Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Chemical Compositions of Essential Oil
2.2. DIZ and MIC of Essential Oil
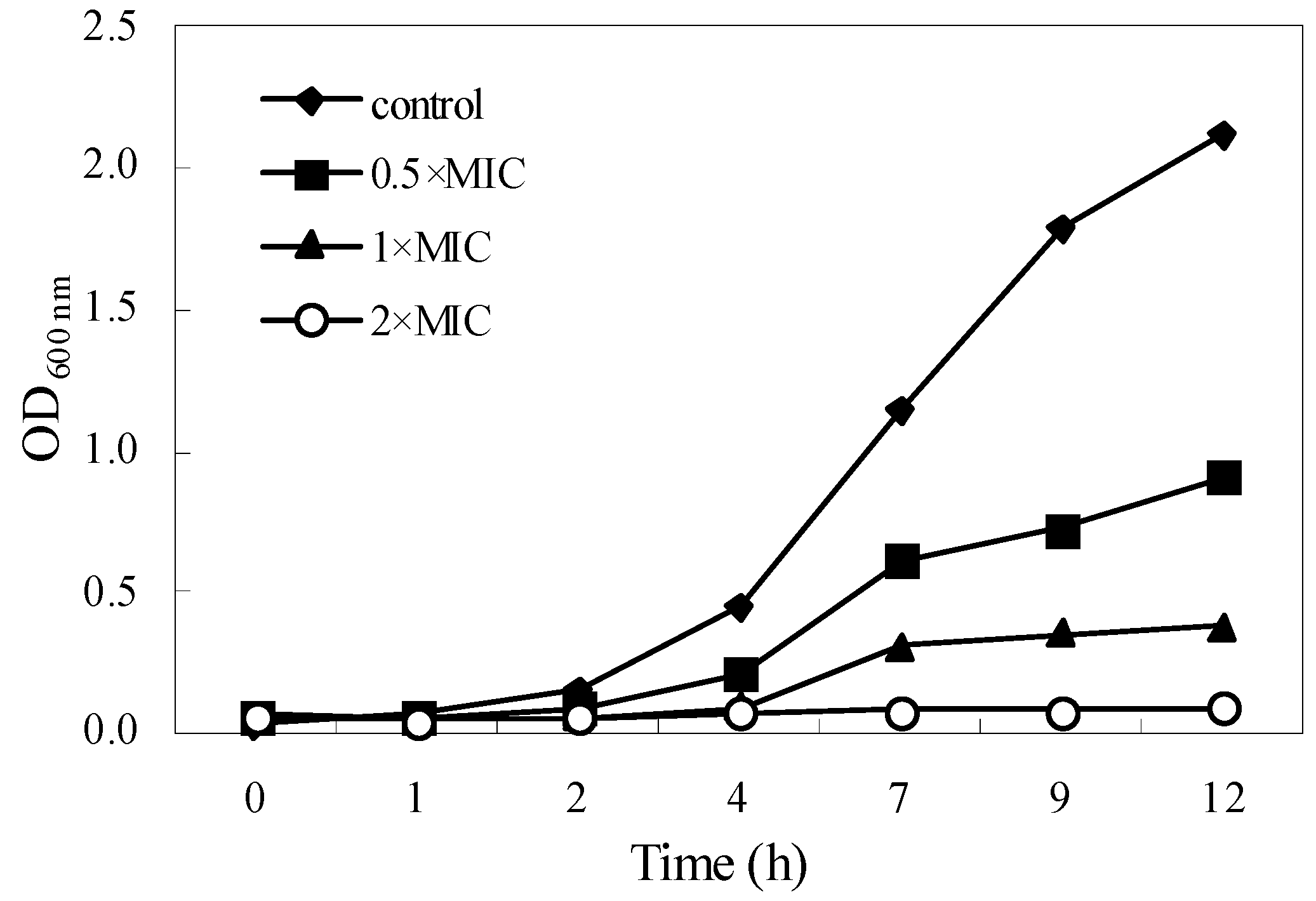
2.3. Kill-Time Analysis
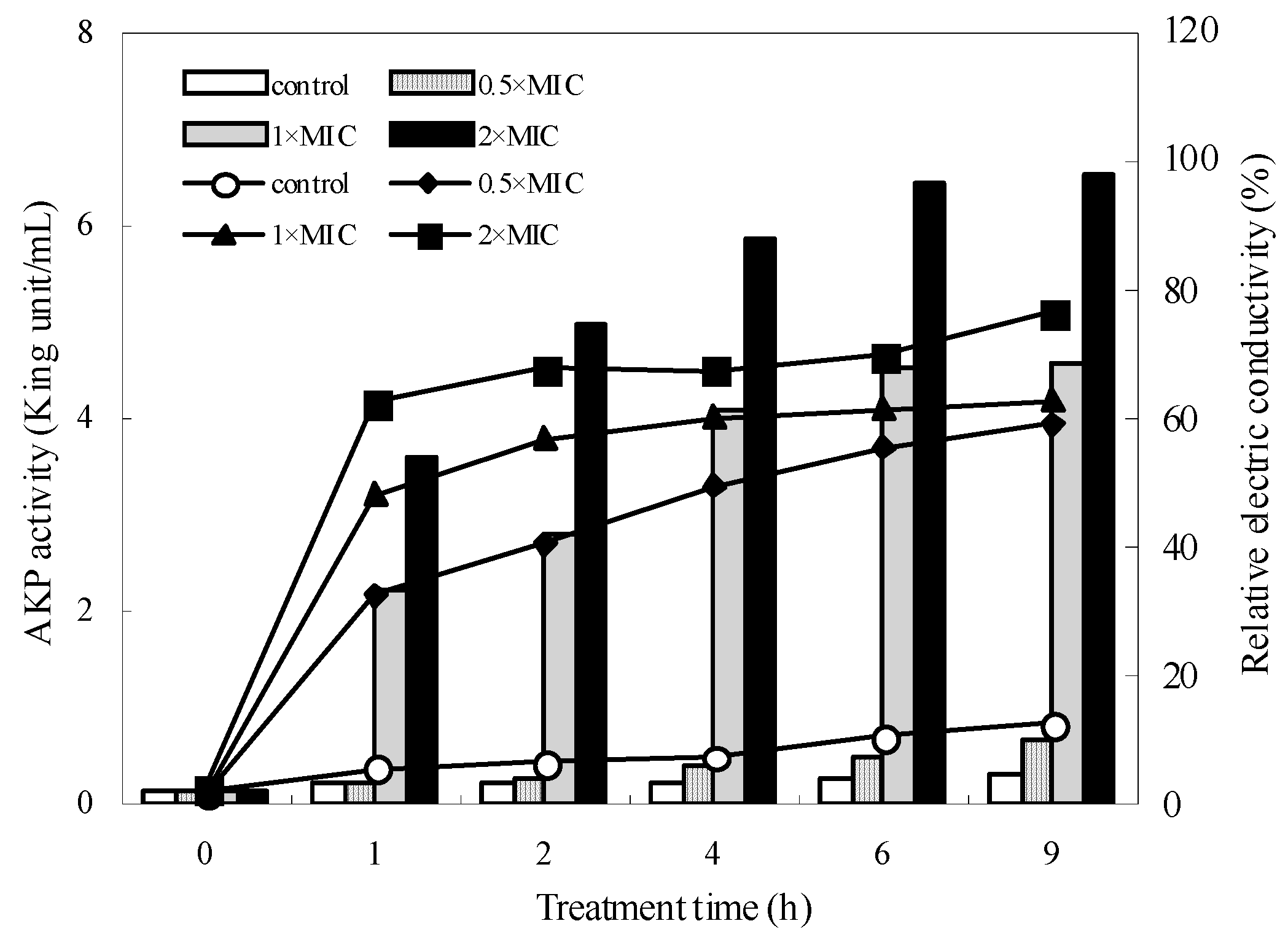
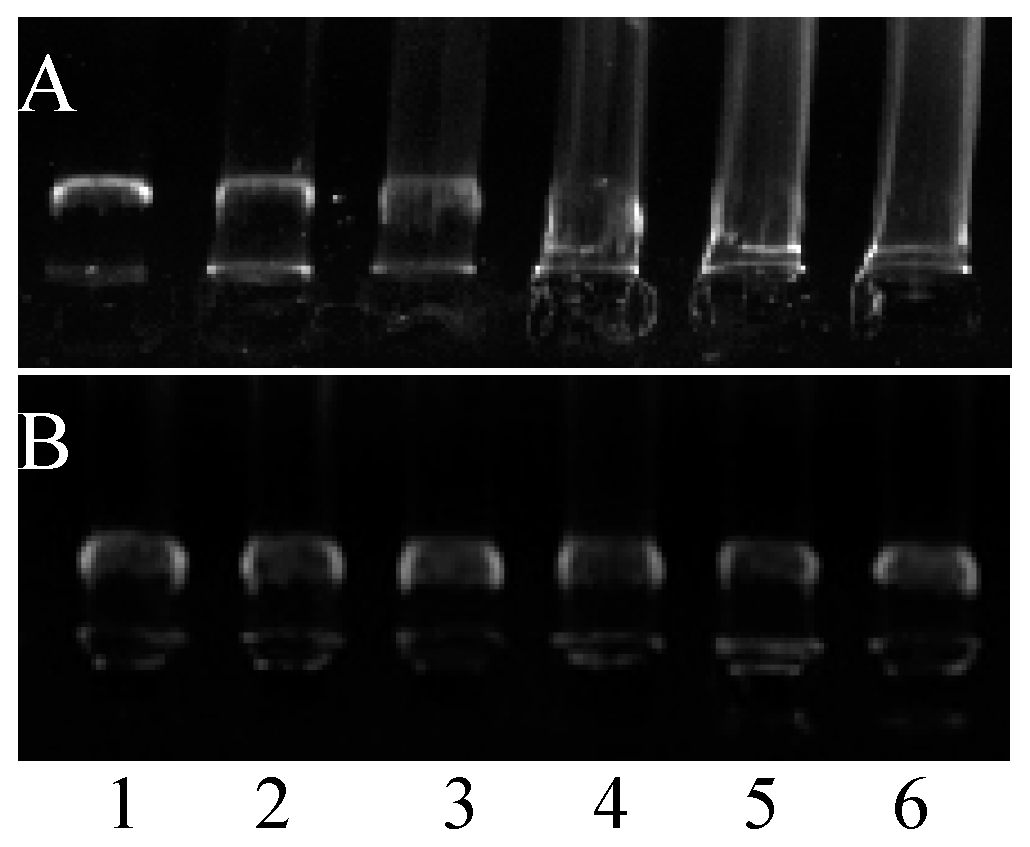
2.4. Cell Permeabilization Assay
2.5. Cell Membrane Permeability
2.6. Integrity of Cell Membrane
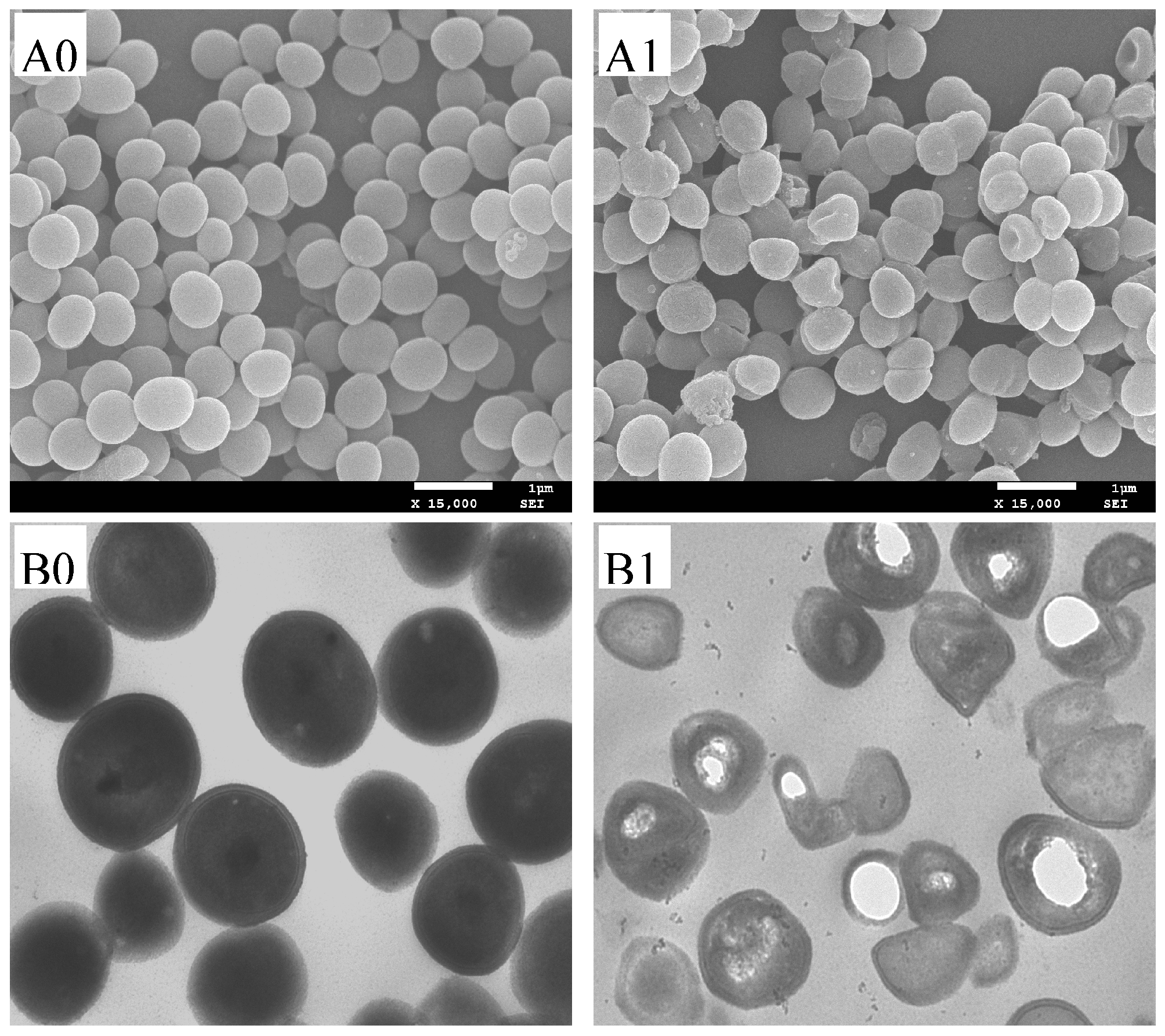
2.7. Microscopic Observations
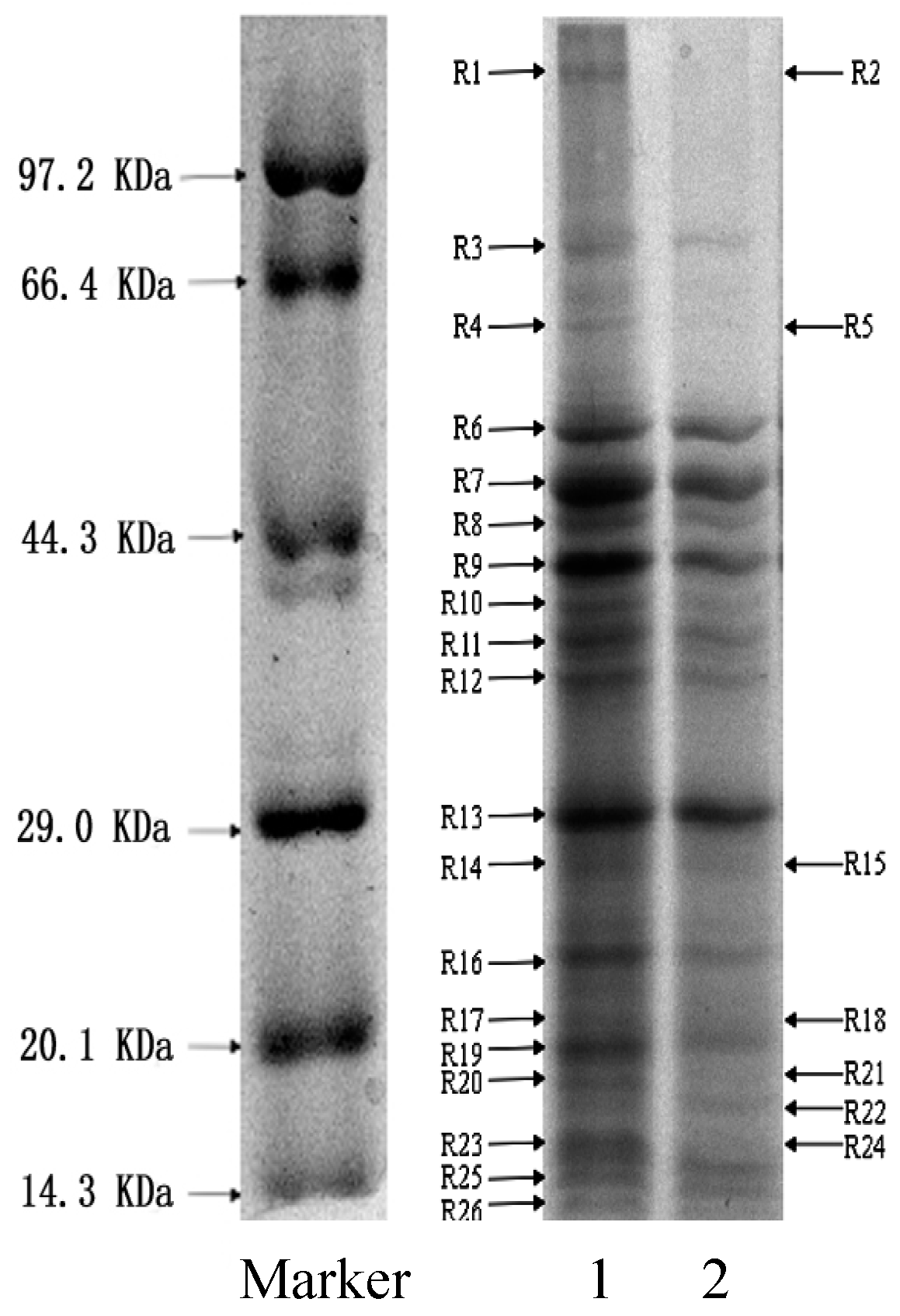
2.8. SDS-PAGE of Proteins from Bacteria
2.9. DNA Synthesis Inhibition and DNA-Binding Assays
3. Discussion
4. Experimental Section
4.1. Plant Materials, Microbial Strain and Culture
4.2. Extraction of Essential Oil
4.3. GC-MS Analysis
4.4. Antibacterial Activity
4.5. Minimum Inhibitory Concentration (MIC)
4.6. Kill-Time Analysis
4.7. Cell Wall Permeabilization Assay
4.8. Permeability of Cell Membrane
4.9. Integrity of Cell Membrane
4.10. Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM)
4.11. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.12. DNA Synthesis Inhibition
4.13. DNA-binding Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bajpai, V.K.; Baek, K.H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Nie, S.P.; Liu, X.Z.; Zhang, H.; Yang, Y.; Yu, Q.; Xie, M.Y. Antimicrobial properties, antioxidant activity and cytotoxicity of ethanol-soluble acidic components from Ganoderma atrum. Food Chem. Toxicol. 2012, 50, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary of polyphenols and the prevention of diseases. Crit. Rev. Food Sci. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Adámez, J.; Fernández-León, M.F.; Velardo-Micharet, B.; González-Gómez, D. In vitro assays of the antibacterial and antioxidant activity of aqueous leaf extracts from different Prunus salicina Lindl. cultivars. Food Chem. Toxicol. 2012, 50, 2481–2486. [Google Scholar] [CrossRef] [PubMed]
- Voon, H.C.; Bhat, R.; Gulam, R. Flower extracts and their essential oils as potential antimicrobial agents. Compr. Rev. Food Sci. Food Saf. 2012, 11, 34–55. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.E.; Gardini, F. Use of natural aroma compounds to improve shelflife and safety of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Ben Ei Hadj Ali, I.; Chaouachi, M.; Bahri, R.; Chaieb, I.; Boussaïd, M.; Harzallah-Skhiri, F. Chemical composition and antioxidant, antibacterial, allelopathic and insecticidal activities of essential oil of Thymus algeriensis Boiss. Et Reut. Ind. Crop Prod. 2015, 77, 631–639. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Kildeaa, M.A.; Allanb, G.L.; Kearney, R.E. Accumulation and clearance of the anaesthetics clove oil and AQUI-STM from the edible tissue of silver perch (Bidyanus bidyanus). Aquaculture 2004, 232, 265–277. [Google Scholar] [CrossRef]
- Özcan, M.M.; Arslan, D. Antioxidant effect of essential oils of rosemary, clove and cinnamon on hazelnut and poppy oils. Food Chem. 2011, 129, 171–174. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Omidbeygi, M.; Barzegar, M.; Hamidi, Z.; Naghdibadi, H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus Xavus in liquid medium and tomato paste. Food Control 2007, 18, 1518–1523. [Google Scholar] [CrossRef]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Custódio, J.B.; Cavaleiro, C.; Sousa, M.C. Anti-giardia activity of Syzygium aromaticum essential oil and eugenol: Effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 2011, 127, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Lu, F.; Ding, Y.; Ye, X.; Ding, Y. Antibacterial effect of cinnamon oil combined with thyme or clove oil. Agric. Sci. China 2011, 10, 1482–1487. [Google Scholar] [CrossRef]
- Ivanovic, J.; Dimitrijevic-Brankovic, S.; Misic, D.; Ristic, M.; Zizovic, I. Evaluation and improvement of antioxidant and antibacterial activities of supercritical extracts from clove buds. J. Funct. Foods 2013, 5, 416–423. [Google Scholar] [CrossRef]
- Hajji, M.; Jarraya, R.; Lassoued, I.; Masmoudi, O.; Damak, M.; Nasri, M. GC/MS and LC/MS analysis, and antioxidant and antimicrobial activities of various solvent extracts from Mirabilis jalapa tubers. Process Biochem. 2010, 45, 1486–1493. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, M.; Zhang, Y.; Liao, H.; Ou Yang, J.; Zeng, Z.; Feng, S.; Li, H. Composition and antimicrobial activity of the volatile oil from bud of Syinga Juliana. C. K. Schneid. Nat. Sci. J. Xiangtan Univ. 2008, 30, 101–105. [Google Scholar]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health. NTP Technical Report on the Carcinogenesis Studies of Eugenol (CAS NO. 97-53-0) in F344/N Rats and B6C3F1 Mice (Feed Studies); NIH Publication No. 84-1779: Bethesda, MD, USA, 1983. [Google Scholar]
- Taher, Y.A.; Samud, A.M.; El-Taher, F.E.; ben-Hussin, G.; Elmezogi, J.S.; Al-Mehdawi, B.F.; Salem, H.A. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J. Med. 2015, 10, 28685–28692. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.M.; Ibrahima, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markhan, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the antimicrobial action of tea tree oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From target to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, S.G.; Ganguly, K.; Chaudhuri, K.; Chakrabarty, A.N. The antibacterial action of diclofenac shown by inhibition of DNA synthesis. Int. J. Antimicrob. Agents 2000, 14, 249–251. [Google Scholar] [CrossRef]
- Haney, E.F.; Petersen, A.P.; Lau, C.K.; Jing, W.; Storey, D.G.; Vogel, H.J. Mechanism of action of puroindoline derived tryptophan-rich antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Truong, V.K.; Wang, F.; Pushpamali, W.A.A.; Wang, J.Y.; Ellis, A.V.; Berndt, C.C.; Crawford, R.J.; Ivanova, E.P. Plasma-enhanced synthesis of bioactive polymeric coatings from monoterpene alcohols: A combined experimental and theoretical study. Biomacromolecules 2010, 11, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Ostrikov, K. Sustainable life cycles of natural-precursor-derived nanocarbons. Chem. Rev. 2016, 116, 163–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Tang, J.H.; Jiang, D.; Fang, H.J.; Liu, Z.B. Bacteriostatic action and mechanism of Sophora flavescens Ait on Escherichia coli 01 C84010. Sci. Agric. Sin. 2006, 39, 1018–1024. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Kubo, I. Combination effects of antibacterial compounds in green tea flavor against Streptococcus mutans. J. Agric. Food Chem. 1993, 41, 1102–1106. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Feng, S.S.; Li, W.Q.; Xu, J.G. Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J. Agric. Food Chem. 2013, 61, 6044–6049. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, I.; Seseña, S.; Palop, L. Identification of lactic acid bacteria from spontaneous fermentation of ‘Almagro’ eggplants by SDS-PAGE whole cell protein fingerprinting. Int. J. Food Microbiol. 2003, 82, 181–189. [Google Scholar] [CrossRef]
- Imura, Y.; Nishida, M.; Matsuzaki, K. Action mechanism of PEGylated magainin 2 analogue peptide. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lin, X.; Long, Y.; Wang, Y.; Zhang, Y.; Mi, H.; Yan, H. A peptide fragment derived from the T-cell antigen receptor protein a-chain adopts b-sheet structure and shows potent antimicrobial activity. Peptides 2009, 30, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
| Compound | Peak Area (%) a | Compound | Peak Area (%) a |
|---|---|---|---|
| 2-Pinene | 0.02 | Eugenol | 76.23 |
| α-Pinene | 0.03 | β-Caryophyllene | 11.54 |
| Eucalyptol | 0.14 | α-Caryophyllene | 0.64 |
| Methyl salicylate | 0.06 | (−)-b-Cadinene | 0.12 |
| Chavicol | 0.09 | β-Selinene | 0.25 |
| 4-Allylanisole | 0.13 | α-Selinene | 0.16 |
| Anethol | 0.11 | Caryophyllene oxide | 4.29 |
| α-Muurolene | 0.01 | Jasmone | 0.07 |
| α-Copaene | 0.05 | Ledol | 0.03 |
| Valencene | 0.01 | Globulol | 0.04 |
| Eugenyl acetate | 1.76 | Cedrene | 0.02 |
| Concentrations | Cell Constituents’ Release | ||
|---|---|---|---|
| Protein (µg/mL) | Reducing Sugar (µg/mL) | Cell Constituents (OD260nm) | |
| Control | 12.2 ± 1.2 d | 12.5 ± 1.4 d | 0.023 ± 0.006 d |
| 0.5 × MIC | 48.86 ± 3.3 c | 32.6 ± 2.6 c | 0.119 ± 0.022 c |
| 1 × MIC | 59.75 ± 4.8 b | 54.3 ± 5.1 b | 0.245 ± 0.052 b |
| 2 × MIC | 78.63 ± 6.1 a | 88.2 ± 4.5 a | 0.517 ± 0.063 a |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules 2016, 21, 1194. https://doi.org/10.3390/molecules21091194
Xu J-G, Liu T, Hu Q-P, Cao X-M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules. 2016; 21(9):1194. https://doi.org/10.3390/molecules21091194
Chicago/Turabian StyleXu, Jian-Guo, Ting Liu, Qing-Ping Hu, and Xin-Ming Cao. 2016. "Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus" Molecules 21, no. 9: 1194. https://doi.org/10.3390/molecules21091194
APA StyleXu, J.-G., Liu, T., Hu, Q.-P., & Cao, X.-M. (2016). Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules, 21(9), 1194. https://doi.org/10.3390/molecules21091194






