Mexicanolide-Type Limonoids from the Roots of Trichilia sinensis
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Bioassay of AChE Inhibitory Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, X.; Di, Y.T.; Hao, X.J. The advances in the limonoid chemistry of the Meliaceae family. Curr. Org. Chem. 2011, 15, 1363–1391. [Google Scholar]
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Chen, B.Y.; Li, H. Flora Republicae Popularis Sinicae. Chinese Flora (Zhongguo Zhiwu Zhi); Science Press: Beijing, China, 1997; Volume 43, p. 34. [Google Scholar]
- Editorial Committee of Chinese Materia Medica. The Administration Bureau of Traditional Chinese Medicine. In Chinese Materia Medica (Zhonghua Bencao); Shanghai Science & Technology Press: Shanghai, China, 2000; Volume 5, pp. 48–49. [Google Scholar]
- Dai, H.F.; Zheng, X.L.; Xing, F.W.; Mei, W.L. Records of Li Folk Medicine; China Science & Technology Press: Beijing, China, 2014; Volume 3, pp. 135–136. [Google Scholar]
- Xu, J.B.; Lin, Y.; Dong, S.H.; Wang, F.; Yue, J.M. Trichinenlides A-T, mexicanolide-type limonoids from Trichilia sinensis. J. Nat. Prod. 2013, 76, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Di, Y.T.; Li, C.S.; Geng, Z.L.; Zhang, Z.; Zhang, Y.; Lu, Y.; Zheng, Q.T.; Yang, S.Y.; Hao, X.J. Tetranortriterpenoids from the leaves of Cipadessa cinerascens. J. Nat. Prod. 2009, 72, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Di, Y.T.; Fang, X.; He, H.P.; Wang, Y.Y.; Li, Y.; Li, S.L.; Hao, X.J. Limonoids from the Leaves of Cipadessa baccifera. J. Nat. Prod. 2010, 73, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kong, L.M.; Li, S.F.; Li, Y.; Zhang, Y.; He, H.P.; Hao, X.J. Five new mexicanolide type limonoids from Heynea trijuga. Nat. Prod. Bioprospect. 2012, 2, 145–149. [Google Scholar] [CrossRef]
- Li, J.; Li, M.Y.; Feng, G.; Xiao, Q.; Sinkkonen, J.; Satyanandamurty, T.; Wu, J. Limonoids from the seeds of a Godavari mangrove, Xylocarpus moluccensis. Phytochemistry 2010, 71, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Okorie, D.A.; Taylor, D.A.H. Limonoids from Swietenia humilis. Phytochemistry 1971, 10, 469–470. [Google Scholar] [CrossRef]
- Yang, D.L.; Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Zhao, Y.X.; Wang, H.; Zuo, W.J.; Dong, W.H.; Wang, Q.H.; Dai, H.F. 2-(2-phenylethyl) chromone derivatives in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. Planta Med. 2013, 79, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
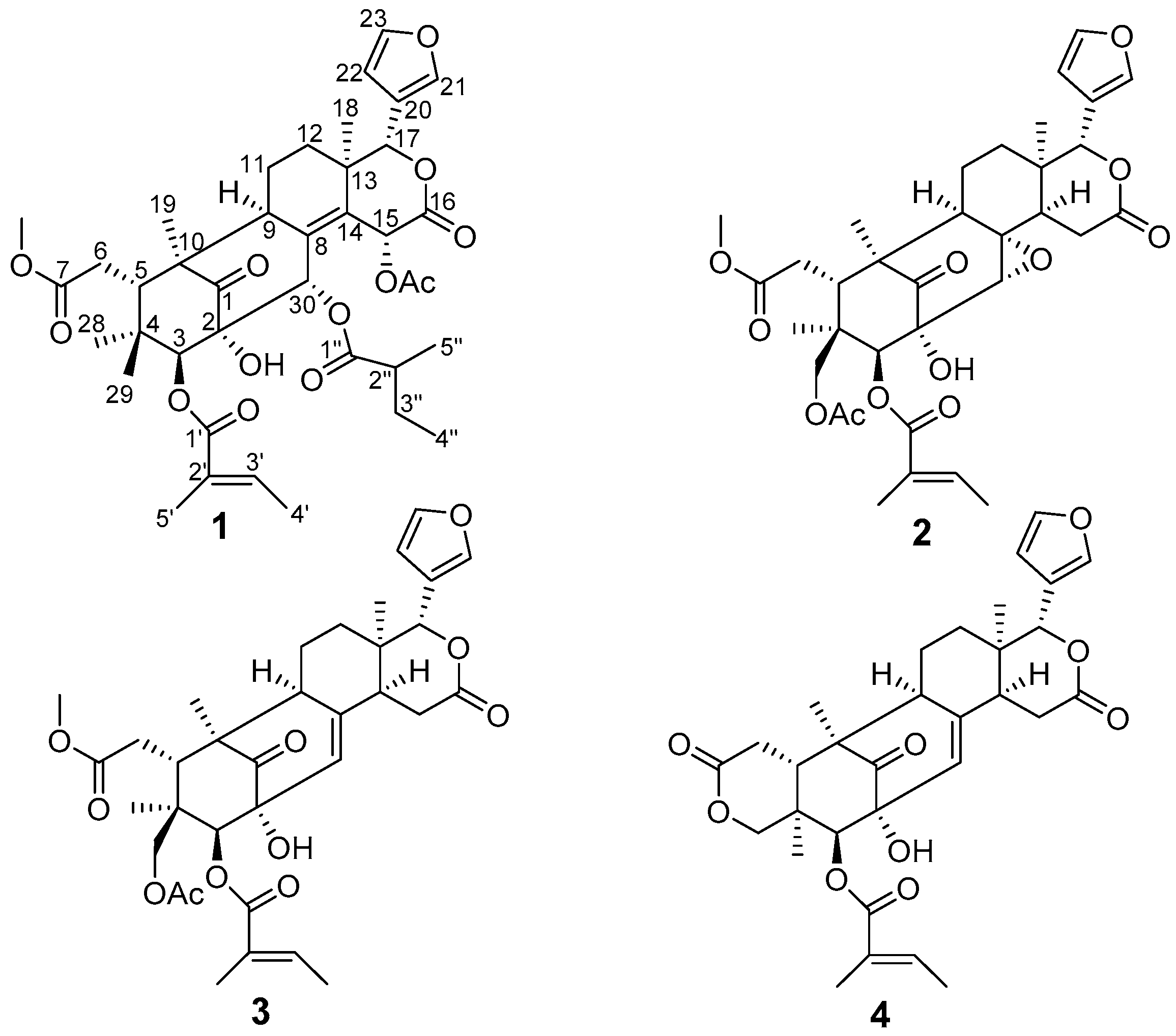
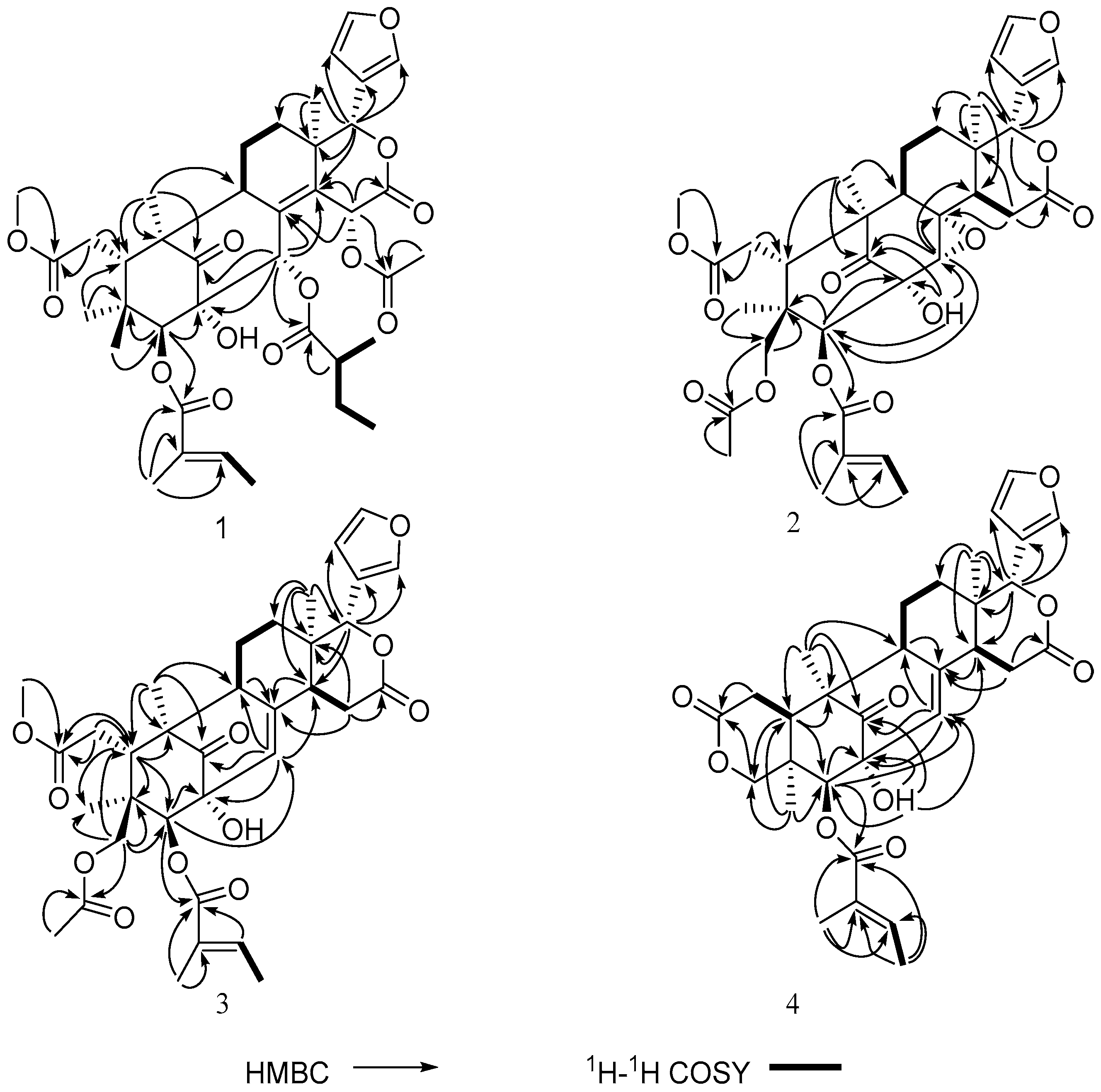
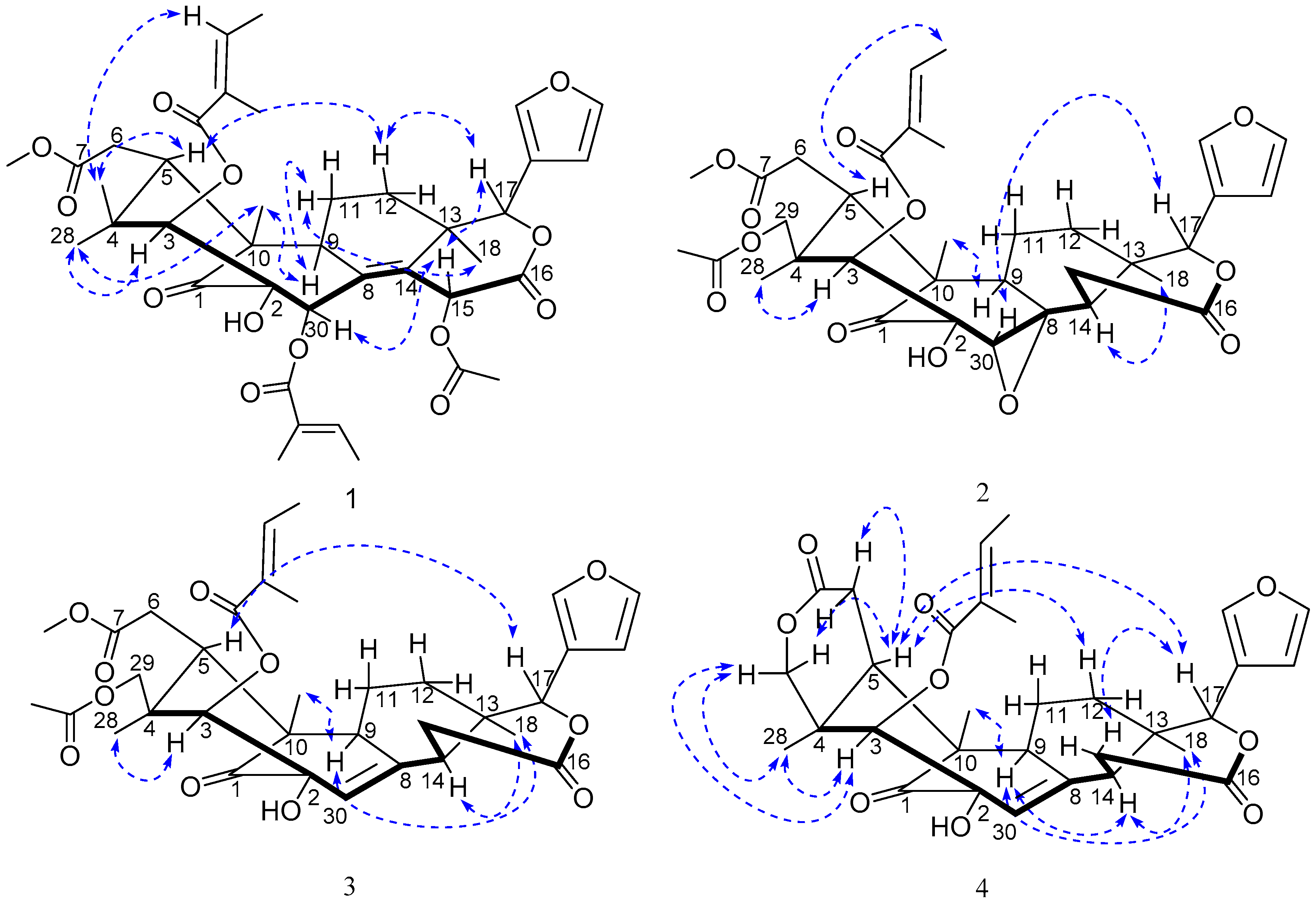
| Proton | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 3 | 5.11 (s) | 5.23 (s) | 5.03 (s) | 5.11 (s) |
| 5 | 3.38 (dd, 10.0, 2.3) | 3.38 (d, 1.4, 9.3) | 3.51 (d, 10.1) | 3.08 (dd, 10.9, 8.0) |
| 6a | 2.31 (m) | 2.43 (m) | 2.51 (dd,17.3, 10.3) | 2.63 (m) |
| 6b | 2.42 (m) | 2.43 (m) | 2.40 (d, 17.3) | 2.61 (m) |
| 9 | 2.41 (m) | 1.94 (m) | 2.24 (m) | 2.32 (m) |
| 11α | 1.77 (m) | 1.80 (m) | 1.66 (dd, 4.0, 13.2) | 1.72 (m) |
| 11β | 1.90 (m) | 1.97 (m) | 2.02 (m) | 1.79 (m) |
| 12α | 1.14 (m) | 1.24 (m) | 1.41 (m) | 1.50 (m) |
| 12β | 1.85 (m) | 1.94 (m) | 1.61 (m) | 1.69 (m) |
| 14 | 1.60 (dd, 13.4, 5.1) | 2.22 (m) | 2.27 (m) | |
| 15α | 3.38 (m) | 2.84 (dd, 18.7, 6.1) | 2.85 (m) | |
| 15β | 6.55 (s) | 2.78 (dd, 16.2, 5.1) | 2.77 (d, 18.7) | 2.85 (m) |
| 17 | 5.68 (s) | 5.17 (s) | 5.56 (s) | 5.37 (s) |
| 18 | 1.11 (s) | 0.99 (s) | 1.09 (s) | 1.02 (s) |
| 19 | 1.28 (s) | 1.20 (s) | 1.27 (s) | 1.24 (s) |
| 21 | 7.65 (s) | 7.49 (s) | 7.81 (s) | 7.46 (br s) |
| 22 | 6.52 (s) | 6.43 (d, 1.0) | 6.46 (s) | 6.36 (br s) |
| 23 | 7.42 (br s) | 7.42 (t, 1.6) | 7.43 (s) | 7.45 (br s) |
| 28 | 0.74 (s) | 0.86 (s) | 0.93 (s) | 1.03 (3H, s) |
| 29a | 0.78 (s) | 3.87 (d, 10.8) | 3.95 (d, 10.6) | 3.92 (d, 11.8) |
| 29b | 3.73 (d, 10.8) | 3.73 (d, 10.6) | 4.24 (d, 11.8) | |
| 30 | 5.76 (s) | 3.42 (s) | 5.31 (s) | 5.40 (s) |
| MeO-7 | 3.74 (s) | 3.76 (s) | 3.77 (s) | |
| 3′ | 6.82 (q, 6.8) | 7.00 (dq, 7.0, 1.3) | 6.94 (q, 7.0) | 6.86 (q, 6.9) |
| 4′ | 1.80 (d, 6.8) | 1.89 (d, 7.0) | 1.72 (d, 7.0) | 1.77 (d, 6.9) |
| 5′ | 2.03 (s) | 1.93 (s) | 1.82 (s) | 1.84 (s) |
| 2′′ | 2.18 (m) | |||
| 3′′ | 1.77 (m) | |||
| 1.49 (m) | ||||
| 4′′ | 0.94 (t, 7.5) | |||
| 5′′ | 1.17 (d, 6.8) | |||
| OAc-15 | 1.96 (s) | |||
| OAc-29 | 1.93 (s) | 1.95 (s) | ||
| OH-2 | 4.06 (s) | 4.17 (s) | 4.12 (s) |
| Carbon | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 213.0 | 212.5 | 214.2 | 213.6 |
| 2 | 79.3 | 78.4 | 77.3 | 77.1 |
| 3 | 85.8 | 82.3 | 82.0 | 82.9 |
| 4 | 39.9 | 42.9 | 42.1 | 37.1 |
| 5 | 41.4 | 40.3 | 39.6 | 38.7 |
| 6 | 33.2 | 33.6 | 32.4 | 31.5 |
| 7 | 174.3 | 173.5 | 173.4 | 169.8 |
| 8 | 133.0 | 63.1 | 137.4 | 137.4 |
| 9 | 47.9 | 55.3 | 56.8 | 56.4 |
| 10 | 52.0 | 48.9 | 49.2 | 48.5 |
| 11 | 18.6 | 19.5 | 20.6 | 21.5 |
| 12 | 28.8 | 33.4 | 34.4 | 34.1 |
| 13 | 39.3 | 36.4 | 37.0 | 36.9 |
| 14 | 139.8 | 45.4 | 45.1 | 44.9 |
| 15 | 64.3 | 32.7 | 29.7 | 29.6 |
| 16 | 167.8 | 171.3 | 168.6 | 168.3 |
| 17 | 80.4 | 78.8 | 76.5 | 76.9 |
| 18 | 17.4 | 26.0 | 21.7 | 21.5 |
| 19 | 17.3 | 16.6 | 16.1 | 15.9 |
| 20 | 120.8 | 120.2 | 120.6 | 120.8 |
| 21 | 142.3. | 141.1 | 142.1 | 141.2 |
| 22 | 110.1 | 110.3 | 109.8 | 109.4 |
| 23 | 143.1 | 143.3 | 143.3 | 143.6 |
| 28 | 23.7 | 14.9 | 14.9 | 16.4 |
| 29 | 19.5 | 66.5 | 66.7 | 73.7 |
| 30 | 74.2 | 67.4 | 129.0 | 128.5 |
| MeO-7 | 52.5 | 52.8 | 52.7 | |
| 1′ | 167.3 | 166.3 | 167.0 | 166.9 |
| 2′ | 131.0 | 127.6 | 127.3 | 127.1 |
| 3′ | 137.4 | 140.4 | 140.5 | 140.5 |
| 4′ | 14.6 | 12.6 | 14.8 | 14.9 |
| 5′ | 12.9 | 15.8 | 11.6 | 12.2 |
| 1′′ | 174.2 | |||
| 2′′ | 40.5 | |||
| 3′′ | 26.6 | |||
| 4′′ | 11.5 | |||
| 5′′ | 14.5 | |||
| OAc-15 | 168.2 | |||
| 21.0 | ||||
| OAc-29 | 170.5 | 170.6 | ||
| 20.6 | 20.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-B.; Mei, W.-L.; Chen, H.-Q.; Guo, Z.-K.; Dai, H.-F.; Wang, Z.-N. Mexicanolide-Type Limonoids from the Roots of Trichilia sinensis. Molecules 2016, 21, 1152. https://doi.org/10.3390/molecules21091152
Liu S-B, Mei W-L, Chen H-Q, Guo Z-K, Dai H-F, Wang Z-N. Mexicanolide-Type Limonoids from the Roots of Trichilia sinensis. Molecules. 2016; 21(9):1152. https://doi.org/10.3390/molecules21091152
Chicago/Turabian StyleLiu, Shou-Bai, Wen-Li Mei, Hui-Qin Chen, Zhi-Kai Guo, Hao-Fu Dai, and Zhu-Nian Wang. 2016. "Mexicanolide-Type Limonoids from the Roots of Trichilia sinensis" Molecules 21, no. 9: 1152. https://doi.org/10.3390/molecules21091152
APA StyleLiu, S.-B., Mei, W.-L., Chen, H.-Q., Guo, Z.-K., Dai, H.-F., & Wang, Z.-N. (2016). Mexicanolide-Type Limonoids from the Roots of Trichilia sinensis. Molecules, 21(9), 1152. https://doi.org/10.3390/molecules21091152






