13C-NMR-Based Metabolomic Profiling of Typical Asian Soy Sauces
Abstract
:1. Introduction
2. Results and Discussion
2.1. 13C-NMR Spectroscopy
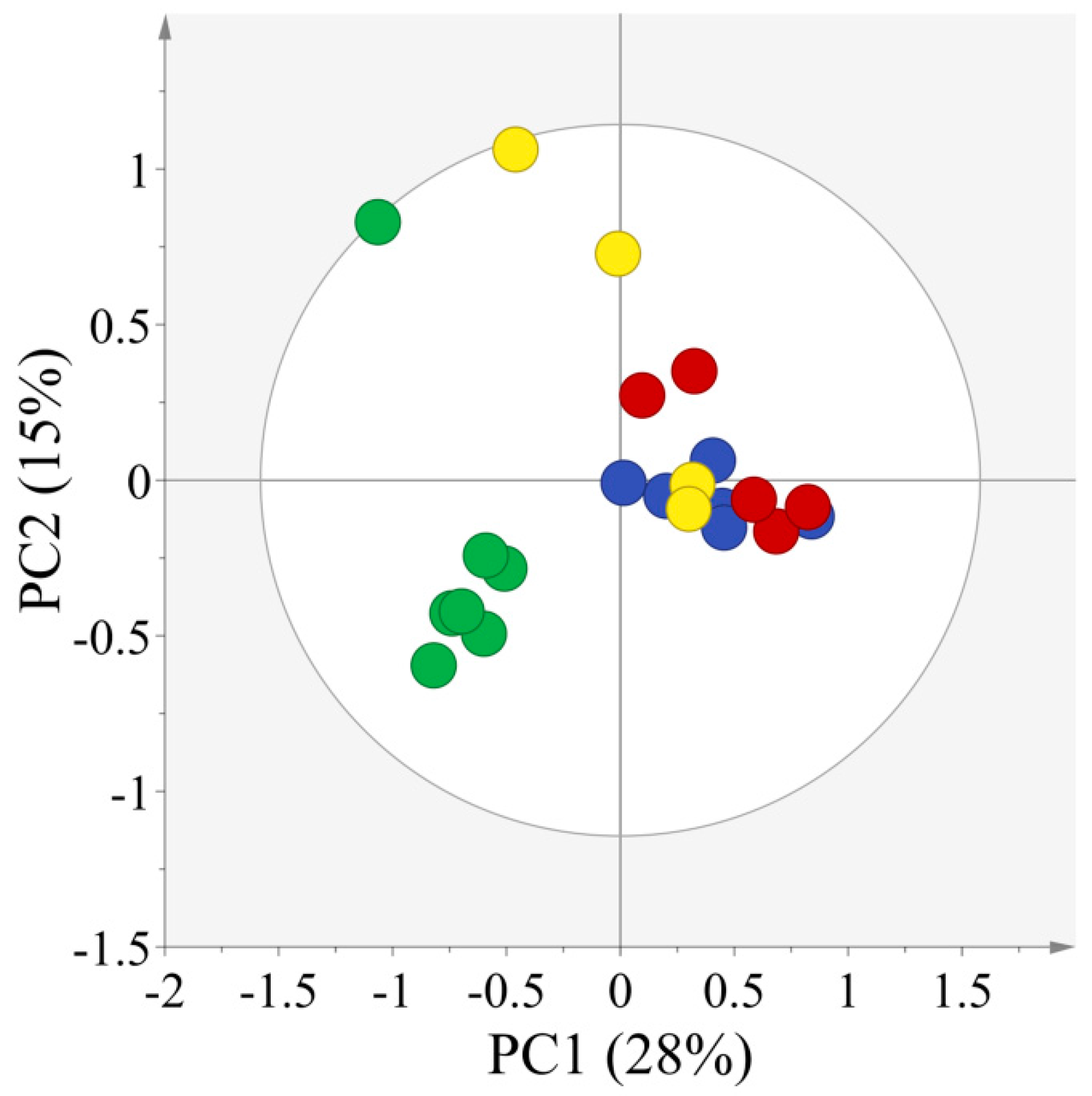
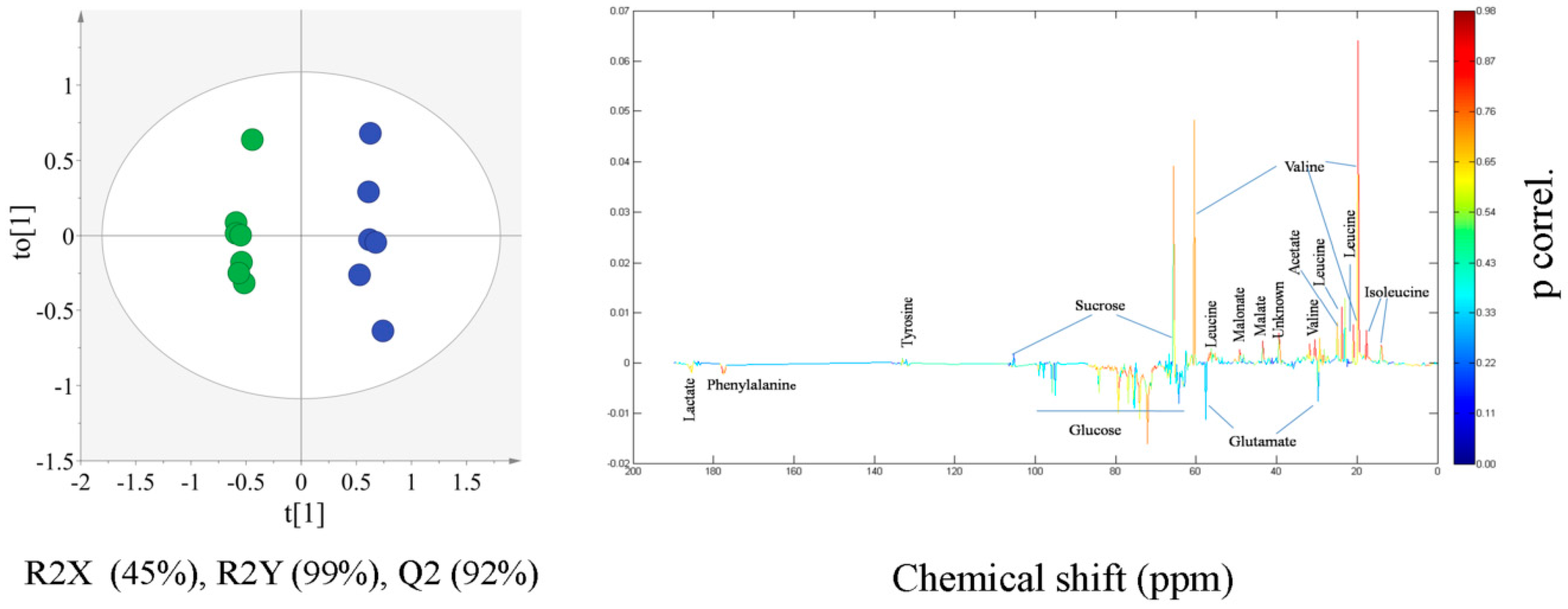
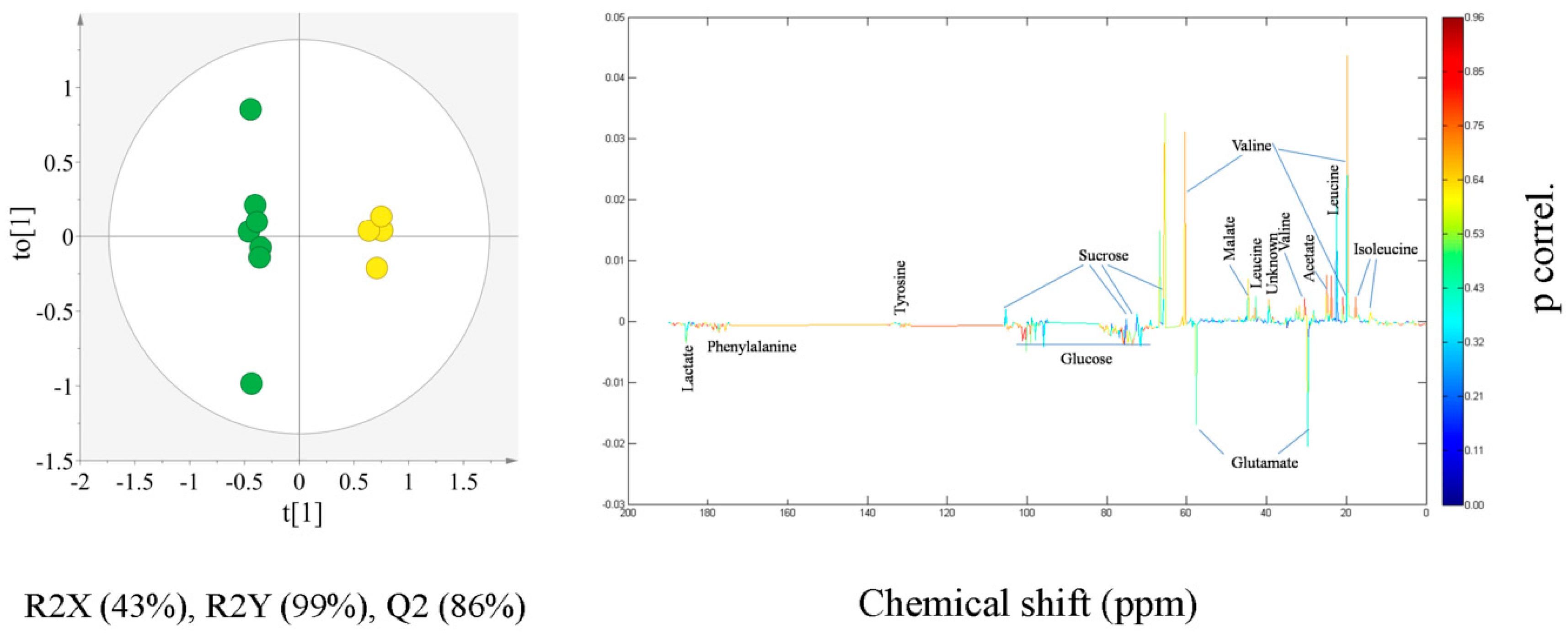
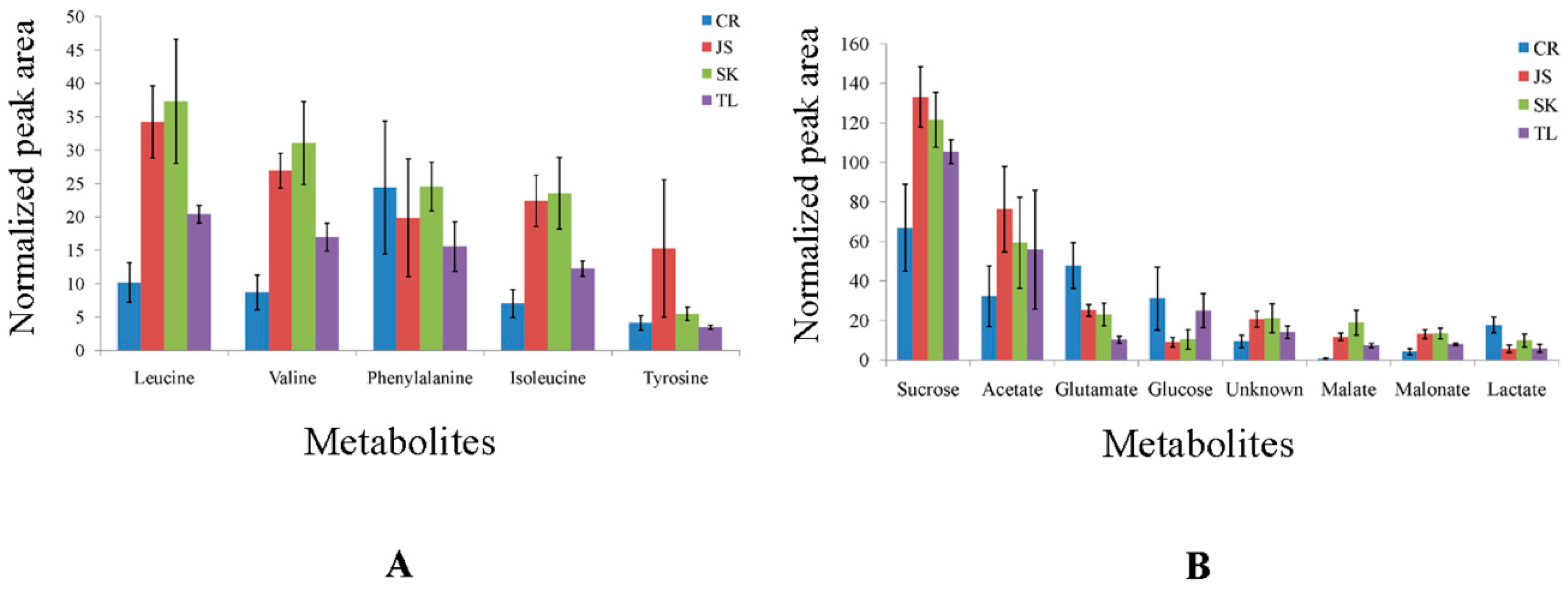
2.2. Multivariate Statistical Analysis
3. Experimental Section
3.1. Chemicals
3.2. Sample Collection and Preparation
3.3. 1H- and 13C-NMR Analysis
3.4. NMR Data Analysis
3.5. Multivariate Data Analysis
3.6. Relative Concentration of Metabolites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franke, B.M.; Gremaud, G.; Hadorn, R.; Kreuzer, M. Geographic origin of meat—Elements of an analytical approach to its authentication. Eur. Food Res. Technol. 2005, 221, 493–503. [Google Scholar] [CrossRef]
- Boto, I.; Phillips, S.; Fay, F.; Vassilakis, G. The Geography of Food: Reconnecting with Origin in the Food System; Centre Technique de Coopération Agricole et Rurale: Brussels, Belgium, 2013; p. 5. [Google Scholar]
- Delamont, S. Appetites and Identities: An Introduction to the Social Anthropology of Western Europe; Routledge: London, UK, 1995. [Google Scholar]
- Cao, X.H.; Hou, L.H.; Lu, M.F.; Wang, C.L.; Zeng, B. Genome shuffling of zygosaccharomyces rouxii to accelerate and enhance the flavour formation of soy sauce. J. Sci. Food Agric. 2010, 90, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Soriano, J.M.; Molto, J.C.; Manes, J. Absence ochratoxin a in soy sauce. Int. J. Food Microbiol. 2004, 97, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Feng, Y.Z.; Cui, C.; Zhao, H.F.; Zhao, M.M. Effects of koji-making with mixed strains on physicochemical and sensory properties of chinese-type soy sauce. J. Sci. Food Agric. 2015, 95, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Zhao, H.F.; Feng, Y.Z.; Zhao, M.M. A comparative study on physicochemical properties of chinese-type soy sauces prepared using pure koji and mixed kojis. Afr. J. Biotechnol. 2010, 9, 6740–6747. [Google Scholar]
- Xu, L.B.; Li, Y.; Xu, N.; Hu, Y.; Wang, C.; He, J.J.; Cao, Y.Z.; Chen, S.G.; Li, D.S. Soy sauce classification by geographic region and fermentation based on artificial neural network and genetic algorithm. J. Agric. Food Chem. 2014, 62, 12294–12298. [Google Scholar] [CrossRef] [PubMed]
- Lioe, H.N.; Wada, K.; Aoki, T.; Yasuda, M. Chemical and sensory characteristics of low molecular weight fractions obtained from three types of japanese soy sauce (shoyu)—Koikuchi, tamari and shiro shoyu. Food Chem. 2007, 100, 1669–1677. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumazawa, K.; Nishimura, O. Isolation and identification of the umami enhancing compounds in japanese soy sauce. Biosci. Biotechnol. Biochem. 2011, 75, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.R.; Abreu Gloria, M.B. Bioactive amines in soy sauce: Validation of method, occurrence and potential health effects. Food Chem. 2012, 133, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.S.; Zhao, Y.; Zhang, G.; Zhang, L.; Li, J.G.; Tang, C.D.; Miao, H.; Ma, J.B.; Zhang, Q.; Wu, Y.N. Occurrence of chloropropanols in soy sauce and other foods in china between 2002 and 2004. Food Addit. Contam. 2007, 24, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Aishima, T. Classification of soy sauce on principal components in gc profiles. Agric. Biol. Chem. 1979, 43, 1905–1910. [Google Scholar]
- Iizuka, K.; Aishima, T. Soy sauce classification by geographic region based on nir spectra and chemometrics pattern recognition. J. Food Sci. 1997, 62, 101–104. [Google Scholar] [CrossRef]
- Solanky, K.S.; Bailey, N.J.; Beckwith-Hall, B.M.; Bingham, S.; Davis, A.; Holmes, E.; Nicholson, J.K.; Cassidy, A. Biofluid 1H-NMR-based metabonomic techniques in nutrition research—metabolic effects of dietary isoflavones in humans. J. Nutr. Biochem. 2005, 16, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Tang, H.R.; Nicholson, J.K.; Hylands, P.J.; Sampson, J.; Holmes, E. A metabonomic strategy for the detection of the metabolic effects of chamomile (Matricaria recutita L.) ingestion. J. Agric. Food Chem. 2005, 53, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.D.; di Camillo, A.; Vlahov, G.; Jones, A.; Bianchi, G.; Rowland, J.; Kell, D.B. Discrimination of the variety and region of origin of extra virgin olive oils using 13C-NMR and multivariate calibration with variable reduction. Anal. Chim. Acta 1997, 348, 357–374. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Koda, M.; Hu, F.; Kato, R.; Miyakawa, T.; Tanokura, M. 13C-NMR-based metabolomics for the classification of green coffee beans according to variety and origin. J. Agric. Food Chem. 2012, 60, 10118–10125. [Google Scholar] [CrossRef] [PubMed]
- Keun, H.C.; Beckonert, O.; Griffin, J.L.; Richter, C.; Moskau, D.; Lindon, J.C.; Nicholson, J.K. Cryogenic probe 13C-NMR spectroscopy of urine for metabonomic studies. Anal. Chem. 2002, 74, 4588–4593. [Google Scholar] [CrossRef] [PubMed]
- Kamal, G.M.; Wang, X.H.; Yuan, B.; Wang, J.; Sun, P.; Zhang, X.; Liu, M. Compositional differences among chinese soy sauce types studied by 13C-NMR spectroscopy coupled with multivariate statistical analysis. Talanta 2016, 158, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xiao, N.; Liu, H.; Zhou, Z.; Mao, X.A.; Liu, M. Optimized quantitative DEPT and quantitative POMMIE experiments for 13C-NMR. Anal. Chem. 2008, 80, 8293–8298. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Furihata, K.; Koda, M.; Hu, F.; Miyakawa, T.; Tanokura, M. Roasting process of coffee beans as studied by nuclear magnetic resonance: Time course of changes in composition. J. Agric. Food Chem. 2012, 60, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.K.; Ahn, H.J.; van den Berg, F.; Lee, C.H.; Hong, Y.S. Metabolomic insight into soy sauce through 1H-NMR spectroscopy. J. Agric. Food Chem. 2009, 57, 6862–6870. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Choi, Y.H.; Verberne, M.; Lefeber, A.W.; Erkelens, C.; Verpoorte, R. Metabolic fingerprinting of wild type and transgenic tobacco plants by 1H-NMR and multivariate analysis technique. Phytochemistry 2004, 65, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lee, J.; Kwon, J.; Lee, K.S.; Ryu, D.H.; Hwang, G.S. Discrimination of the geographical origin of beef by 1H-NMR-based metabolomics. J. Agric. Food Chem. 2010, 58, 10458–10466. [Google Scholar] [CrossRef] [PubMed]
- Son, H.S.; Hwang, G.S.; Park, W.M.; Hong, Y.S.; Lee, C.H. Metabolomic characterization of malolactic fermentation and fermentative behaviors of wine yeasts in grape wine. J. Agric. Food Chem. 2009, 57, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Luh, B.S. Industrial-production of soy-sauce. J. Ind. Microbiol. 1995, 14, 467–471. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Bhavnani, B.R. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (aif) and this process is inhibited by equine estrogens. BMC. Neurosci. 2006. [Google Scholar] [CrossRef]
- Stratton, K.R.; Worley, P.F.; Litz, J.S.; Parsons, S.J.; Huganir, R.L.; Baraban, J.M. Electroconvulsive treatment induces a rapid and transient increase in tyrosine phosphorylation of a 40-kilodalton protein associated with microtubule-associated protein-2 kinase-activity. J. Neurochem. 1991, 56, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Deijen, J.B.; Orlebeke, J.F. Effect of tyrosine on cognitive function and blood-pressure under stress. Brain Res. Bull. 1994, 33, 319–323. [Google Scholar] [CrossRef]
- Deijen, J.B.; Wientjes, C.J.E.; Vullinghs, H.F.M.; Cloin, P.A.; Langefeld, J.J. Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res. Bull. 1999, 48, 203–209. [Google Scholar] [CrossRef]
- Hentges, D.J. Influence of pH on inhibitory activity of formic and acetic acids for shigella. J. Bacteriol. 1967, 93, 2029. [Google Scholar] [PubMed]
- Noda, F.; Hayashi, K.; Mizunuma, T. Antagonism between osmophilic lactic-acid bacteria and yeasts in brine fermentation of soy sauce. Appl. Environ. Microbiol. 1980, 40, 452–457. [Google Scholar] [PubMed]
- Radler, F.; Brohl, K. The metabolism of several carboxylic-acids by lactic-acid bacteria. Z. Lebensm. Unters. Forsch. B Food Res. Technol. 1984, 179, 228–231. [Google Scholar] [CrossRef]
- Nishimura, I.; Igarashi, T.; Enomoto, T.; Dake, Y.; Okuno, Y.; Obata, A. Clinical efficacy of halophilic lactic acid bacterium tetragenococcus halophilusth221 from soy sauce moromi for perennial allergic rhinitis. Allergol. Int. 2009, 58, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Kobayashi, M.; Tsukiyama, R.-I.; Fujimoto, M.; Suzuki, M.; Tsuji, K.; Yamamoto, K. Stimulatory effect of shoyu polysaccharides from soy sauce on the intestinal immune system. Int. J. Mol. Med. 2008, 22, 243–247. [Google Scholar] [PubMed]
- Villar, M.; de Ruiz Holgado, A.P.; Sanchez, J.J.; Trucco, R.E.; Oliver, G. Isolation and characterization of pediococcus halophilusfrom salted anchovies (Engraulis anchoita). Appl. Environ. Microbiol. 1985, 49, 664–666. [Google Scholar] [PubMed]
- Masuda, S.; Yamaguchi, H.; Kurokawa, T.; Shirakami, T.; Tsuji, R.F.; Nishimura, I. Immunomodulatory effect of halophilic lactic acid bacterium tetragenococcus halophilus th221 from soy sauce moromi grown in high-salt medium. Int. J. Food Microbiol. 2008, 121, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, M.; Igarashi, Y. Nondecarboxylating and decarboxylating isocitrate dehydrogenases: Oxalosuccinate reductase as an ancestral form of isocitrate dehydrogenase. J. Bacteriol. 2008, 190, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Ebbels, T.M.; Lindon, J.C.; Coen, M. Processing and modeling of nuclear magnetic resonance (NMR) metabolic profiles. Methods Mol. Biol. 2011, 708, 365–388. [Google Scholar] [PubMed]
- Defernez, M.; Colquhoun, I.J. Factors affecting the robustness of metabolite fingerprinting using 1H-NMR spectra. Phytochemistry 2003, 62, 1009–1017. [Google Scholar] [CrossRef]
- Witjes, H.; Melssen, W.J.; in't Zandt, H.J.; van der Graaf, M.; Heerschap, A.; Buydens, L.M. Automatic correction for phase shifts, frequency shifts, and lineshape distortions across a series of single resonance lines in large spectral data sets. J. Magn. Reson. 2000, 144, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Vogels, J.T.W.E.; Tas, A.C.; Venekamp, J.; VanderGreef, J. Partial linear fit: A new nmr spectroscopy preprocessing tool for pattern recognition applications. J. Chemometr. 1996, 10, 425–438. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Cheng, J.; Wang, J.; Xu, F. A processing method for spectrum alignment and peak extraction for NMR spectra. Chin. J. Magn. Reson. 2015, 2, 382–392. [Google Scholar]
- Sample Availability: Not available.
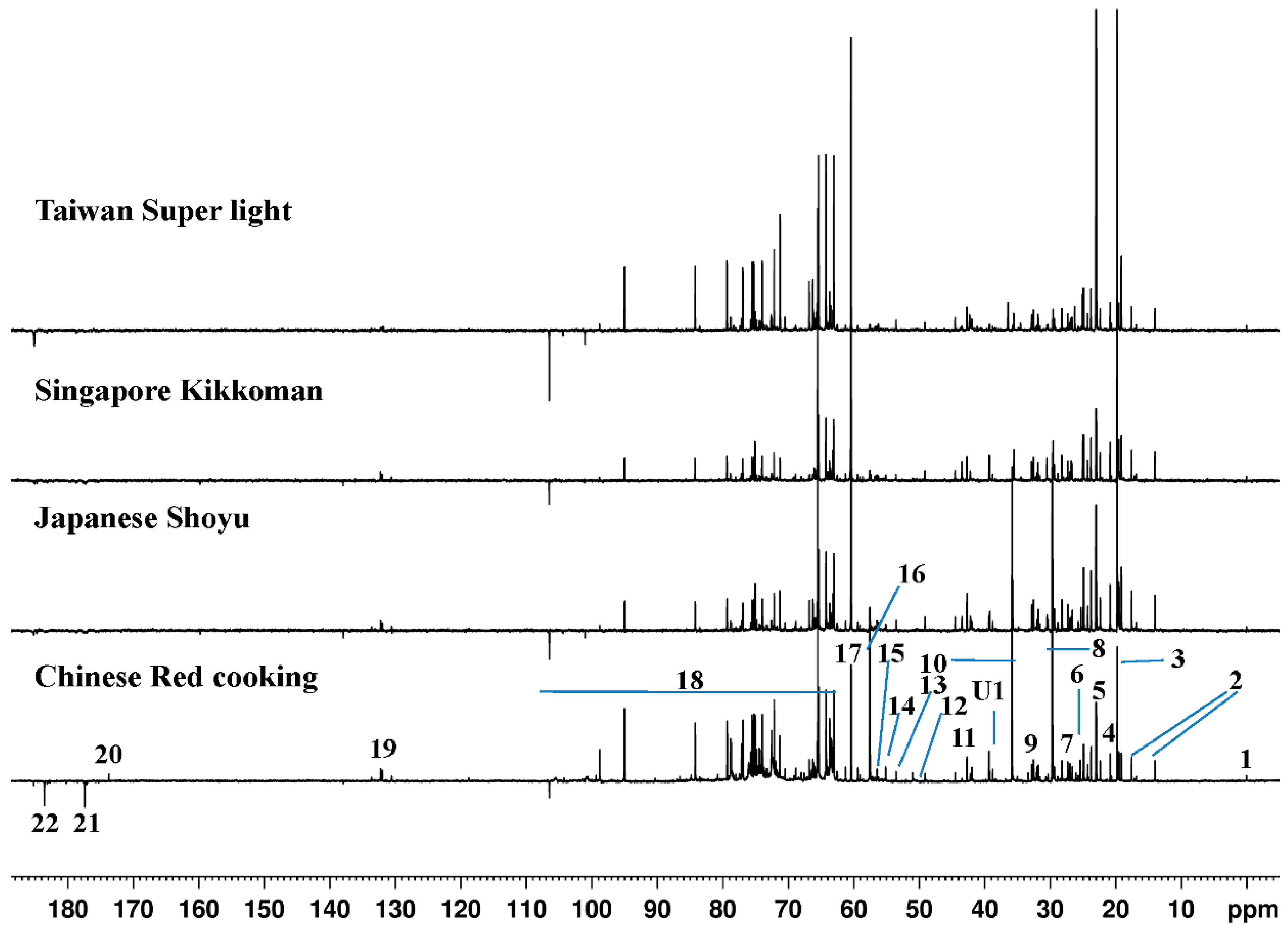

| Compound | 13C-NMR Chemical Shift | Group |
|---|---|---|
| Isoleucine | 13.98, 17.54 * | –C6H3, –C5H3 |
| Alanine | 19.74 | –C3H3 |
| Valine | 20.81 *, 60.38 | –C4H3, –C2H |
| Threonine | 22.33 | –C4H3 |
| Acetate/Acetic acid | 22.97 *, 185 | –CH3, –CO |
| Glutamate | 29.67, 57.53 * | –C5H3, –C3H |
| Leucine | 26.72, 42.68 * | –C4H, C3H3 |
| Unknown | 39.23–39.35 * | — |
| Malate | 44 * | C4H3 |
| Malonate | 49.10 * | C2H3 |
| Betaine | 56.22 | N(CH3)3 |
| Choline | 56.43 | N(CH3)3 |
| Aspartate | 56.70 | –C3H |
| Glucose | 63–105 (94.99 *) | — |
| Sucrose | 63–105 (65.48 *) | — |
| Tyrosine | 131–132 * | –C3H |
| Phenylalanine | 176–177 * | –C=O |
| Lactate | 184 * | –C=O |
| Soy Sauce Type | Raw Materials | Fermentation Process |
|---|---|---|
| Chinese red cooking | Steamed soybean and wheat, caramel color and or molasses, spice extracts, MSG, sodium benzoate. | Prolonged fermentation of steamed soybeans and wheat with A. oryzae. |
| Japanese Shoyu (Tamari) | Less wheat and more soybean, alcohol as preservative. | Stronger alcoholic fermentation with yeast. |
| Singapore Kikkoman | Low salt, water, soybean, less wheat, rice, alcohol. | Brine fermentation with yeast or Aspergillus and lactic acid bacteria. |
| Taiwan (China) light | Black soybeans, wheat, water, less salt, sodium benzoate. | Japanese-style fermentation. |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, G.M.; Yuan, B.; Hussain, A.I.; Wang, J.; Jiang, B.; Zhang, X.; Liu, M. 13C-NMR-Based Metabolomic Profiling of Typical Asian Soy Sauces. Molecules 2016, 21, 1168. https://doi.org/10.3390/molecules21091168
Kamal GM, Yuan B, Hussain AI, Wang J, Jiang B, Zhang X, Liu M. 13C-NMR-Based Metabolomic Profiling of Typical Asian Soy Sauces. Molecules. 2016; 21(9):1168. https://doi.org/10.3390/molecules21091168
Chicago/Turabian StyleKamal, Ghulam Mustafa, Bin Yuan, Abdullah Ijaz Hussain, Jie Wang, Bin Jiang, Xu Zhang, and Maili Liu. 2016. "13C-NMR-Based Metabolomic Profiling of Typical Asian Soy Sauces" Molecules 21, no. 9: 1168. https://doi.org/10.3390/molecules21091168
APA StyleKamal, G. M., Yuan, B., Hussain, A. I., Wang, J., Jiang, B., Zhang, X., & Liu, M. (2016). 13C-NMR-Based Metabolomic Profiling of Typical Asian Soy Sauces. Molecules, 21(9), 1168. https://doi.org/10.3390/molecules21091168






