Abstract
Elevated blood concentrations of homocysteine have been well established as a risk factor for cardiovascular diseases and neuropsychiatric diseases, yet the etiologic relationship of homocysteine to these disorders remains poorly understood. Protein N-homocysteinylation has been hypothesized as a contributing factor; however, it has not been examined globally owing to the lack of suitable detection methods. We recently developed a selective chemical method to label N-homocysteinylated proteins with a biotin-aldehyde tag followed by Western blotting analysis, which was further optimized in this study. We then investigated the variation of protein N-homocysteinylation in plasma from rats on a vitamin B12 deficient diet. Elevated “total homocysteine” concentrations were determined in rats with a vitamin B12 deficient diet. Correspondingly, overall levels of plasma protein N-homocysteinylation displayed an increased trend, and furthermore, more pronounced and statistically significant changes (e.g., 1.8-fold, p-value: 0.03) were observed for some individual protein bands. Our results suggest that, as expected, a general metabolic correlation exists between “total homocysteine” and N-homocysteinylation, although other factors are involved in homocysteine/homocysteine thiolactone metabolism, such as the transsulfuration of homocysteine by cystathionine β-synthase or the hydrolysis of homocysteine thiolactone by paraoxonase 1 (PON1), may play more significant or direct roles in determining the level of N-homocysteinylation.
1. Introduction
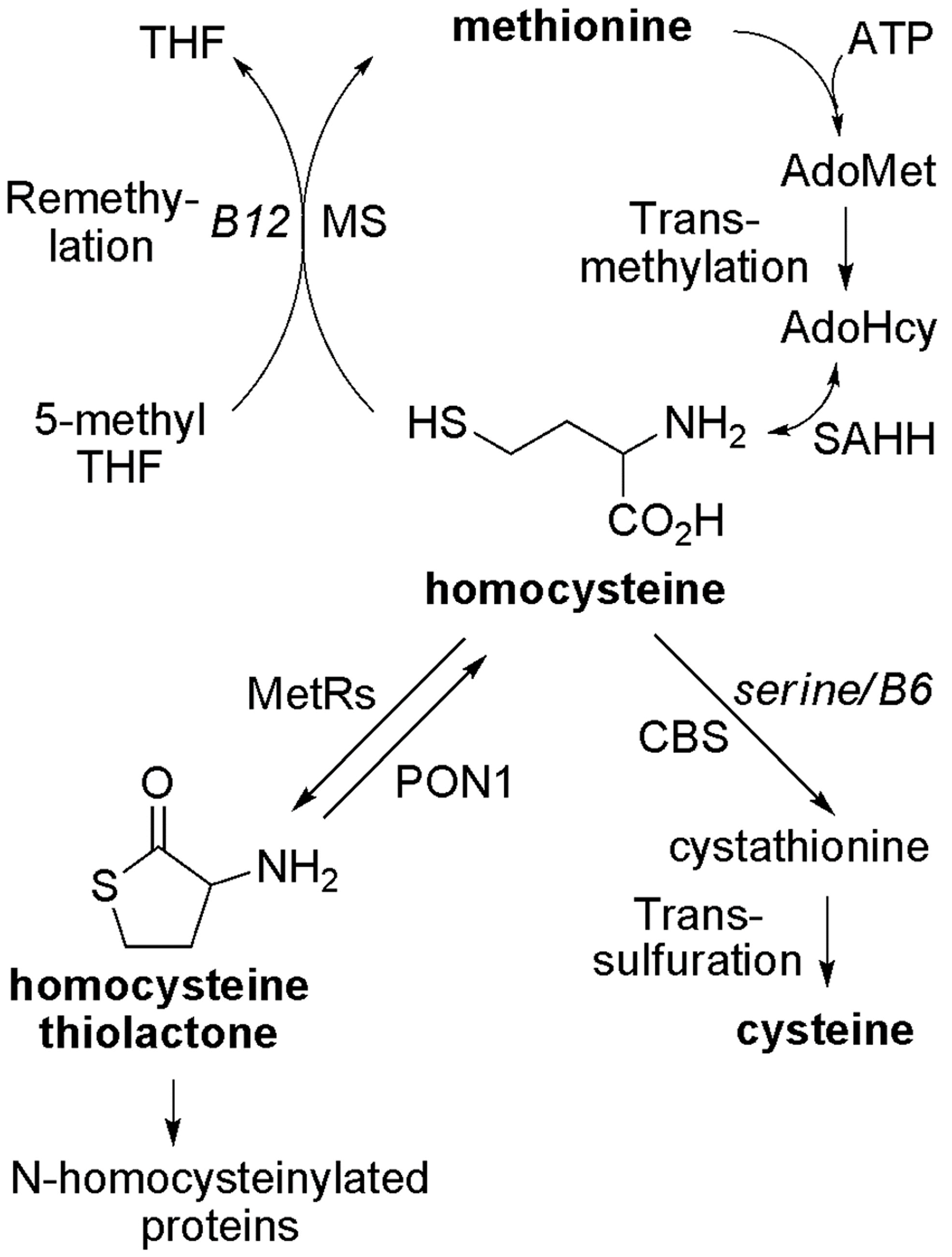
An abnormally increased concentration of homocysteine (Hcy) in blood or urine, i.e., hyperhomocysteinemia or homocystinuria, has been well recognized as a risk factor for cardiovascular, neuropsychiatric diseases, and other conditions [1,2,3,4,5,6]. As illustrated in Figure 1, in mammals, Hcy can be metabolized through two main pathways: methylation and transsulfuration [7,8,9,10,11]. In addition, Hcy can also be converted back to S-adenosyl-homocysteine (AdoHcy or SAH) because of the reversible transformation catalyzed by AdoHcy hydrolase (EC 3.3.1.1) [12,13]. It has been reported that methionine-rich diets (e.g., animal proteins), genetic defects of enzymes such as cystathionine β-synthase (CBS), deficiencies of nutritional factors (folate, vitamin B6 and B12), or a combination of such factors can lead to an increase of plasma “total homocysteine” (“total Hcy”) [7,8,14,15,16,17,18,19], which includes protein S-homocysteinylation (disulfides) and some free small molecule forms (e.g., free Hcy and its mixed disulfides with cysteine or glutathione) [3,20]. However, the pathophysiological consequences of homocysteinemia remain unclear.
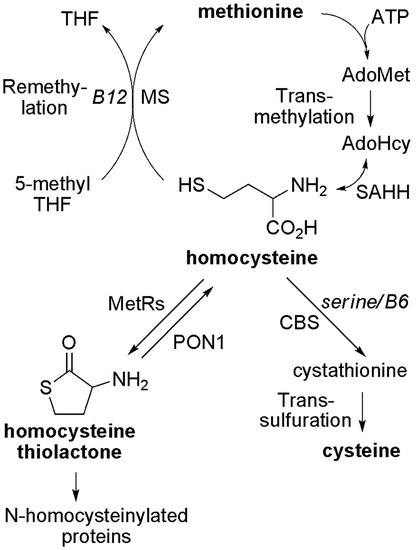
Figure 1.
Metabolism of homocysteine in mammals. AdoHcy, S-adenosylhomocysteine; AdoMet, S-adenosylmethionine; ATP, adenosine-5′-triphosphate; CBS, cystathionine β-synthase; Hcy, homocysteine; Hcy TL, homocysteine thiolactone; MetRS, methionyl-tRNA synthetase; MS, methionine synthase; PON1, paraoxonase 1; SAHH, S-adenosylhomocysteine hydrolase; THF, tetrahydrofolate.
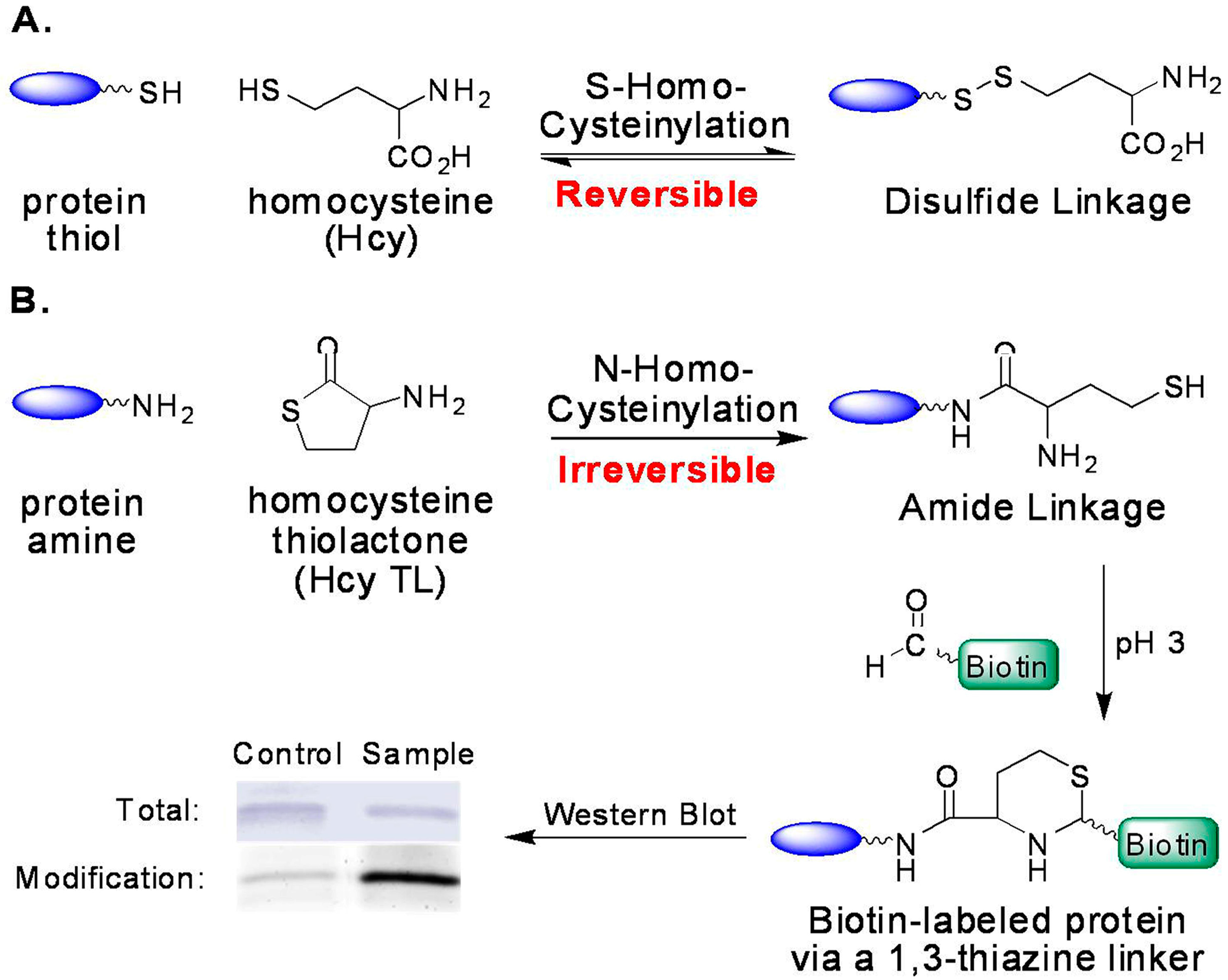
Possible mechanisms of Hcy toxicity have been proposed, such as the induction of oxidative stress and the ensuing-alteration of protein structure and loss of protein function, or the inhibition of transmethylation by the accumulation of AdoHcy—the common methylation product and a potent inhibitor for most methyltransferases [21,22,23,24,25,26]. Another possibility is that homocysteine is covalently attached to proteins, including S-homocysteinylation by forming mixed disulfides with cysteine residues and N-homocysteinylation by forming homocystamides (Figure 2) [27,28,29]. Protein S-homocysteinylation has been identified in several plasma proteins, for example, S-homocysteinylated transthyretin [30,31]. It is worth noting that “total Hcy” is a misnomer (historical usage), as it includes more than 70% of protein S-homocysteinylation (disulfides), but excludes other important Hcy species, such as protein N-homocysteinylation in human plasma [3,20,22]. First reported by Jakubowski, protein N-homocysteinylation results from the non-enzymatic acylation of the amino groups in proteins (either on the side chain of lysines and/or the N-termini) by homocysteine thiolactone (Hcy thiolactone or Hcy TL), which is produced as a byproduct from the editing process of methionyl-tRNA synthetase (Figure 1 and Figure 2) [29,32,33,34].
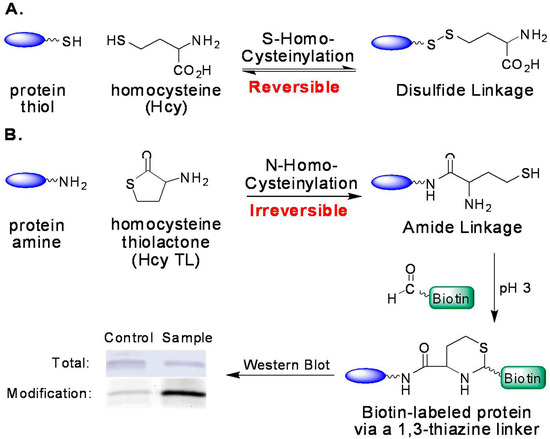
Figure 2.
Formation of protein homocysteinylation and detection of N-homocysteinylation via selective tagging with aldehydes. (A) Reversible formation of S-homocysteinylation (disulfide); and (B) irreversible formation of N-homocysteinylation (amide) and its detection.
In comparison to S-homocysteinylation, protein N-homocysteinylation is irreversible (chemically stable with no enzyme found to reverse the process) and, thus, may accumulate in proteins, particularly in long-lived ones [35,36]. In human plasma, N-homocysteinylation was reported ~0.2–0.5 μM, and the ratio of “total Hcy” to total N-homocysteinylation was found to be 7:1 to 10:1 [27,37,38]. Several human proteins have been examined more closely; human serum albumin and hemoglobin contain 0.3% and 0.6% N-homocysteinylation (mol of modification/mol of total protein), and 0.06% and 1.0% S-homocysteinylation, respectively [39]. Another publication reported that the levels of N-homocysteinylated (N-Hcy) proteins in plasma were increased due to the mutations or deletions in the cystathionine β-synthase (CBS) or methylenetetrahydrofolate reductase genes in mice [40].
Like other protein modifications, protein N-homocysteinylation may affect protein cleavage, crosslinking, aggregation, autoimmune response, and function [41,42,43,44,45,46,47,48,49,50,51,52]. In addition, thiols are highly reactive and may undergo myriad transformations under physiological conditions (such as redox, alkylation, and even desulfurization) [53,54,55,56,57]. One example is homocystamide-induced protein oxidative damage by triggering the formation of free radicals, discovered by Strongin’s laboratory [58]. It has also been observed that the formation of protein aggregates from N-homocysteinylated acidic proteins (e.g., α-lactalbumin) induces the tertiary structural changes and functional alterations [59]. Recently, it was reported that N-Hcy proteins affected gene expression in human vascular endothelial cells, which is related to cardiovascular development and neurological disease [60]. Thus, Jakubowski and others have hypothesized that protein N-homocysteinylation is an important contributor to the pathological consequences of hyperhomocysteinemia, either together with or independent of elevated concentrations of plasma “total Hcy” (free Hcy and disulfides) [29,39,45,61].
Methods for the quantification of N-Hcy proteins have been developed. The first and most commonly used is the complete chemical hydrolysis of proteins, followed by subsequent analysis of free homocysteine using HPLC coupled with fluorescence or UV detection [37,62]; however, the modified sites cannot be identified based on current methods of amino acid analysis. Alternatively, an immunological assay (ELISA and dot blotting) using polyclonal antibodies was reported [36,63], but the specificity remains to be established.
Until now, lack of a systematic analysis of N-Hcy proteins in complex systems has prevented a more thorough understanding of molecular and pathobiological consequences in hyperhomocysteinemia. Toward this end, we have developed a chemical method to selectively derivatize N-Hcy groups with different aldehyde tags under mildly acidic conditions [64]. For example, by introducing a biotin-containing aldehyde tag onto the N-homocystamide group (Figure 2B), Western blotting coupled with a chemiluminescence assay can be used to both detect and quantify N-homocysteinylation of different proteins. In comparison to antibodies against N-Hcy proteins, the biotin-aldehyde is commercially available and inexpensive. Hence, our method makes it feasible for global profiling and quantitative analysis of N-Hcy proteins in complex systems [64], including proteomic studies from other laboratories [65,66]. In this study, we investigated changes in protein N-homocysteinylation associated with variation of “total Hcy” concentrations in plasma from rats. Taken together, this proteomic study reveals that protein N-homocysteinylation may be affected by homocysteine metabolism and, furthermore, opens new avenues by which to discover potential biomarkers and to understand better the underlying molecular mechanisms.
2. Results and Discussions
2.1. Optimized Conditions for Aldehyde Tag Labeling
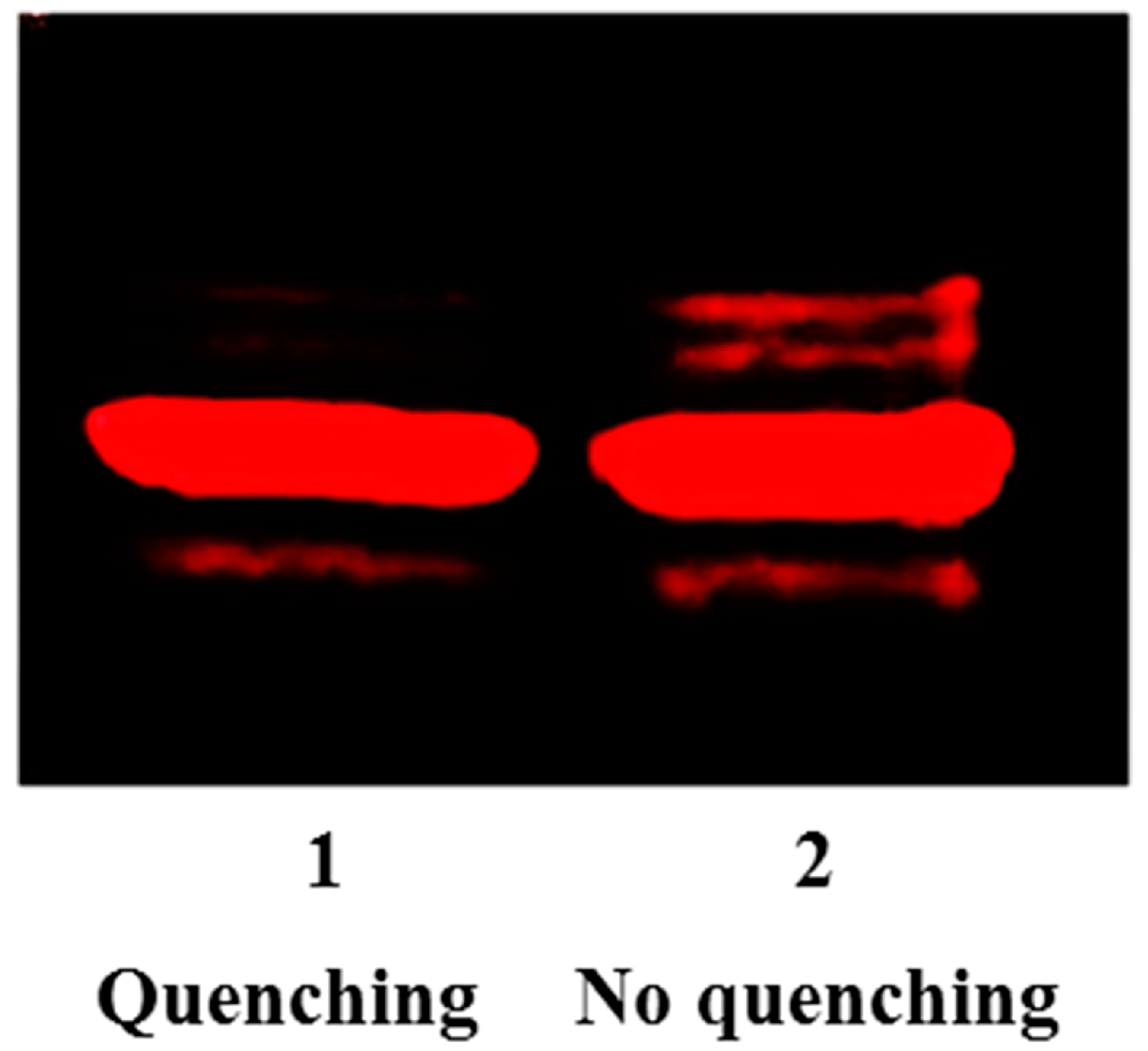
As we previously demonstrated, the coupling between aldehydes and N-homocystamide is highly specific under mildly acidic conditions (pH 2 to 4), because competing amines are protonated and rendered inactive [64,67]. However, the pH values of the loading buffer and running buffer for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) are around 6.8 and 8.3, respectively; under these conditions, significant percentages of amines in proteins are present in the neutral form, favoring the formation of a protein Schiff base with the excess aldehyde labeling reagent. Hence, in this study, cysteamine was added to the solution to quench the excess aldehyde reagent. As a beta-amino thiol, cysteamine reacts with aldehyde to form 1,3-thiazolidine at pH 3.0 (Figure S2 in Supplementary Materials). Under our conditions, after quenching with cysteamine, no aldehyde reagent was left in the solution to react with free amino groups during the subsequent sample handling steps, thereby eliminating non-specific labeling (Figure 3). Moreover, compared to antibody-based assays, our labeling method uses a commercially available biotin-aldehyde and streptavidin without the need of more expensive primary and secondary antibodies, and moreover, only requires one-step Western blotting (i.e., no need for secondary antibodies). Altogether, our method significantly reduces both cost and time, and also improves both accuracy and reproducibility.
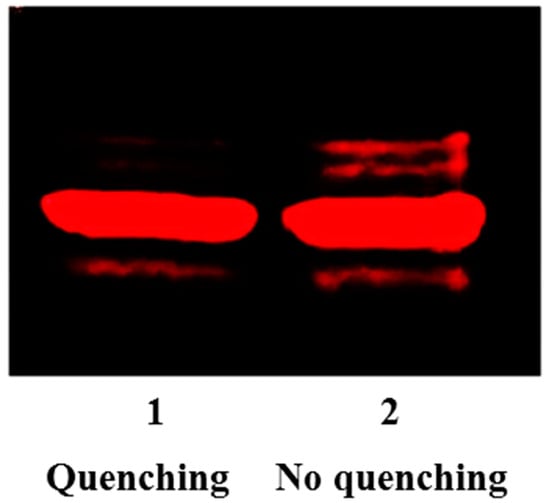
Figure 3.
Fluorescence intensities of rhodamine-aldehyde labeled N-Hcy myoglobin with and without cysteamine quenching reaction. Lane 1: 7.8 µM modified myoglobin including 3.1 µM N-Hcy myoglobin and 4.7 µM native myoglobin with cysteamine (500 µM) quenching; Lane 2: 7.8 µM modified myoglobin including 3.1 µM N-Hcy myoglobin and 4.7 µM native myoglobin without cysteamine quenching.
2.2. Increased Level of Protein N-Homocysteinylation in Plasma from Rats on B12 Deficient Diet
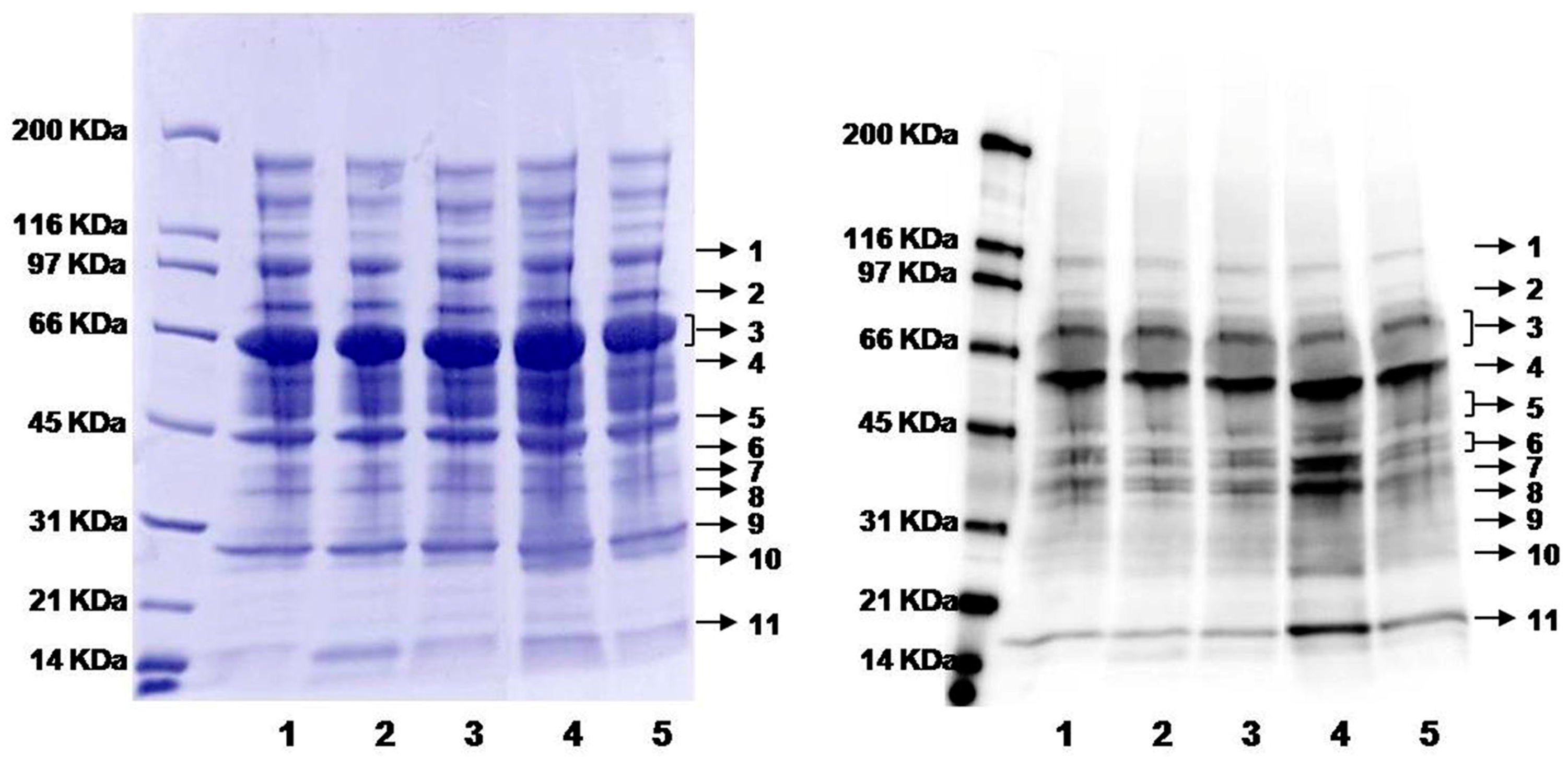
As described in Figure 1, nutrients such as folate, vitamin B12 and B6 affect the concentration of homocysteine [7,17]. A diet deficient in vitamin B12 has been observed to cause hyperhomocysteinemia in humans and animals [68,69,70,71]. Rats on vitamin B12-deficient or control diets (six each) were analyzed in this work. The plasma concentration of “total Hcy” was 31.1 ± 10.0 (standard deviation, SD) and 4.7 ± 0.8 µM for rats on B12-deficient and control diets, respectively (Table 1), a difference of 6.6-fold. As expected, the overall level of protein N-homocysteinylation shows an increased trend with 1.3-fold rising (see overall intensity in Table 1 and Figure S3), while the abundance of plasma proteins (total and individual) remained about the same (Coomassie blue image in Figure 4). More strikingly, several individual bands displayed much larger differences in protein modifications (Figure 4 and Table 1). For example, normalized to the control group (1.0 ± 0.4), band 11 has a ratio of 1.8 ± 0.7 for B12-defecient rats with a p-value of 0.03 (Table 1). Moreover, it should be pointed out that plasma samples were blinded to treatment and the nature of the samples revealed only after all analysis had been completed as shown above. Thus, our method is well suited for large scale quantification of protein N-homocysteinylation in biological samples. So far, this method has been applied to determine the relative levels of N-homocysteinylation in serum from autistic children with elevated levels of Hcy to investigate the relationship between N-homocysteinylation and neurophysiological disorders [66], in embryos from mice with the folate-responsive neural tube defects to check the correlation between folate and N-homocysteinylation in the embryos [65], and in plasma from mice with heterozygous deficiency (Cbs+/−) of cystathionine β-synthase (CBS) to study the correlation between CBS deficiency and protein N-homocysteinylation (preliminary data from our laboratories shown in Figure S4) [72,73]. Further studies would be focused on the identification of N-Hcy proteins and modified sites in order to discover the potential N-Hcy protein biomarkers. This assay could be accomplished by enrichment of N-Hcy protein digests using aldehyde resin coupled with liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis [64]. For future work, quantitative mass spectrometry approaches such as isotopic labeling (e.g., iTRAQ) can be combined with ours [74]. Such a combination should also allow us to perform both identification of the modified sites and quantification at the same time.

Table 1.
Chemiluminescence intensities of biotin-labeled proteins (N-homocysteinylation) and “total homocysteine” concentration of rat plasma.
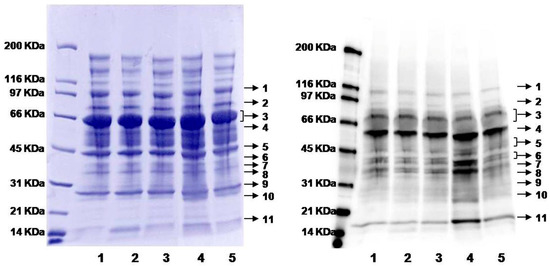
Figure 4.
Gel images of plasma proteins from rats on B12-deficient and control diets. Left image: Coomassie blue staining showing total protein loading; right image: chemiluminescence from Western blotting showing the levels of biotin-labeling (protein N-homocysteinylation). Lanes 1, 2, and 3: plasma from individual rats on control diet; lanes 4 and 5: plasma from individual rats on a B12-deficient diet. Proteins were divided into eleven individual protein bands (indicated by the arrows) for subsequent analysis.
2.3. Possible Contributing Factors to N-Homocysteinylation
As shown in our work, whereas there were increases in N-homocysteinylation when the blood concentrations of “total Hcy” (disulfides) were elevated, the magnitude of the former is smaller. This finding is not unexpected, considering the formation of “total Hcy” (free Hcy and disulfides) and N-homocysteinylation are influenced by multiple and different factors. As illustrated in Figure 1, except the nutritional factors, the activities of different enzymes which involve in the conversion of Hcy to methionine, cysteine and Hcy thiolactone could also contribute to the variation of protein N-homocysteinylation. Hcy thiolactone can be hydrolyzed back to Hcy by paraoxonase 1 (PON1, EC 3.1.8.1) [35]. Therefore, activity of PON1 likely plays a direct and significant role in Hcy thiolactone metabolism and, hence, N-homocysteinylation. For example, PON1 is present in serum, and there is little protein synthesis in serum (hence, little formation of Hcy thiolactone) [75,76]. Thus, the steady-state concentration of Hcy thiolactone is extremely low (0.1–26 nM), while “total Hcy” is in the 5–15 µM range [22]; as such, the level of N-homocysteinylation is expected to be lower than the “total Hcy”, as we observed here. Conversely, in tissues and within cellular compartments, where protein synthesis is more active, Hcy thiolactone formation is likely to be higher as well. Ultimately, the counterbalance of the formation and hydrolysis of Hcy thiolactone, not simply the concentration of homocysteine, determines the level of N-homocysteinylation. In addition, as mentioned in Section 1, the inactivation of cystathionine β-synthase (CBS) in transgenic mice (Cbs−/−) caused 50- to 140-fold elevation of “total Hcy” and six- to 10-fold increase of N-Hcy proteins in serum [40]. As such, patients with genetically-deficient enzymes could cause a more striking increase of N-Hcy proteins, and N-Hcy proteins would be more effectively identified using LC-MS/MS. Recently, N-Hcy fibrinogen and its modified sites have been identified in the plasma from CBS-deficient patients [77]. Based on our results and the published reports, we propose that differences on enzyme activity (e.g., PON1 and CBS) are likely to alter plasma N-homocysteinylation status more distinctly, which can be tested using our method. Finally, given the simplicity, low-cost, and robustness of our method, our method can be easily employed for clinical applications [66].
3. Materials and Methods
3.1. Plasma
Plasma from rats on vitamin B12-deficient and control diets (six in each group) were from Dr. Ligi Paul at Tufts University. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University (Protocol No. SE-56) and conducted according to the Guide for the Care and Use of Laboratory Animals (1996). Measurement of “total Hcy” in plasma was performed as reported [78]. Protein concentrations of plasma were assayed according to the Bradford method using concentrated dye (500-0006) from Bio-Rad (Hercules, CA, USA); and bovine serum albumin (A3059) purchased from Sigma-Aldrich (St. Louis, MO, USA) was used as a standard.
3.2. Chemicals and Reagents
Horseradish peroxidase streptavidin (SA-5004) was from Vector Laboratories (Burlingame, CA, USA). SuperSignal West Pico chemiluminescent substrate was from Thermo Scientific (Rockford, IL, USA). Myoglobin from equine skeletal muscle (M0630) was from Sigma-Aldrich. N-Homocysteinylated myoglobin was prepared as we previously reported [64]. Biotinyl-Asp-Glu-Val-Asp-aldehyde (CAS registry number: 178603-73-1) was from Bachem Americas (N-1470, Torrance, CA, USA; see Figure S1 for its structure in Support Information). Rhodamine-aldehyde (10 mM) in 50% 200-proof ethanol was synthesized as previously described [64]. EZ-Run prestained protein ladder (BP3603) was from Fisher Scientific. Natural unstained protein ladder (161-0317) was from Bio-Rad. Biotinylated protein markers were from Sigma-Aldrich (B2787) and Bio-Rad (161-0319), respectively. All reagents were ACS grade and used as received without further purification. All incubations were carried out in an Eppendorf Thermomixer® (Eppendorf North America, Hauppauge, NY, USA) at 25 °C unless specified otherwise.
3.3. Optimization of Labeling with Cysteamine Quenching
Hcy thiolactone-modified myoglobin (13 µM including 5.2 µM N-Hcy myoglobin (~40%) and 7.8 µM native myoglobin) was incubated with 200 µM Rhodamine-aldehyde (pH 3), containing 50 mM citric acid, 500 µM tris(2-carboxyethyl)phosphine (TCEP), at 25 °C in the dark for 8 h [64]. Aliquots (20 µL) were removed and stored at 4 °C. Cysteamine (15 mM, 1 µL) was added to the remaining solution to quench the excess aldehyde for 3–14 h. Solutions (12 µL) with or without cysteamine quenching were mixed with loading buffer (8 µL, 5% SDS, and 25% glycerol) for SDS-PAGE analysis. Gels were analyzed using a Molecular Dynamics Storm840 imaging system (GE Healthcare, Piscataway, NJ, USA). Fluorescence was recorded with an excitation wavelength at 450 nm and an emission wavelength at 520 nm using a Strom Scanner Control version 5.03 (Amersham Bioscience, Piscataway, NJ, USA), and data were visualized by ImageQuant TL 7.0 (GE Healthcare, Pittsburgh, PA, USA).
3.4. Labeling Proteins with Biotin-Aldehyde
Rat plasma (2 mg/mL protein from each sample, final concentration) was incubated with 250 µM biotin-aldehyde in 200 mM citric acid, 2 mM TCEP, pH 3, in the dark at 25 °C for 5 h. To quench the labeling reaction, cysteamine (1 mM, final concentration) in 50 mM citric acid and 2 mM TCEP, pH 3, was added to each sample and incubated for additional 3 h. Reaction solutions were stored at −80 °C before analysis.
3.5. Western Blotting of Biotin-Labeled Proteins
Labeling reactions (35 μL) were mixed with 2× Laemmli loading buffer (35 µL) containing 350 mM dithiothreitol (DTT) and then boiled in water for 5 min. For each reaction, two aliquots (30 µL) were loaded into two separate precast Tris-HCl gels (4%–15%, Bio-Rad) for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). One gel was used for Coomassie blue staining and the other for Western blotting. For blotting, proteins from the gel were transferred onto an Immun-Blot PVDF Membrane (0.2 µm, Bio-Rad) using transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol, pH 8.3). The membranes were next blocked in 2% bovine serum albumin (BSA) in TBST (25 mM Tris, 137 mM NaCl, 3 mM KCl, 0.1% Tween-20, pH 7.4) for 1 h. After blocking, the membrane was washed with TBST for 3 × 10 min and incubated with 0.5 µg/mL streptavidin-horseradish peroxidase (HRP) in 20 mL TBST for 1 h. The membrane was then washed again in TBST for 5 × 6 min and incubated in PBS (68 mM NaCl, 1 mM KCl, 5 mM Na2HPO4, and 1 mM KH2PO4, pH 7.4) for 10 min. After incubation, the buffer was discarded, and the chemiluminescence signal was developed by the addition of 1 mL SuperSignal West Pico chemiluminescent substrate for 1 min. Chemiluminescence was detected by FluorChem Imager SP (Alpha Innotech, San Leandro, CA, USA), and the image was analyzed by ImageQuant TL 7.0 (GE Healthcare). The experiment was conducted in duplicate for each sample.
3.6. Data Analysis for the Degree of Modification
Protein N-homocysteinylation in overall level and in each protein band's level (See Figure 4) were determined from the chemiluminescent intensity, which was normalized by using the Coomassie blue staining intensity of overall protein bands in order to minimize the intensity variation from the protein loading amount. Eventually, the normalized chemiluminescent intensity was compared to the control group and the statistical analysis was performed using two-tailed t-test analysis by GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
4. Conclusions
For the first time, global analysis of protein N-homocysteinylation was performed in the plasma from rats with perturbed homocysteine metabolism. As expected, a general correlation between “total homocysteine” and N-homocysteinylation was observed. Interestingly, more pronounced and statistically significant changes were identified for some individual protein bands. While larger sets of samples should be analyzed before a conclusive interpretation can be drawn, our method has been shown to be suitable for quantitative analysis. Moreover, our results suggest that other factors directly involved in homocysteine/homocysteine thiolactone metabolism, such as the activity of cystathionine β-synthase (CBS) or paraoxonase 1 (PON1), may play more direct and pronounced roles in N-homocysteinylation and facilitate the identification of N-Hcy proteins.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/9/1195/s1.
Acknowledgments
We thank Penny Beuning, Barry Karger and Alex Makriyannis at Northeastern University for access to their instruments. This is a contribution number 1038 from the Barnett Institute. The work is supported by the National Institutes of Health (GM101396 to Z.S.Z., HL061795, HG007690 and GM107618 to J.L.), the American Heart Association Predoctoral Fellowship (09PRE2300071 to T.Z.) and the China Scholarship Council Fund (201408330488 to J.M.).
Author Contributions
J.L., S.D., R.C.D., Z.S.Z. and J.M. participated in the interpretation of the studies and the revision of the manuscript; T.Z. drafted the manuscript, conducted the experiments and data analysis. L.P.P. and D.E.H. prepared the plasma and serum samples and contributed to the data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brattstrom, L.; Wilcken, D.E. Homocysteine and cardiovascular disease: Cause or effect? Am. J. Clin. Nutr. 2000, 72, 315–323. [Google Scholar] [PubMed]
- Cacciapuoti, F. Hyper-homocysteinemia: A novel risk factor or a powerful marker for cardiovascular diseases? Pathogenetic and therapeutical uncertainties. J. Thromb. Thrombolysis 2011, 32, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R.J.; Jacobsen, D.W. Homocysteine in Health and Disease; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001; p. 526. [Google Scholar]
- Maron, B.A.; Loscalzo, J. The treatment of hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Arrastia, R. Homocysteine and neurologic disease. Arch. Neurol. 2000, 57, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Pathways and regulation of homocysteine metabolism in mammals. Semin. Thromb. Hemost. 2000, 26, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.G.; Smith, A.E.; Zhou, Z.S.; Taurog, R.E.; Bandarian, V.; Evans, J.C.; Ludwig, M. Cobalamin-dependent and cobalamin-independent methionine synthases: Are there two solutions to the same chemical problem? Helv. Chim. Acta 2003, 86, 3939–3954. [Google Scholar] [CrossRef]
- Gui, S.; Wooderchak-Donahue, W.L.; Zang, T.; Chen, D.; Daly, M.P.; Zhou, Z.S.; Hevel, J.M. Substrate-induced control of product formation by protein arginine methyltransferase 1. Biochemistry 2013, 52, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Peariso, K.; Penner-Hahn, J.E.; Matthews, R.G. Identification of the zinc ligands in cobalamin-independent methionine synthase (MetE) from Escherichia coli. Biochemistry 1999, 38, 15915–15926. [Google Scholar] [CrossRef] [PubMed]
- Mosley, S.L.; Bakke, B.A.; Sadler, J.M.; Sunkara, N.K.; Dorgan, K.M.; Zhou, Z.S.; Seley-Radtke, K.L. Carbocyclic pyrimidine nucleosides as inhibitors of S-adenosylhomocysteine hydrolase. Bioorg. Med. Chem. 2006, 14, 7967–7971. [Google Scholar] [CrossRef] [PubMed]
- Deth, R.C.; Hodgson, N.W.; Trivedi, M.S.; Muratore, C.R.; Waly, M.I. Autsim: A Neuroepigenetic Disorder. Autsim Sci. Dig. 2011, 3, 9–19. [Google Scholar]
- Blom, H.J.C.; Fowler, B.; Koch, H.G. Disorders of homocysteine metabolism: From rare genetic defects to common risk factors. Eur. J. Pediatr. 1998, 157 (Suppl. 2), S39–S142. [Google Scholar] [CrossRef]
- Goyette, P.; Sumner, J.S.; Milos, R.; Duncan, A.M.; Rosenblatt, D.S.; Matthews, R.G.; Rozen, R. Human methylenetetrahydrofolate reductase: Isolation of cDNA mapping and mutation identification. Nat. Genet. 1994, 7, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H.; Finkelstein, J.D.; Irreverre, F.; Laster, L. Homocysteinuria: An enzymatic defect. Science 1964, 143, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Jacques, P.F.; Wilson, P.W.; Rush, D.; Rosenberg, I.H. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. J. Am. Med. Assoc. 1993, 270, 2693–2698. [Google Scholar] [CrossRef]
- Verhoef, P.; van Vliet, T.; Olthof, M.R.; Katan, M.B. A high-protein diet increases postprandial but not fasting plasma total homocysteine concentrations: A dietary controlled, crossover trial in healthy volunteers. Am. J. Clin. Nutr. 2005, 82, 553–558. [Google Scholar] [PubMed]
- Handy, D.E.; Loscalzo, J. Homocysteine and atherothrombosis: Diagnosis and treatment. Curr. Atheroscler. Rep. 2003, 5, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. An overview of homocysteine metabolism. In Homocysteine in Protein Structure/Function and Human Disease: Chemical Biology of Homocysteine-Containing Proteins; Springer: New York, NY, USA, 2013; pp. 7–17. [Google Scholar]
- Olszewski, A.J.; McCully, K.S. Homocysteine metabolism and the oxidative modification of proteins and lipids. Free Radic. Biol. Med. 1993, 14, 683–693. [Google Scholar] [CrossRef]
- Perla-Kajan, J.; Twardowski, T.; Jakubowski, H. Mechanisms of homocysteine toxicity in humans. Amino Acids 2007, 32, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D.; Lombardi, C.; Acanfora, F.; Satta, E.; Cesare, C.M.; Violetti, E.; Romano, M.M.; De Santo, N.G. Possible mechanisms of homocysteine toxicity. Kidney Int. Suppl. 2003, 84, S137–S140. [Google Scholar] [CrossRef] [PubMed]
- Cannon, L.M.; Butler, F.N.; Wan, W.; Zhou, Z.S. A stereospecific colorimetric assay for (S,S)-adenosylmethionine quantification based on thiopurine methyltransferase-catalyzed thiol methylation. Anal. Biochem. 2002, 308, 358–363. [Google Scholar] [CrossRef]
- Biastoff, S.; Teuber, M.; Zhou, Z.S.; Drager, B. Colorimetric activity measurement of a recombinant putrescine N-methyltransferase from Datura stramonium. Planta Med. 2006, 72, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Joseph, L.; Devi, S.; Kennedy, R.H. Effect of anti-oxidant treatment on hyperhomocysteinemia-induced myocardial fibrosis and diastolic dysfunction. J. Heart Lung Transplant. 2008, 27, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Satta, E.; Acanfora, F.; Lombardi, C.; Ingrosso, D.; de Santo, N.G. Increased plasma protein homocysteinylation in hemodialysis patients. Kidney Int. 2006, 69, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Acanfora, F.; Luciano, M.G.; Pulzella, P.; Capasso, R.; Satta, E.; Cinzia, L.; Pollastro, R.M.; Iannelli, S.; Ingrosso, D.; et al. Plasma protein homocysteinylation in uremia. Clin. Chem. Lab. Med. 2007, 45, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Molecular basis of homocysteine toxicity in humans. Cell. Mol. Life. Sci. 2004, 61, 470–487. [Google Scholar] [CrossRef] [PubMed]
- Sass, J.O.; Nakanishi, T.; Sato, T.; Sperl, W.; Shimizu, A. S-homocysteinylation of transthyretin is detected in plasma and serum of humans with different types of hyperhomocysteinemia. Biochem. Biophys. Res. Commun. 2003, 310, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Sengupta, S.; McComb, M.E.; Theberge, R.; Wilson, W.G.; Costello, C.E.; Jacobsen, D.W. In vitro and in vivo interactions of homocysteine with human plasma transthyretin. J. Biol. Chem. 2003, 278, 49707–49713. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Proofreading in vivo: Editing of homocysteine by methionyl-tRNA synthetase in the yeast Saccharomyces cerevisiae. EMBO J. 1991, 10, 593–598. [Google Scholar] [PubMed]
- Jakubowski, H.; Goldman, E. Synthesis of homocysteine thiolactone by methionyl-tRNA synthetase in cultured mammalian cells. FEBS Lett. 1993, 317, 237–240. [Google Scholar] [CrossRef]
- Jakubowski, H. Aminoacyl-tRNA synthetases and the evolution of coded peptide synthesis: The Thioester World. FEBS Lett. 2016, 590, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Perla-Kajan, J.; Jakubowski, H. Paraoxonase 1 protects against protein N-homocysteinylation in humans. FASEB J. 2010, 24, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Perla-Kajan, J.; Stanger, O.; Luczak, M.; Ziolkowska, A.; Malendowicz, L.K.; Twardowski, T.; Lhotak, S.; Austin, R.C.; Jakubowski, H. Immunohistochemical detection of N-homocysteinylated proteins in humans and mice. Biomed. Pharmacother. 2008, 62, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. New method for the determination of protein N-linked homocysteine. Anal. Biochem. 2008, 380, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Uji, Y.; Motomiya, Y.; Hanyu, N.; Ukaji, F.; Okabe, H. Protein-bound homocystamide measured in human plasma by HPLC. Clin. Chem. 2002, 48, 941–944. [Google Scholar] [PubMed]
- Jakubowski, H. Homocysteine is a protein amino acid in humans. Implications for homocysteine-linked disease. J. Biol. Chem. 2002, 277, 30425–30428. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H.; Perla-Kajan, J.; Finnell, R.H.; Cabrera, R.M.; Wang, H.; Gupta, S.; Kruger, W.D.; Kraus, J.P.; Shih, D.M. Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. FASEB J. 2009, 23, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Protein N-homocysteinylation: Implications for atherosclerosis. Biomed. Pharmacother. 2001, 55, 443–447. [Google Scholar] [CrossRef]
- Paoli, P.; Sbrana, F.; Tiribilli, B.; Caselli, A.; Pantera, B.; Cirri, P.; de Donatis, A.; Formigli, L.; Nosi, D.; Manao, G.; et al. Protein N-homocysteinylation induces the formation of toxic amyloid-like protofibrils. J. Mol. Biol. 2010, 400, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Perla, J.; Lacinski, M.; Trzeciak, W.; Kazmierski, R.; Jakubowski, H. Autoantibodies against N-homocysteinylated proteins in humans: Implications for atherosclerosis. Stroke J. Cereb. Circ. 2004, 35, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Sauls, D.L.; Lockhart, E.; Warren, M.E.; Lenkowski, A.; Wilhelm, S.E.; Hoffman, M. Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: A potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry 2006, 45, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Sauls, D.L.; Warren, M.; Hoffman, M. Homocysteinylated fibrinogen forms disulfide-linked complexes with albumin. Thromb. Res. 2011, 127, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Zang, T.; Spahr, C.; Cheetham, J.; Ren, D.; Zhou, Z.S. Discovery of Undefined Protein Crosslinking Chemistry: A Comprehensive Methodology Utilizing 18O-labeling and Mass Spectrometry. Anal. Chem. 2013, 85, 5900–5908. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Nayak, N.; Majee, S.M.; Lowenson, J.; Schafermeyer, K.R.; Eliopoulos, A.C.; Lloyd, T.D.; Dinkins, R.; Perry, S.E.; Forsthoefel, N.R.; et al. Substrates of the Arabidopsis thaliana Protein Isoaspartyl Methyltransferase 1 Identified Using Phage Display and Biopanning. J. Biol. Chem. 2010, 285, 37281–37292. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Dai, S.; Karger, B.L.; Zhou, Z.S. Analysis of isoaspartic Acid by selective proteolysis with Asp-N and electron transfer dissociation mass spectrometry. Anal. Chem. 2010, 82, 7485–7491. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cheetham, J.; Cauchon, N.; Ostovic, J.; Ni, W.; Ren, D.; Zhou, Z.S. Protein isoaspartate methyltransferase-mediated 18O-labeling of isoaspartic acid for mass spectrometry analysis. Anal. Chem. 2012, 84, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ni, W.; Patananan, A.N.; Clarke, S.G.; Karger, B.L.; Zhou, Z.S. Integrated proteomic analysis of major isoaspartyl-containing proteins in the urine of wild type and protein l-isoaspartate O-methyltransferase-deficient mice. Anal. Chem. 2013, 85, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Cheetham, J.; Ren, D.; Zhou, Z.S. Discovery and characterization of a photo-oxidative histidine-histidine cross-link in IgG1 antibody utilizing 18O-labeling and mass spectrometry. Anal. Chem. 2014, 86, 4940–4948. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Moulton, K.R.; Auclair, J.R.; Zhou, Z.S. Mildly acidic conditions eliminate deamidation artifact during proteolysis: Digestion with endoprotease Glu-C at pH 4.5. Amino Acids 2016, 48, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Smith, A.E.; Matthews, R.G. l-selenohomocysteine: One-step synthesis from l-selenomethionine and kinetic analysis as substrate for methionine synthases. Bioorg. Med. Chem. Lett. 2000, 10, 2471–2475. [Google Scholar] [CrossRef]
- Zang, T.; Lee, B.W.; Cannon, L.M.; Ritter, K.A.; Dai, S.; Ren, D.; Wood, T.K.; Zhou, Z.S. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorg. Med. Chem. Lett. 2009, 19, 6200–6204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rejtar, T.; Zhou, Z.S.; Karger, B.L. Desulfurization of cysteine-containing peptides resulting from sample preparation for protein characterization by mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhou, Z.S. Vinyl sulfonium as novel proteolytic enzyme inhibitor. Bioorg. Med. Chem. Lett. 2001, 11, 2331–2335. [Google Scholar] [CrossRef]
- Chumsae, C.; Gifford, K.; Lian, W.; Liu, H.; Radziejewski, C.H.; Zhou, Z.S. Arginine modifications by methylglyoxal: Discovery in a recombinant monoclonal antibody and contribution to acidic species. Anal. Chem. 2013, 85, 11401–11409. [Google Scholar] [CrossRef] [PubMed]
- Sibrian-Vazquez, M.; Escobedo, J.O.; Lim, S.; Samoei, G.K.; Strongin, R.M. Homocystamides promote free-radical and oxidative damage to proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.S.; Kumar, T.; Singh, L.R. N-homocysteinylation induces different structural and functional consequences on acidic and basic proteins. PLoS ONE 2014, 9, e116386. [Google Scholar] [CrossRef] [PubMed]
- Gurda, D.; Handschuh, L.; Kotkowiak, W.; Jakubowski, H. Homocysteine thiolactone and N-homocysteinylated protein induce pro-atherogenic changes in gene expression in human vascular endothelial cells. Amino Acids 2015, 47, 1319–1339. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Pathophysiological consequences of homocysteine excess. J. Nutr. 2006, 136 (Suppl. 6), 1741S–1749S. [Google Scholar] [PubMed]
- Jakubowski, H. Quantification of urinary S- and N-homocysteinylated protein and homocysteine-thiolactone in mice. Anal. Biochem. 2016, 508, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.; Parthasarathy, S.; Joseph, J.; Kalyanaraman, B. Generation and initial characterization of a novel polyclonal antibody directed against homocysteine thiolactone-modified low density lipoprotein. J. Lipid Res. 1998, 39, 925–933. [Google Scholar] [PubMed]
- Zang, T.; Dai, S.; Chen, D.; Lee, B.W.; Liu, S.; Karger, B.L.; Zhou, Z.S. Chemical methods for the detection of protein N-homocysteinylation via selective reactions with aldehydes. Anal. Chem. 2009, 81, 9065–9071. [Google Scholar] [CrossRef] [PubMed]
- Fathe, K.; Person, M.D.; Finnell, R.H. The application of a chemical determination of N-homocysteinylation levels in developing mouse embryos: Implication for folate responsive birth defects. J. Nutr. Biochem. 2015, 26, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, N.W.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.M.; Al-Farsi, O.; Ali, A.; Ouhtit, A.; Zang, T.Z.; Zhou, Z.S.; Deth, R.C. Decreased glutathione and elevated hair mercury levels are associated with nutritional deficiency-based autism in Oman. Exp. Biol. Med. 2014, 239, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, J.F.; Gillies, L.A.; Sun, H.G.; Dai, S.; Zang, T.; Klaene, J.J.; Kim, B.J.; Lowenson, J.D.; Clarke, S.G.; Karger, B.L.; et al. Chemo-enzymatic detection of protein isoaspartate using protein isoaspartate methyltransferase and hydrazine trapping. Anal. Chem. 2008, 80, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Guttormsen, A.B.; Schneede, J.; Ueland, P.M.; Refsum, H. Kinetics of total plasma homocysteine in subjects with hyperhomocysteinemia due to folate or cobalamin deficiency. Am. J. Clin. Nutr. 1996, 63, 194–202. [Google Scholar] [PubMed]
- Herrmann, M.; Wildemann, B.; Wagner, A.; Wolny, M.; Schorr, H.; Taban-Shomal, O.; Umanskaya, N.; Ross, S.; Garcia, P.; Hübner, U.; et al. Experimental folate and vitamin B12 deficiency does not alter bone quality in rats. J. Bone Miner. Res. 2009, 24, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Stangl, G.I.; Schwarz, F.J.; Jahn, B.; Kirchgessner, M. Cobalt-deficiency-induced hyperhomocysteinaemia and oxidative status of cattle. Br. J. Nutr. 2000, 83, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Troen, A.M.; Shea-Budgell, M.; Shukitt-Hale, B.; Smith, D.E.; Selhub, J.; Rosenberg, I.H. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12474–12479. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, R.T.; Forgione, M.A.; Cap, A.; Leopold, J.A.; Rudd, M.A.; Trolliet, M.; Heydrick, S.; Stark, R.; Klings, E.S.; Moldovan, N.I.; et al. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J. Clin. Investig. 2000, 106, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Heydrick, S.; Zhang, Y.Y.; Bierl, C.; Cap, A.; Loscalzo, J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Reidegeld, K.A.; Meyer, H.E.; Warscheid, B. Protein labeling by iTRAQ: A new tool for quantitative mass spectrometry in proteome research. Proteomics 2007, 7, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Camps, J.; Marsillach, J.; Joven, J. The paraoxonases: Role in human diseases and methodological difficulties in measurement. Crit. Rev. Clin. Lab. Sci. 2009, 46, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.L.; Bly, C.G.; Watson, M.L.; Bale, W.F. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J. Exp. Med. 1951, 94, 431–453. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Marczak, L.; Kubalska, J.; Graban, A.; Jakubowski, H. Identification of N-homocysteinylation sites in plasma proteins. Amino Acids 2014, 46, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Araki, A.; Sako, Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B 1987, 422, 43–52. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).