Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L.
Abstract
:1. Introduction
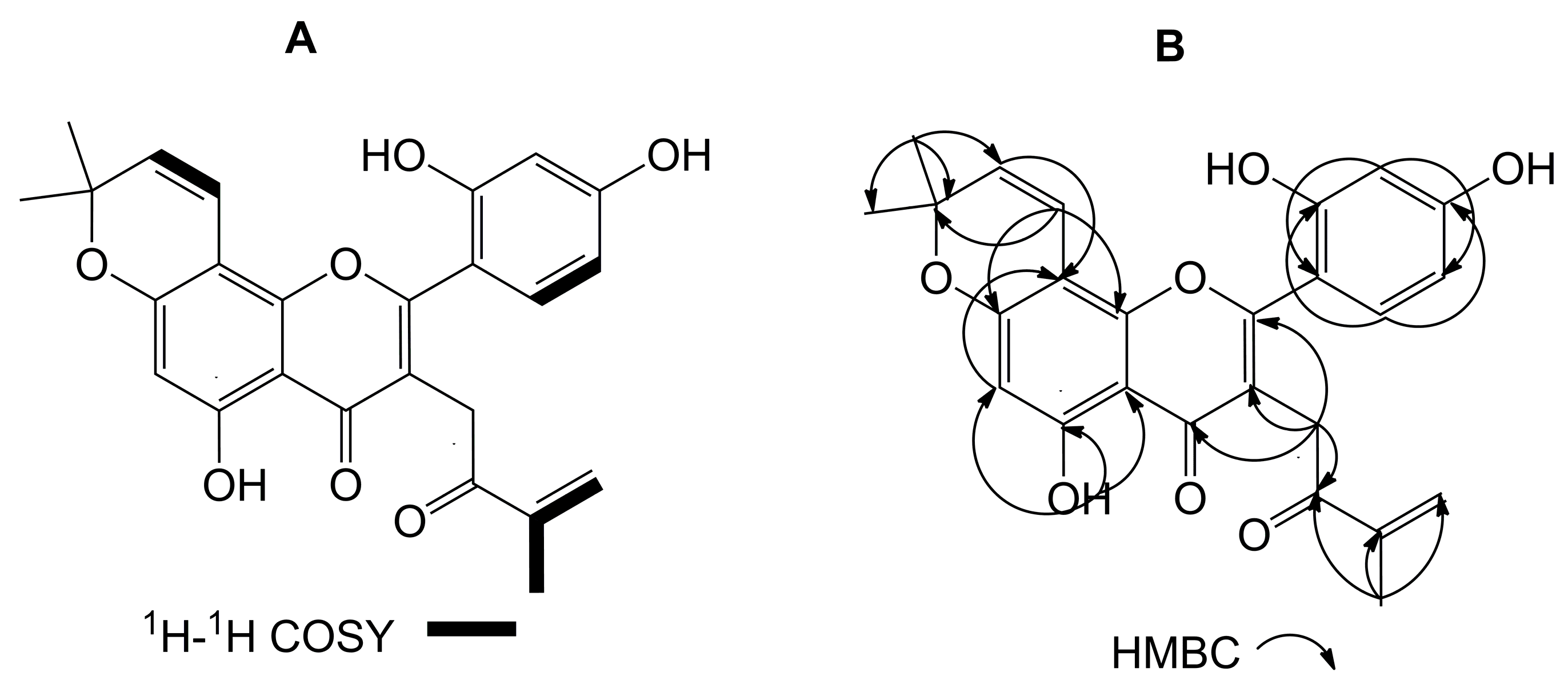
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Mushroom Tyrosinase Inhibitory Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Artés, F.; Castañer, M.; Gil, M.I. Revisión: EI pardeamientoenzimático en frutas y hortalizas mínimamente procesadas review: Enzymatic browning in minimally processed fruit and vegetables. Food Sci. Technol. Int. 1998, 4, 377–389. [Google Scholar] [CrossRef]
- Lerner, A.B.; Fitzpatrick, T.B. Biochemistry of melanin formation. Phys. Rev. 1950, 30, 91–126. [Google Scholar]
- Iozumi, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993, 100, 806–811. [Google Scholar] [CrossRef]
- Solano, F.; Stefania, B.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2007, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Fu, Y.W.; Zhang, Q.Z.; Xu, D.H.; Wang, B.; Lin, D.J. Identification and effect of two flavonoids from root bark of Morus alba against Ichthyophthirius multifiliis in grass carp. J. Agric. Food Chem. 2015, 63, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Choi, S.W.; Jang, Y.J.; Lee, Y.J.; Leem, H.H.; Kim, E.O. Analysis of functional constituents in mulberry (Morus alba L.) twigs by different cultivars, producing areas, and heat processings. Prev. Nutr. Food Sci. 2013, 18, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2001, 49, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Meng, X.; Kim, A.; Wang, M.; Simon, J.E. Developing a long-lasting tyrosinase inhibitor from Morus alba L. Cosmet. Toilet. 2006, 121, 91–102. [Google Scholar]
- Li, H.; Cheng, K.W.; Cho, C.H.; He, Z.; Wang, M. Oxyresveratrol as an antibrowning agent for cloudy apple juices and fresh-cut apples. J. Agric. Food Chem. 2007, 55, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Tan, H.Y.; Chen, J.; Wang, M. Characterization of tyrosinase inhibitors in the twigs of Cudrania tricuspidata and their structure-activity relationship study. Fitoterapia 2013, 84, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Cheng, K.W.; Zhu, Q.; Wang, X.C.; Lin, Z.X.; Wang, M. Tyrosinase inhibitory constituents from the roots of Morus nigra: A structure-activity relationship study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Fukai, T.; Yamada, S.; Katayanagi, M. Phenolic constituents of the cultivated mulberry tree (Morus alba L.). Chem. Pharm. Bull. 1976, 24, 2898–2900. [Google Scholar] [CrossRef]
- Nomura, T.; Kukai, T.; Katayanagi, M. Studies on the constituents of the cultivated mulberry tree. III. Isolation of four new flavones, kuwanon A, B, C and oxydihydromorusin from the root bark of Morus alba L. Chem. Pharm. Bull. 1978, 26, 1453–1458. [Google Scholar] [CrossRef]
- Deshpande, V.H.; Parthasarathy, P.C.; Venkataraman, K. Four analogs of artocarpin and cycloartocarpin from Morus alba. Tetrahedron Lett. 1968, 14, 1715–1719. [Google Scholar] [CrossRef]
- Ferlinahayati; Syah, Y.M.; Juliawaty, L.D; Achmad, S.A.; Hakim, E.H.; Takayama, H.; Said, I.M.; Latip, J. Phenolic constituents from the wood of Morus australis with cytotoxic activity. Z. Naturforsch. C Biosci. 2008, 63, 35–39. [Google Scholar] [CrossRef]
- Lee, C.S.; Wang, J.S.; Chen, K.C.S. Chemical constituents from the roots of Zizyphus jujuba Mill. var. spinosa. J. Chin. Chem. Soc. 1995, 42, 77–82. [Google Scholar] [CrossRef]
- Encarnacion, D.R.; Nogueiras, C.L.; Salinas, V.H.A.; Anthoni, U.; Nielsen, P.H.; Christophersen, C. Isolation of eriodictyol identical with huazhongilexone from Solanum hindsianum. Acta Chem. Scand. 1999, 53, 375–377. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Xu, Y.; Qin, C.; Zhang, S.; Gu, X.; Lin, Y.; Xie, G.; Wang, M.; Chen, J. Characterization of antiproliferative activity constituents from Artocarpus heterophyllus. J. Agric. Food Chem. 2014, 62, 5519–5527. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Duarte, I.F.; Almeida, C.; Delgadillo, I.; Goodfelllow, B.J.; Gil, A.M.; Morris, G.A. High-resolution NMR and diffusion-ordered spectroscopy of port wine. J. Agric. Food Chem. 2004, 52, 3736–3743. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M.; Nagao, S.; Munoz, L.; Ishikawa, S.; Masamune, T.; Shirata, A.; Takahashi, K. The structure of phytoalexins produced in diseased mulberry. Koen Yoshishu-Tennen Yuki Kagobutsu Toronkai 1979, 22, 275–282. [Google Scholar]
- Takasugi, M.; Nagao, S.; Masamune, T.; Shirata, A.; Takahashi, K. Structure of moracin A and B, new phytoalexins from diseased mulberry. Tetrahedron Lett. 1978, 9, 797–798. [Google Scholar] [CrossRef]
- Takasugi, M.; Nagao, S.; Ueno, S.; Masamune, T.; Shirata, A.; Takahashi, K. Studies on phytoalexins of the Moraceae. 2. Moracin C and D, new phytoalexins from diseased mulberry. Chem. Lett. 1978, 11, 1239–1240. [Google Scholar] [CrossRef]
- Hano, Y.; Suzuki, S.; Nomura, T.; Ueda, S. Constituents of the cultivated mulberry tree. Part XLI. Two new phenolic compounds, kuwanols C and D, from the root bark of a mulberry tree redifferentiated from the callus tissues. Heterocycles 1989, 29, 807–813. [Google Scholar]
- Jeong, J.Y.; Jo, Y.H.; Kim, S.B.; Liu, Q.; Lee, J.W.; Mo, E.J.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Pancreatic lipase inhibitory constituents from Morus alba leaves and optimization for extraction conditions. Bioorg. Med. Chem. Lett. 2015, 25, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Cheng, K.W.; To, J.T.K.; Li, H.; Wang, M. Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as anti-browning agent. Mol. Nutr. Food Res. 2008, 52, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhang, Y.N.; He, J.L.; Zhang, S.; Zeng, M.M.; Chen, J.; Zheng, Z.P. Preparation of tyrosinase inhibitors and antibrowning agents using green technology. Food Chem. 2016, 197, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Dong, X.; Yuan, K.; Lan, S.; Zhu, Q.; Wang, M.; Chen, J. Preparation, characterization, and preliminary antibrowning evaluations of norartocarpetin microemulsions. J. Agric. Food Chem. 2015, 63, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–17 are available from the authors.
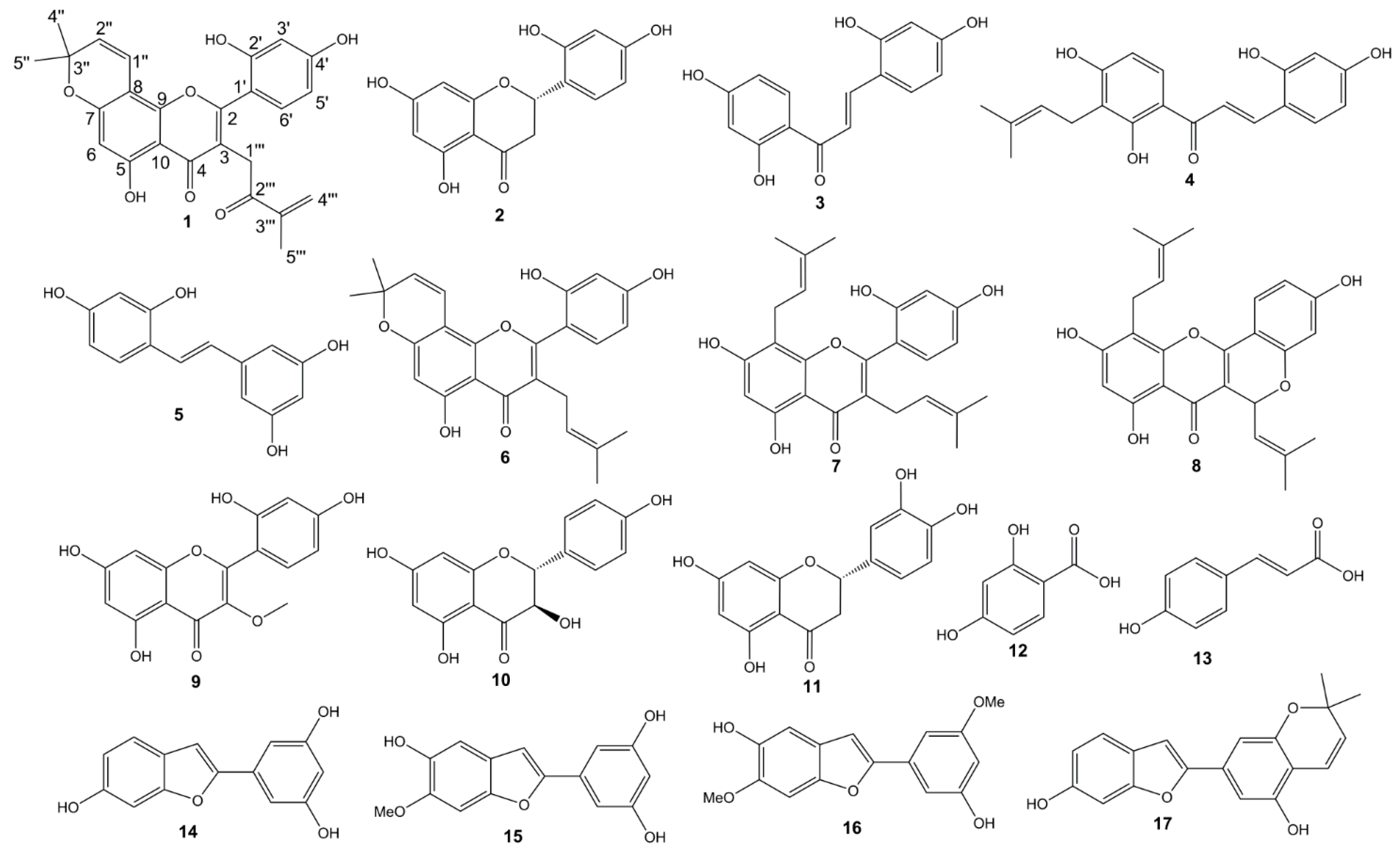
| Compounds | IC50 (µM) ± SD |
|---|---|
| Morusone (1) | 290.00 ± 7.90 |
| Steppogenin (2) | 0.98 ± 0.01 |
| 2,4,2′,4′-Tetrahydroxychalcone (3) | 0.07 ± 0.02 |
| Morachalcone A (4) | 0.08 ± 0.02 |
| Oxyresveratrol (5) | 0.10 ± 0.01 |
| Morusin (6) | >100 |
| Kuwanon C (7) | 52.00 ± 2.50 |
| Cyclomulberrin (8) | 66.30 ± 7.20 |
| 5,7,2′,4′-Tetrahydroxy-3-methoxyflavone (9) | >150 |
| Dihydrokaempferol (10) | >200 |
| Eriodictyol (11) | >150 |
| 2,4-Dihydroxybenzoic acid (12) | >200 |
| p-Coumaric acid (13) | >200 |
| Moracin M (14) | 8.00 ± 0.22 |
| Moracin J (15) | 76.34 ± 0.86 |
| Moracin B (16) | 34.40 ± 1.7 |
| Moracin D (17) | >200 |
| Kojic acid | 58.30 ± 1.60 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Tao, G.; Chen, J.; Zheng, Z.-P. Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L. Molecules 2016, 21, 1130. https://doi.org/10.3390/molecules21091130
Zhang L, Tao G, Chen J, Zheng Z-P. Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L. Molecules. 2016; 21(9):1130. https://doi.org/10.3390/molecules21091130
Chicago/Turabian StyleZhang, Long, Guanjun Tao, Jie Chen, and Zong-Ping Zheng. 2016. "Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L." Molecules 21, no. 9: 1130. https://doi.org/10.3390/molecules21091130
APA StyleZhang, L., Tao, G., Chen, J., & Zheng, Z.-P. (2016). Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L. Molecules, 21(9), 1130. https://doi.org/10.3390/molecules21091130






