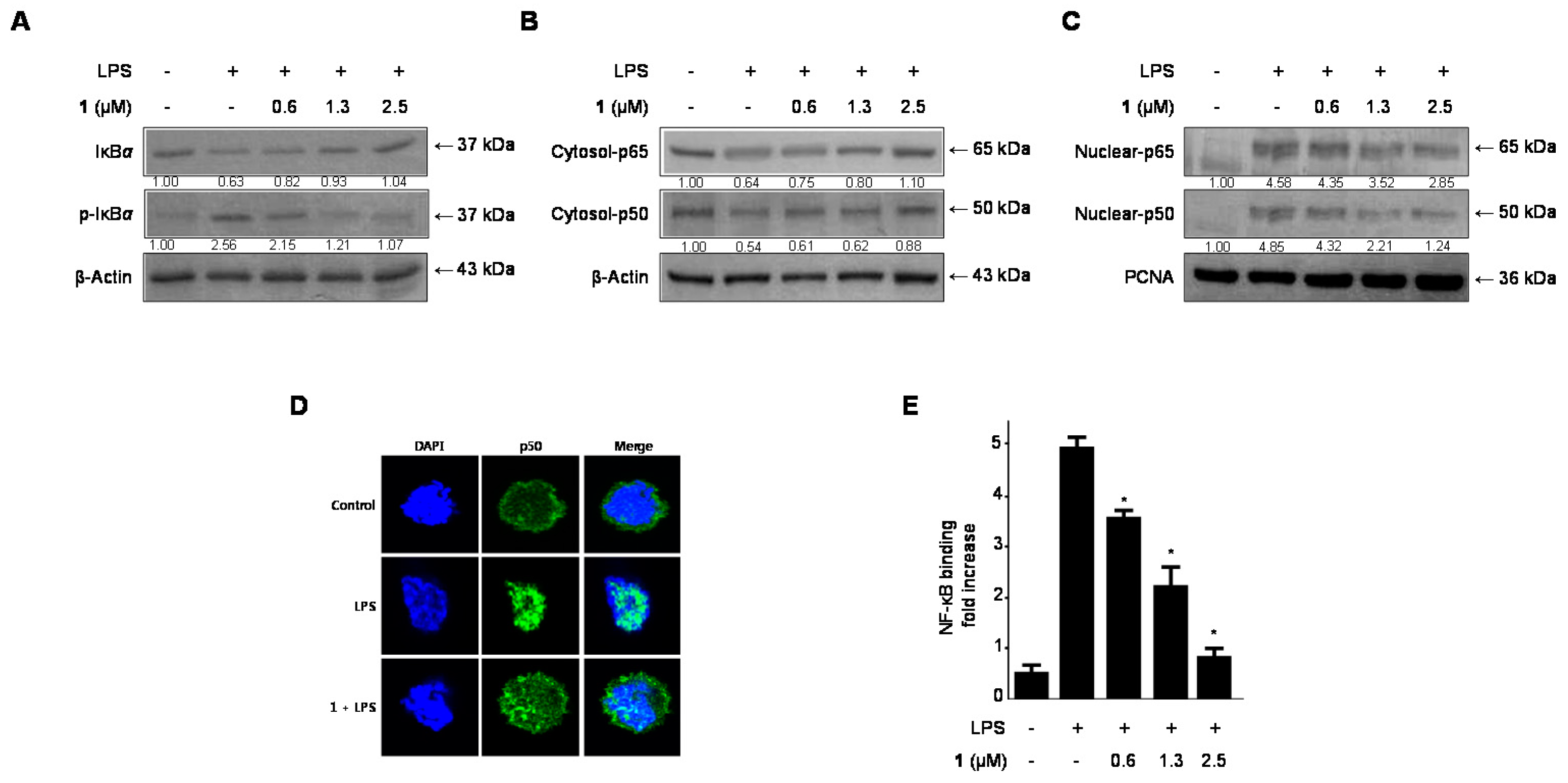
2.5. Effects of Cudratricusxanthone A (1) on IκB-α Levels, NF-κB Nuclear Translocation, and NF-κB DNA Binding Activity in LPS-Stimulated BV2 Microglia
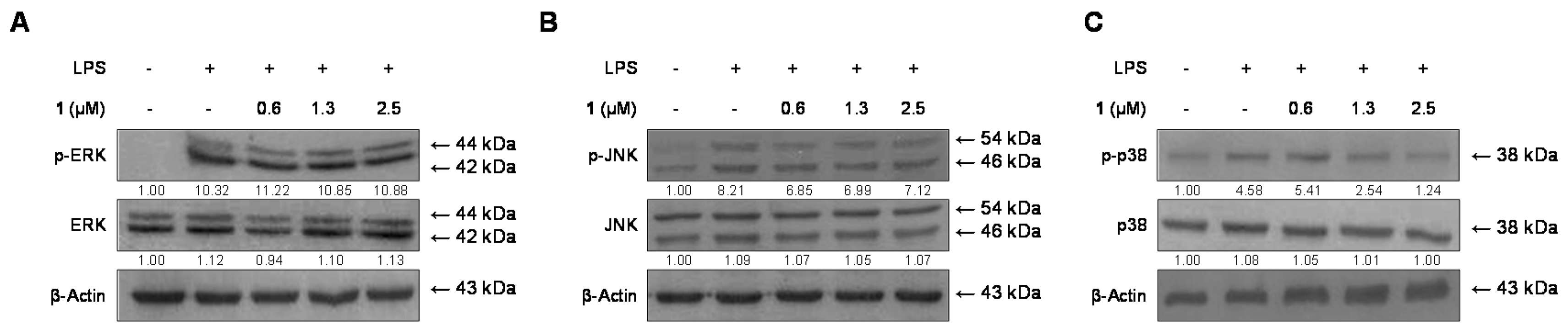
2.6. Effects of Cudratricusxanthone A (1) on the Phosphorylation of MAPKs in BV2 Microglia Stimulated with LPS
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Cell Culture and Viability Assay
4.3. Quantitative Reverse Transcription Polymerase Chain Reaction (PCR)
4.4. DNA Binding Activity of NF-κB
4.5. Preparation of Cytosolic and Nuclear Fractions
4.6. Nitrite (NO Production) Determination
4.7. Western Blot Analysis
4.8. NF-κB Localization and Immunofluorescence
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.D. In the Dictionary of Chinese Drugs; Shanghai Science and Technological Publisher: Shanghai, China; Shougakukan: Tokyo, Japan, 1985; Volume 2, p. 2383. [Google Scholar]
- Fujimoto, T.; Hano, Y.; Nomura, T. Components of root bark of Cudrania tricuspidata l.1,2 structures of four new isoprenylated xanthones, cudraxanthones A, B, C and D. Planta Med. 1984, 50, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Lee, J.H.; Lee, S.T.; Lee, H.S.; Lee, W.S.; Jeong, T.S.; Park, K.H. Antioxidant and cytotoxic activities of xanthones from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2005, 15, 5548–5552. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Hong, S.S.; Hwang, J.S.; Jeong, S.H.; Hwang, J.H.; Lee, M.H.; Lee, M.K.; Lee, D.; Ro, J.S.; Hwang, B.Y. Monoamine oxidase inhibitory constituents from the fruits of Cudrania tricuspidata. Arch. Pharm. Res. 2005, 28, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Hiep, N.T.; Kim, D.W.; Hwang, B.Y.; Lee, H.J.; Mar, W.; Lee, D. Neuroprotective xanthones from the root bark of Cudrania tricuspidata. J. Nat. Prod. 2014, 77, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Park, Y.D.; Han, J.M.; Im, K.R.; Lee, B.W.; Jeong, I.Y.; Jeong, T.S.; Lee, W.S. Anti-atherosclerotic and anti-inflammatory activities of catecholic xanthones and flavonoids isolated from Cudrania tricuspidata. Bioorg. Med. Chem. Lett. 2006, 16, 5580–5583. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, N.T.; Yoon, C.S.; Cho, K.H.; Kang, D.G.; Lee, H.S.; Kim, Y.C.; Oh, H. Protein tyrosine phosphatase 1B inhibitors from the roots of Cudrania tricuspidata. Molecules 2015, 20, 11173–11183. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.H.; Kim, H.C.; Cui, J.M.; Kim, Y.C. Hepatoprotective constituents of Cudrania tricuspidata. Arch. Pharm. Res. 2005, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Lee, D.S.; Kim, Y.C. Cudratricusxanthone A from Cudrania tricuspidata suppresses pro-inflammatory mediators through expression of anti-inflammatory heme oxygenase-1 in RAW264.7 macrophages. Int. Immunopharmacol. 2009, 9, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; An, R.B.; Pae, H.O.; Chung, H.T.; Yoon, K.H.; Kang, D.G.; Lee, H.S.; Kim, Y.C. Cudratricusxanthone A protects mouse hippocampal cells against glutamate-induced neurotoxicity via the induction of heme oxygenase-1. Planta Med. 2008, 74, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.È.; Stevens, B.; Sierra, A.; Wake, H.; Bessis, A.; Nimmerjahn, A. The role of microglia in the healthy brain. J. Neurosci. 2011, 31, 16064–16069. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Progr. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.U.; de Vellis, J. Microglia in health and disease. J. Neurosci. Res. 2005, 81, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease: A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.A.; Schena, M.; Chai, A.; Shalon, D.; Bedilion, T.; Gilmore, J.; Woolley, D.E.; Davis, R.W. Discovery and analysis of inflammatory disease-related genes using cDNA microarrays. Proc. Natl. Acad. Sci. USA 1997, 94, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ghosh, S. Toll-like receptor-mediated NF-kappaB activation: A phylogenetically conserved paradigm in innate immunity. J. Clin. Investig. 2001, 107, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, F.; Manning, A.M. Multiple signals converging on NF-κB. Curr. Opin. Cell Biol. 1999, 11, 226–232. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol. Reprod. 2002, 67, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M. Map kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar] [PubMed]
- Rajapakse, N.; Kim, M.M.; Mendis, E.; Kim, S.K. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-kappaB signaling. Immunology 2008, 123, 348–357. [Google Scholar] [PubMed]
- Chen, L.F.; Greene, W.C. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Biol. 2004, 5, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Hong, S.S.; Han, X.H.; Hwang, J.S.; Lee, D.; Lee, H.; Yun, Y.P.; Kim, Y.; Ro, J.S.; Hwang, B.Y. Prenylated xanthones from the root bark of Cudrania tricuspidata. J. Nat. Prod. 2007, 70, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Ku, S.K.; Lee, W.; Kwak, S.; Baek, Y.D.; Min, B.W.; Jeong, G.S.; Bae, J.S. Antiplatelet, anticoagulant, and profibrinolytic activities of cudratricusxanthone A. Arch. Pharm. Res. 2014, 37, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Jeong, G.S. Cudratricusxanthone A protect pancreatic beta cells from cytokines-mediated toxicity through the inhibition of NF-κB and STAT pathways. Int. Immunopharmacol. 2014, 21, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Choi, E.; Lee, Y.M.; Jeong, G.S.; Lee, S. In vitro inhibition of human cytochrome P450 by cudratricusxanthone A. Food Chem. Toxicol. 2015, 81, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Lynch, M.A. The multifaceted profile of activated microglia. Mol. Neurobiol. 2009, 40, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Pratico, D.; Trojanowski, J.Q. Inflammatory hypotheses: Novel mechanisms of Alzheimer's neurodegeneration and new therapeutic targets. Neurobiol. Aging 2000, 21, 441–445. [Google Scholar] [CrossRef]
- Hald, A.; Lotharius, J. Oxidative stress and inflammation in Parkinson’s disease: Is there a causal link. Exp. Neurol. 2005, 193, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Rankine, E.L.; Hughes, P.M.; Botham, M.S.; Perry, V.H.; Felton, L.M. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. Eur. J. NeuroSci. 2006, 24, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Ham, Y.M.; Yoo, B.S.; Moon, J.Y.; Koh, J.; Hyun, C.G. Oenothera laciniata inhibits lipopolysaccharide induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. J. Biosci. Bioeng. 2009, 107, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Boje, K.M.; Arora, P.K. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992, 587, 250–256. [Google Scholar] [CrossRef]
- Chao, C.C.; Hu, S.; Molitor, T.W.; Shaskan, E.G.; Peterson, P.K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 1992, 149, 2736–2741. [Google Scholar] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, T.; Wang, Y.; Wang, Y.; He, L.; Jiang, Z.; Huang, Z.; Liao, H.; Li, J.; Saavedra, J.M.; et al. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation. Brain Behav. Immun. 2015, 50, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Ohshima, H. Nitrative DNA damage in inflammation and its possible role in carcinogenesis. Nitric Oxide 2006, 14, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-kappaB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.F.; Hibbs, J.B., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991, 3, 65–70. [Google Scholar] [CrossRef]
- Murakami, A. Chemoprevention with phytochemicals targeting inducible nitric oxide synthase. Forum Nutr. 2009, 61, 193–203. [Google Scholar] [PubMed]
- Yoon, C.S.; Kim, D.C.; Lee, D.S.; Kim, K.S.; Ko, W.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effect of aurantiamide acetate from the marine fungus Aspergillus sp. SF-5921: Inhibition of NF-κB and MAPK pathways in lipopolysaccharide-induced mouse BV2 microglial cells. Int. Immunopharmacol. 2014, 23, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Lee, H.S.; Ko, W.; Lee, D.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-inflammatory effect of methylpenicinoline from a marine isolate of Penicillium sp. (SF-5995): Inhibition of NF-κB and MAPK pathways in lipopolysaccharide-induced RAW264.7 macrophages and BV2 microglia. Molecules 2014, 19, 18073–18089. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Yoon, C.S.; Quang, T.H.; Ko, W.; Kim, J.S.; Oh, H.; Kim, Y.C. Prenylated flavonoids from Cudrania tricuspidata suppress lipopolysaccharide-induced neuroinflammatory activities in BV2 microglial cells. Int. J. Mol. Sci. 2016, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the cudratricusxanthone A are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, C.-S.; Kim, D.-C.; Quang, T.H.; Seo, J.; Kang, D.G.; Lee, H.S.; Oh, H.; Kim, Y.-C. A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-κB and p38 MAPK Pathways in BV2 Microglia. Molecules 2016, 21, 1240. https://doi.org/10.3390/molecules21091240
Yoon C-S, Kim D-C, Quang TH, Seo J, Kang DG, Lee HS, Oh H, Kim Y-C. A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-κB and p38 MAPK Pathways in BV2 Microglia. Molecules. 2016; 21(9):1240. https://doi.org/10.3390/molecules21091240
Chicago/Turabian StyleYoon, Chi-Su, Dong-Cheol Kim, Tran Hong Quang, Jungwon Seo, Dae Gill Kang, Ho Sub Lee, Hyuncheol Oh, and Youn-Chul Kim. 2016. "A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-κB and p38 MAPK Pathways in BV2 Microglia" Molecules 21, no. 9: 1240. https://doi.org/10.3390/molecules21091240
APA StyleYoon, C.-S., Kim, D.-C., Quang, T. H., Seo, J., Kang, D. G., Lee, H. S., Oh, H., & Kim, Y.-C. (2016). A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-κB and p38 MAPK Pathways in BV2 Microglia. Molecules, 21(9), 1240. https://doi.org/10.3390/molecules21091240




