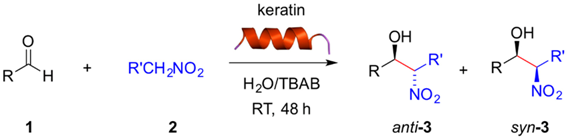
Abstract
Here we describe a preliminary investigation on the ability of natural keratin to catalyze the nitroaldol (Henry) reaction between aldehydes and nitroalkanes. Both aromatic and heteroaromatic aldehydes bearing strong or moderate electron-withdrawing groups were converted into the corresponding β-nitroalcohol products in both DMSO and in water in the presence of tetrabutylammonium bromide (TBAB) as a phase transfer catalyst. Negligible background reactions (i.e., negative control experiment in the absence of keratin protein) were observed in these solvent systems. Aromatic aldehydes bearing electron-donating groups and aliphatic aldehydes showed poor or no conversion, respectively. In general, the reactions in water/TBAB required twice the amount of time than in DMSO to achieve similar conversions. Moreover, comparison of the kinetics of the keratin-mediated nitroaldol (Henry) reaction with other biopolymers revealed slower rates for the former and the possibility of fine-tuning the kinetics by appropriate selection of the biopolymer and solvent.
1. Introduction
Keratin is one of the most abundant non-food proteins and represents a group of insoluble, cysteine-rich and stable filament-forming materials derived primarily from wool, hair, feathers, beaks, hooves, horns and nails [1]. Depending on their sulfur content, keratins can be classified as soft or hard keratins. Soft keratins, with a cysteine content up to 2% and found in outer layers of the epidermis and hair core, have a weaker mechanical and chemical stability. Hard keratins with a cysteine content of 10%–14% in hair, wool and skin, exhibit a higher resistance to thermal and chemical influences due to higher content of disulfide bridges [2]. Keratins contain a central ~310 residue domain with four segments in α-helical conformation that are separated by three short linker segments in β-turn conformation [3]. The complete amino acid sequence of keratins obtained from different sources has been reported in several publications [4,5,6,7], making the protein a suitable candidate to be tested as a catalyst. Recently, keratinous materials have attracted increasing attention as an important source of renewable biomaterials, especially since keratin wastes have been estimated to be more than 5 million tons per year [8], being used after degradation for different applications including scaffolds for tissue engineering [9,10] and support matrices for catalytic metal nanoparticles [11], among others [12].
On the other hand, the nitroaldol (Henry) reaction is a highly versatile C-C bond-forming reaction between an aldehyde or ketone and a nitroalkane to yield β-nitroalcohols, which can be easily modified into valuable building blocks [13,14,15,16,17]. As part of one of our research programs devoted to understanding the potential catalytic role of different biopolymers [18,19,20,21], we report here for the first time the catalytic properties of keratin protein towards the nitroaldol (Henry) reaction as well as a critical comparison with the performance of other natural polymers in the same process.
2. Results and Discussion
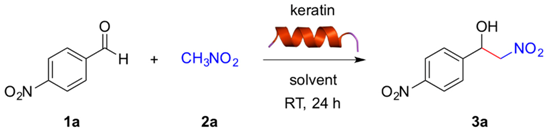
The nitroaldol (Henry) reaction between 4-nitrobenzaldehyde (1a, 0.1 mmol) and nitromethane (2a, 1.0 mmol) was chosen as a model reaction to demonstrate the potential ability of keratin to promote C-C bond formation under mild conditions. Preliminary experiments using 10 mg of keratin in different solvents showed no formation of the desired β-nitroalcohol 3a in toluene, THF or CH3CN (Table 1, entries 1–3), and very low yields of 3a (ca. 10%) were obtained in CH2Cl2, CHCl3 or H2O (Table 1, entries 4, 5 and 7). However, DMSO and water containing tetrabutylammonium bromide (TBAB) as phase-transfer catalyst were found to be appropriate solvents allowing the selective formation of 3a in modest yields at room temperature with no or very low background reaction (Table 1, entry 6 and entry 8). Thus, formation of 3a could be unequivocally ascribed to the intrinsic catalytic activity of the protein. These results are similar to those obtained with other biopolymers [19]. We found that the keratin loading could be reduced to 2 mg without detriment in the product yield (data not shown). However, 10 mg were used in most experiments for practical reasons. In addition, the reaction can be scaled up (i.e., 1 mmol of 1a) without detriment on the product yield.

Table 1.
Initial solvent screening for the nitroaldol (Henry) reaction between 1a and 2a a.
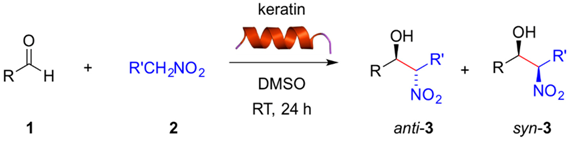
With these preliminary results in hand, we examined the substrate scope of the reaction using different aldehydes in combination with nitromethane or nitroethane both in DMSO (Table 2) and H2O/TBAB (Table 3) at room temperature. Aromatic aldehydes bearing strong electron-withdrawing substituents were easily converted in DMSO into the corresponding β-nitroalcohols 3a–f in excellent yields regardless the position of the substituent (ortho-, meta-, para-) (Table 2, entries 1–6). Weaker acceptor substituents afforded the corresponding product 3 in moderate yields (Table 2, entries 7, 8, 10, 12, 15). Interestingly, here the meta-position (Table 2, entry 9) of the substituent seemed to be more favored, giving rise to 3i in good yield and twice as much than the para-substituted substrate (Table 2, entry 8). In contrast, benzaldehyde or aromatic aldehydes bearing electron-donating substituents yielded only trace amounts of the expected product (Table 2, entries 11, 13,14). Although we did not perform these experiments under longer reaction times and/or higher temperatures, it is expected that these factors could enhance to some extent the yields as we have observed with other biopolymers [19]. Heteroaromatic aldehydes such as 2-pyridinecarboxaldehyde (Table 2, entry 16) lead to the desired product in excellent yield, whereas furfural (Table 2, entry 18) and 1-naphthaldehyde (Table 2, entry 19) proceeded only with low yields.

Table 2.
Keratin-catalyzed nitroaldol (Henry) reaction in DMSO a.

Table 3.
Keratin-catalyzed nitroaldol (Henry) reaction in H2O/tetrabutylammonium bromide (TBAB) a.
Regarding the nature of the nucleophile, the use of nitroethane instead of nitromethane provided slightly higher yields of the desired product albeit with no or little diastereoselectivity favoring the syn diastereomer (Table 2, entries 2, 6, 17 vs. 1, 5, 16, respectively). Slight anti-diastereoselectivy was observed only with 3-nitrobenzaldehyde (Table 2, entry 4). Relative configurations were assigned by comparison with 1H-NMR data reported in the literature. For instance, in the model reaction between 1a and 2b, the anti diastereomer is characterized by a doublet at 4.85 ppm (J = 8.3 Hz), whereas the syn diastereomer shows the doublet at 5.41 ppm (J = 2.4 Hz). These results are similar to those obtained with other biopolymers such as gelatin [19] and suggest that the acidity of the nitroalkane (nitroethane pKa = 8.6; nitromethane pKa = 10.2) constitutes in most cases here a more important factor than steric effects [22]. It is also worth mentioning that aliphatic aldehydes such as isobutyraldehyde were not converted (data not shown). Moreover, despite the α-helical chiral structure of the keratin, HPLC analysis of the reaction mixtures showed no enantioselection, as we have previously observed with other biocatalysts in the same reaction [19,20,21].
Although the desired β-nitroalcohols 3 were also obtained in H2O/TBAB, the reactions took two times longer than in DMSO to achieve similar conversions. In H2O/TBAB the substituent position in the aldehyde 1 was found to have higher influence than in DMSO. For example, strong electron-withdrawing substituents in ortho- and para-position favored the reaction (Table 3, entries 1 and 5), whereas a significant decrease of the yield was observed with the same substituent placed in meta-position (Table 3, entry 3). The reasons for the differences observed in H2O/TBAB vs. DMSO may be related to the solubilization of the reactants and/or involve important variations in the mechanistic pathways, which remain unclear and will be investigated in future research. Furthermore, the expected products were obtained in good yields also with 4-cyanobenzaldehyde (Table 3, entry 12) and 2-pyridine carboxaldehyde (Table 3, entry 17). Other aldehydes were converted less efficiently. Furthermore, the use of nitroethane instead of nitromethane provided significant higher yields of the desired product (Table 3, entries 2, 6, 13, 18 vs. 1, 5, 12, 17, respectively) albeit with almost negligible diastereoselectivity (Table 3, entries 6, 18). It is worth to mention that the replacement of TBAB by other ionic materials such as 1-butyl-3-methylimidazolium hexaflorophosphate (BMIM-PF6) afforded the desired compound albeit in lower yield even at longer reaction time (Table 3, entry 1).
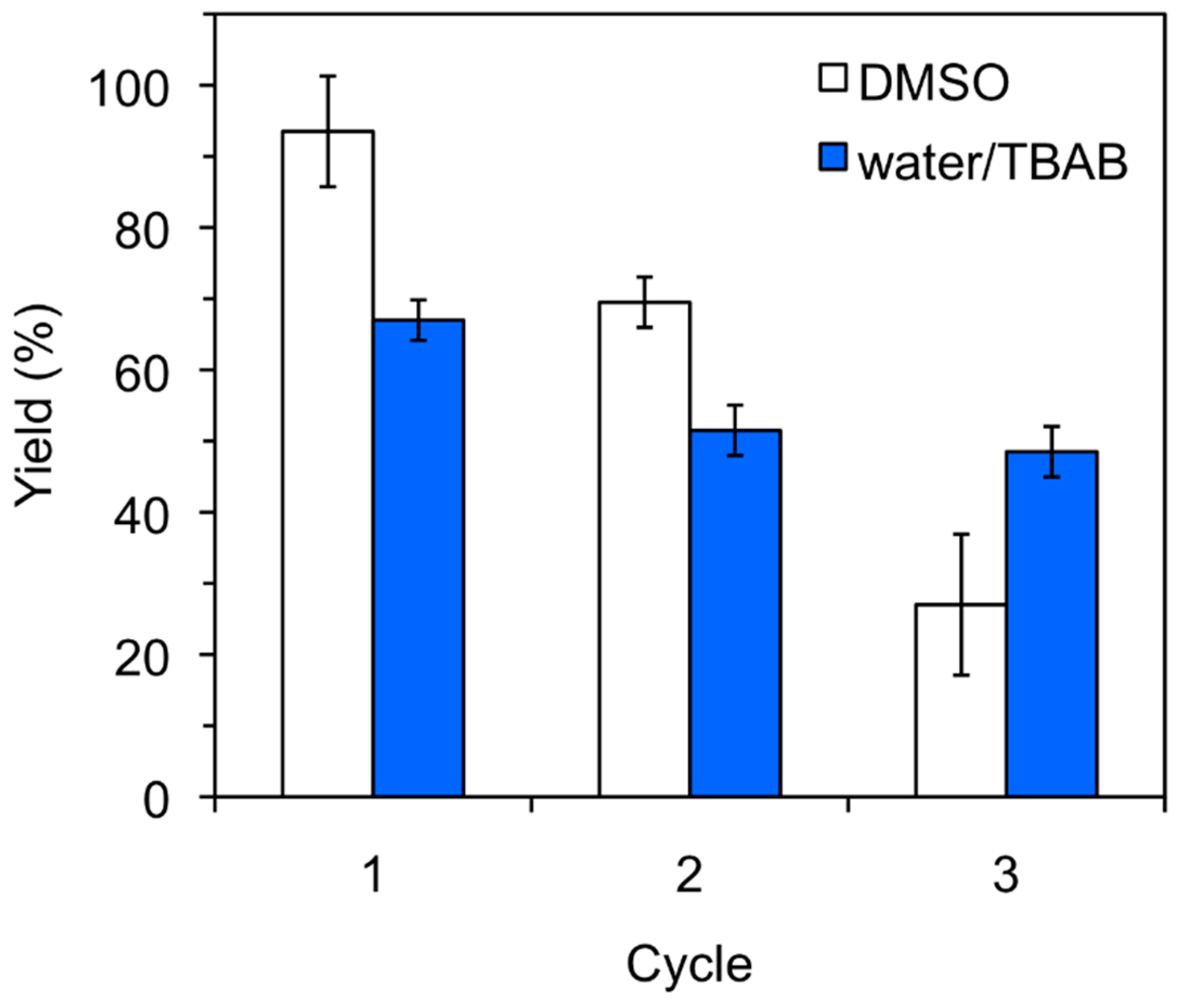
The heterogeneous nature of the protein catalyst allowed its recovery from the reaction mixture and its reuse for further cycles. However, a gradual deactivation of the catalyst could be observed in both organic and aqueous media, although the reduction of the catalytic activity was clearly more pronounced in DMSO (Figure 1). Such behavior has also been observed with other biopolymers [19,20,21] and could be associated to loss of catalyst loading (e.g., inefficient isolation by filtration) after each cycle and/or formation of intermediate linear or cyclic aminals that could block catalytic sites of the protein. These potential competitive processes are somehow more critical in DMSO than in aqueous solution; however, the reasons behind these differences remain unclear.
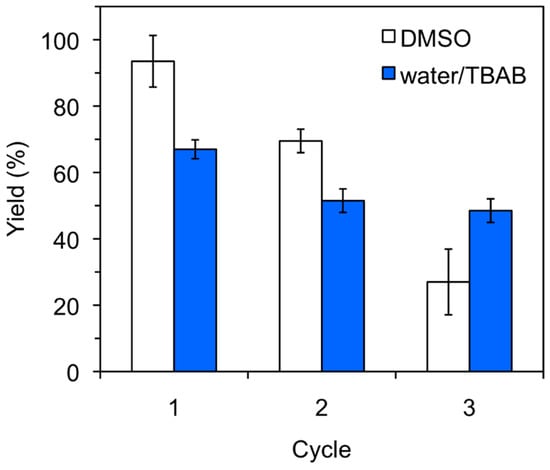
Figure 1.
Typical recycling experiments for keratin-catalyzed nitroaldol (Henry) reaction in DMSO and H2O/TBAB. Reaction conditions: 4-Nitrobenzaldehyde (1a, 15.1 mg, 0.1 mmol), nitromethane (2a, 54 μL, 1.0 mmol), solvent (0.5 mL), keratin powder (10 mg), room temperature, 24–48 h. Yields correspond to 1H-NMR values obtained from at least two experiments. The error bars represent the standard deviation of the measurements. For the experiments in H2O/TBAB, addition of new TBAB after each cycle (i.e., 32.2 mg, 0.1 mmol) was necessary in order to ensure a constant concentration during the reactions as confirmed by NMR analyses of the reaction mixtures.
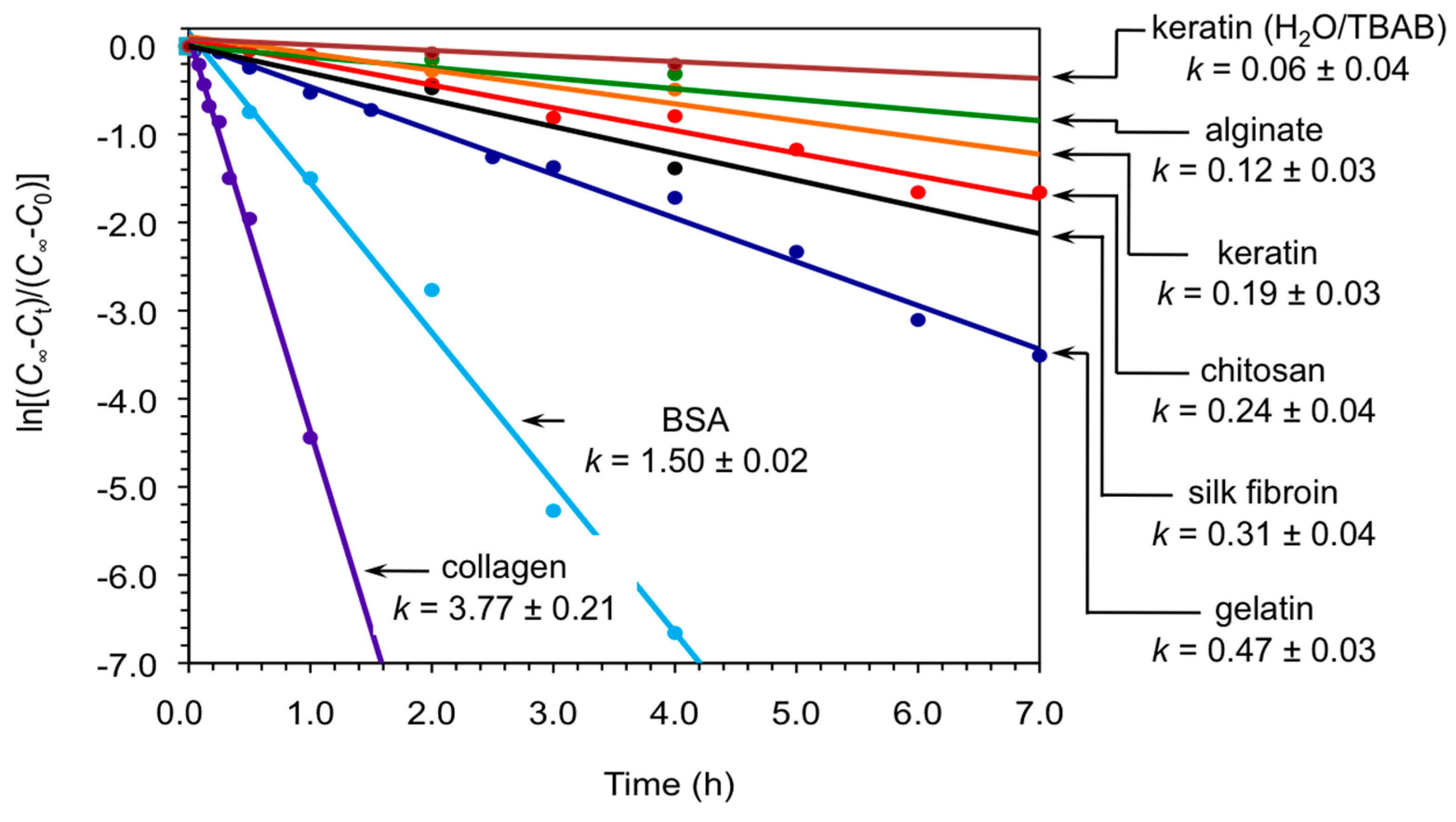
At this point, we carried out kinetic analyses of the model reaction between 1a and 2a catalyzed by keratin in both DMSO and H2O/TBAB under the described conditions. The results were compared with the performance of other biopolymers as biocatalysts for the same reaction that we have previously studied in our group and others (i.e., chitosan [18], gelatin [19], collagen [19], bovine serum albumin (BSA) [19,23], silk fibroin [20] and alginate [21]) under optimized conditions. Figure 2 shows the first-order kinetics corresponding to each biopolymer within the first hours of reaction. In general, powdered keratin displayed rate constants in the range of aerogel calcium alginate [21] and significantly below the other biopolymers demonstrating the possibility of fine-tuning the kinetics of the nitroaldol (Henry) reaction by appropriate selection of the biopolymer and solvent system. It is worth mentioning that although these biopolymers are not superior to standard base catalysts such as tetramethylethylenediamine [16], the former avoided the formation of byproducts and allowed to work under ecofriendly and heterogeneous conditions. However, far beyond the interest of these materials as standard catalyst, their mechanism of action to promote C-C bond formation reactions may be more relevant within the context of biological evolution.
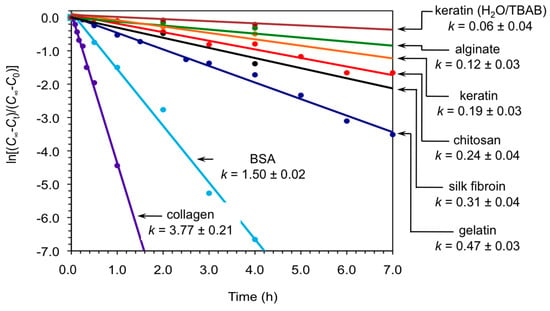
Figure 2.
First-order kinetics plots for the nitroaldol (Henry) reaction between 1a and 2a catalyzed by different biopolymers in powder form. Unless otherwise indicated, reactions were made in DMSO as described in the text and experimental section. Apparent rate constants are given in units of h−1. Each data point represents the average of at least two independent measurements. Cinfi = final concentration, at infinite time; Ct = concentration at given time t; C0 = initial concentration at t = zero time.
3. Materials and Methods
3.1. Materials
Unless otherwise indicated, analytical grade solvents and reactants were commercially available and used as received without further purification. Deionized water was used for experiments in aqueous solutions. Aldehydes (purity by GC > 98%) were purchased from TCI Europe (TCI Europe, Zwijndrecht, Belgium). Keratin extracted from wool (CAS 69430-36-0, Cat. Nr. AB 250197) was purchased from ABCR. Tetrabutylammonium bromide (CAS 1643-19-2, Cat Nr. 86860) was purchased from Fluka (Fluka Chemical Corp., Milwaukee, WI, USA). 1-Buthyl-3-methylimidazolium hexafluorophosphate (BMIM-PF6) was purchased from TCI Europe (CAS 174501-64-5, Cat. Nr. B2320).
3.2. Methods
1H-NMR spectra were recorded on Avance 300 or Avance 400 spectrometers (Bruker, Billerica, MA, USA) at 25 °C. Chemical shifts for 1H-NMR were reported as δ, parts per million, relative to external standards. Yields were determined by 1H-NMR analyses of the crude product in CDCl3 using dimethyl acetamide (0.1 mmol, 9.2 µL) as internal standard after complete work-up of the reaction. Enantiomeric excess was evaluated by chiral HPLC (Agilent Technologies, Santa Clara, CA, USA) (column: Phenomenex (Phenomenex, Aschaffenburg, Germany) Lux Cellulose-1, 4.6 mm × 250 mm, 5 µm, eluents: n-heptane, i-propanol 70:30, flow: 0.5 mL/min). Relative configurations were assigned by comparison with 1H-NMR data reported in the literature. For example, in the model reaction between 1a and 2b, the anti diastereomer was identified by a doublet at 4.85 ppm (J = 8.3 Hz), whereas the syn diastereomer showed the doublet at 5.41 ppm (J = 2.4 Hz). For kinetics calculations, the 1H-NMR analyses of the reaction mixtures were performed in the presence of an internal standard as above indicated. In general, given yield values correspond to the average of at least two independent measurements with STDV ± 2%–4%. Among various kinetics models, the straight lines shown in the kinetics plots correspond to the best fit of the first-order model (e.g., (nitromethane) ≥ (aldehyde)).
3.3. General Procedure for Keratin-Catalyzed Nitroaldol (Henry) Reaction
Keratin (10 mg) was added in one portion to a mixture of 4-nitrobenzaldehyde (1a, 0.1 mmol, 15.1 mg), nitromethane (2a, 1.0 mmol, 54 μL) and solvent (0.5 mL) placed into a 4-mL screw-capped vial. The resulting reaction mixture was gently shaked in an orbital shaker (150 rpm) for the appropriate time at room temperature. After completion, water (1 mL) was added. The reaction mixture was extracted with EtOAc (4 × 1.5 mL), dried over anhydrous sodium sulfate, filtrated and evaporated under reduced pressure. Yield was determined by 1H-NMR of the crude product in CDCl3 using dimethylacetamide (9.2 μL, 0.1 mmol) as internal standard. All β-nitroalcohol products are known and the spectroscopic data obtained from NMR analysis of the reaction mixtures were in agreement with those reported in the literature.
3.4. Typical Recycling Procedure
After reaction and extraction with EtOAc, the aqueous phase with remaining catalyst was freeze-dried prior addition of the reaction substrates and solvent for the next run.
3.5. Kinetic Studies
Reaction conversions were unequivocally calculated by 1H-NMR analysis of the reaction mixtures according to the integration of characteristic signals of the species in the reaction mixture in the presence of a suitable internal standard. Each experimental point represents the average of at least two experiments. Among various kinetics models, lines presented in the kinetic plots show best-fits of the first-order model for each case (i.e., (NO2R) ≥ (aldehyde)). Due to the fact that not all reactions reached 100% yield, data fitting was made according to the variation of ln((Ct − C∞ )/(C∞ − C0)) with time, where Ct is the concentration at a given time t; C∞ the final concentration (at infinite time) and C0 the initial concentration (at t = zero time). For reaction conversions close to 100%, plots of ln(Ct/C0) versus time provided consistent results (C∞ = 0). Under these considerations, minor differences were observed between the exponential and linear fits. All errors reported for the rate constants k were calculated by graphical analysis. Kinetics data for other biopolymers have been previously reported by us and were used for comparison: Chitosan [18], gelatin [19], collagen [19], BSA [19], freeze-dried silk fibroin [20] and calcium alginate aerogel [21]. Sample preparation and details of the reactions can be found in the corresponding references.
4. Conclusions
In conclusion, we have demonstrated that keratin proteins are able to promote C-C bond formation via the nitroaldol (Henry) reaction between various aldehydes and nitroalkanes. Appropriate control experiments demonstrated the intrinsic catalytic activity of the keratin. Both aromatic and heteroaromatic aldehydes having strong or moderate electron-withdrawing groups were converted exclusively into the corresponding β-nitroalcohol products in both DMSO and in water in the presence of TBAB as phase transfer catalyst. In contrast, aromatic aldehydes bearing electron-donating groups and aliphatic aldehydes showed poor or no conversion, respectively. In general, the reactions in water/TBAB required twice the amount of reaction time than in DMSO to achieve similar conversions. Although the heterogeneous nature of the reaction allowed for the recovery and reuse of the keratin, a gradual deactivation of the catalyst was observed after each cycle. Comparative kinetic studies with other biopolymers revealed that the rate of the nitroaldol (Henry) reaction strongly depends on the nature of the biopolymer. The effect of different forms of keratins on the ability to catalyze this and other C-C bond forming reactions, as well as detailed mechanistic studies in both organic and aqueous medium, including different ionic liquids, are currently under study in our laboratories and the results will be reported in due course.
Acknowledgments
D.D.D. thanks the Deutsche Forschungsgemeinschaft (DFG) for the Heisenberg Professorship Award. A.P. thanks the SINCHEM program for her PhD Grant. SINCHEM is a Joint Doctorate program selected under the Erasmus Mundus Action 1 Program—FPA 2013-0037.
Author Contributions
M.H. and A.P. performed the experiments. F.Q. and N.T. contributed to results discussion. D.D.D. designed the experiments. M.H. and D.D.D. wrote the paper. All authors took part in data analysis and discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Kornillowics-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, M.S.; Chung, B.M.; Leahy, D.J.; Coulombe, P.A. Structural basis for heteromeric assembly and perinuclear organization of keratin filaments. Nat. Struct. Mol. Biol. 2012, 19, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Idler, W.W.; Steven, A.C.; Roop, D.R.; Steinert, P.M. The complete sequence of the human intermediate filament chain keratin 10. Subdomainal divisions and model for folding of end domain sequences. J. Biol. Chem. 1988, 263, 15584–15589. [Google Scholar] [PubMed]
- O′Donnell, J. The complete amino acid sequence of a feather keratin from Emu (Dromaius Novae-Hollandiae). Aust. J. Bioi. Sci. 1973, 26, 415–437. [Google Scholar] [CrossRef]
- Arai, K.M.; Takahashi, R.; Yokote, Y.; Akahane, K. Amino-acid sequence of feather keratin from fowl. Eur. J. Biochem. 1983, 132, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Rice, R.H.; Roop, D.R.; Trus, B.L.; Steven, A.C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature 1983, 302, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Tonin, C.; Aluigi, A.; Zoccola, M. Characterisation of keratin biomass from butchery and wool industry wastes. J. Mol. Struct. 2009, 938, 35–40. [Google Scholar]
- Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008, 29, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.M.; Ellenburg, M.D.; de Guzman, R.C.; van Dyke, M. Keratin hydrogels support the sustained release of bioactive ciprofloxacin. J. Biomed. Mater. Res. A 2011, 98A, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Hengchang, M.; Zhikang, B.; Guobin, H.; Ningning, Y.; Yufei, X.; Zengming, Y.; Wei, C.; Yuan, M. Nanoparticulate palladium catalyst stabilized by supported on feather keratin for Suzuki coupling reaction. Chin. J. Catal. 2013, 52, 578–584. [Google Scholar]
- Karthikeyan, R.; Balaji, S.; Sehgal, P.K. Industrial applications of keratins—A review. J. Sci. Ind. Res. 2007, 52, 710–715. [Google Scholar]
- Luzzio, F.A. The Henry reaction: Recent examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; Laso, A. Recent advances in the catalytic asymmetric nitroaldol (Henry) reaction. Eur. J. Org. Chem. 2007, 2007, 2561–2574. [Google Scholar] [CrossRef]
- Boruwa, J.; Gogoi, N.; Saikia, P.P.; Barua, N.C. Catalytic asymmetric Henry reaction. Tetrahedron Asymmetry 2006, 17, 3315–3326. [Google Scholar] [CrossRef]
- Sharma, K.K.; Biradar, A.V.; Asefa, T. Substituent- and catalyst-dependent selectivity to aldol or nitrostyrene products in a heterogeneous base-catalyzed Henry reaction. ChemCatChem 2010, 2, 61–66. [Google Scholar] [CrossRef]
- Ballini, R.; Gabrielli, S.; Palmieri, A.; Petrini, M. Nitroalkanes as key compounds for the synthesis of amino derivatives. Curr. Org. Chem. 2011, 15, 1482–1506. [Google Scholar] [CrossRef]
- Kühbeck, D.; Saidulu, G.; Reddy, K.R.; Díaz, D.D. Critical assessment of the efficiency of chitosan biohydrogel beads as recyclable and heterogenous organocatalyst for C-C bond formation. Green Chem. 2012, 14, 378–392. [Google Scholar] [CrossRef]
- Kühbeck, D.; Dhar, B.B.; Schön, E.-M.; Cativiela, C.; Gotor-Fernández, V.; Díaz, D.D. C-C bond formation catalyzed by natural gelatin and collagen proteins. Beilstein J. Org. Chem. 2013, 9, 1111–1118. [Google Scholar]
- Kühbeck, D.; Ghosh, M.; Gupta, S.S.; Díaz, D.D. Investigation of C-C Bond formation mediated by Bombyx Mori silk fibroin materials. ACS Sustain. Chem. Eng. 2014, 2, 1510–1517. [Google Scholar] [CrossRef]
- Kühbeck, D.; Mayr, J.; Häring, M.; Hofmann, M.; Quignard, F.; Díaz, D.D. Evaluation of the nitroaldol reaction in the presence of metal ion-crosslinked alginates. New J. Chem. 2015, 39, 2306–2315. [Google Scholar]
- Akutu, K.; Kabashima, H.; Seki, T.; Hattori, H. Nitroaldol reaction over solid base catalysts. Appl. Catal. A 2003, 247, 65–74. [Google Scholar] [CrossRef]
- Busto, E.; Gotor-Fernández, V.; Gotor, V. Protein-mediated nitroaldol addition in aqueous media. Catalytic promiscuity or unspecific catalysis? Org. Process Res. Dev. 2011, 15, 236–240. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).