Corynoline Isolated from Corydalis bungeana Turcz. Exhibits Anti-Inflammatory Effects via Modulation of Nfr2 and MAPKs
Abstract
:1. Introduction
2. Results
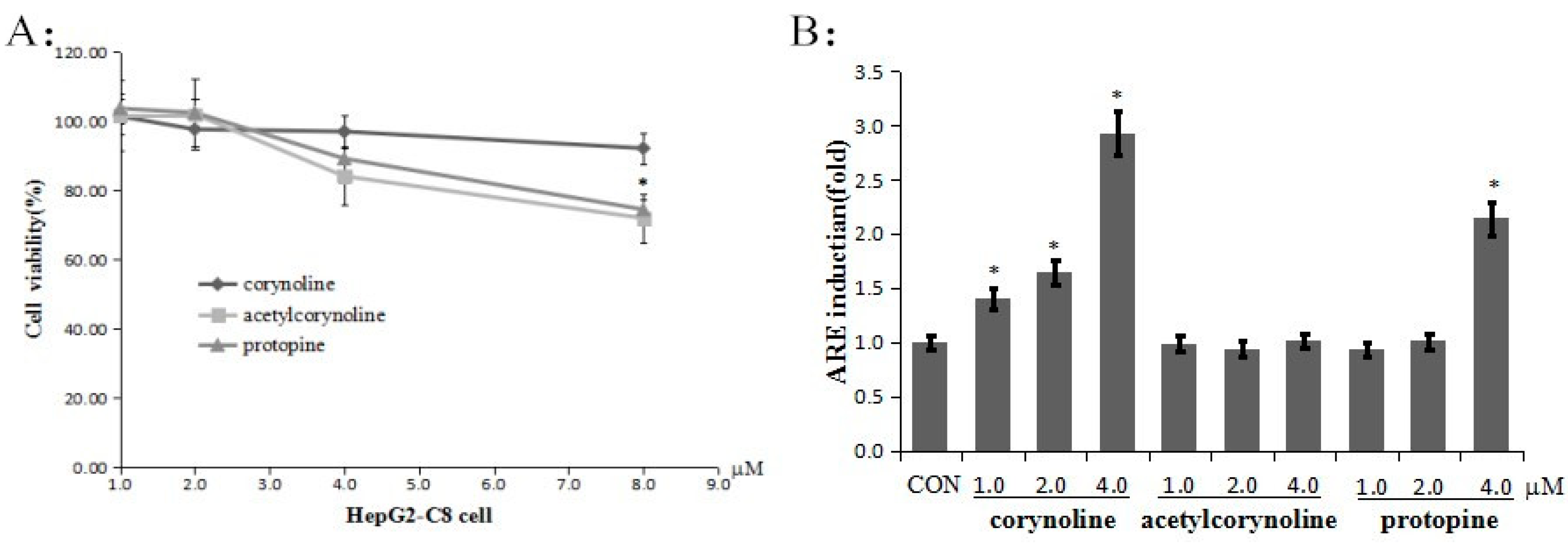
2.1. Corynoline, Acetylcorynoline and Protopine Induce Transcriptional Activation of ARE-Luciferase
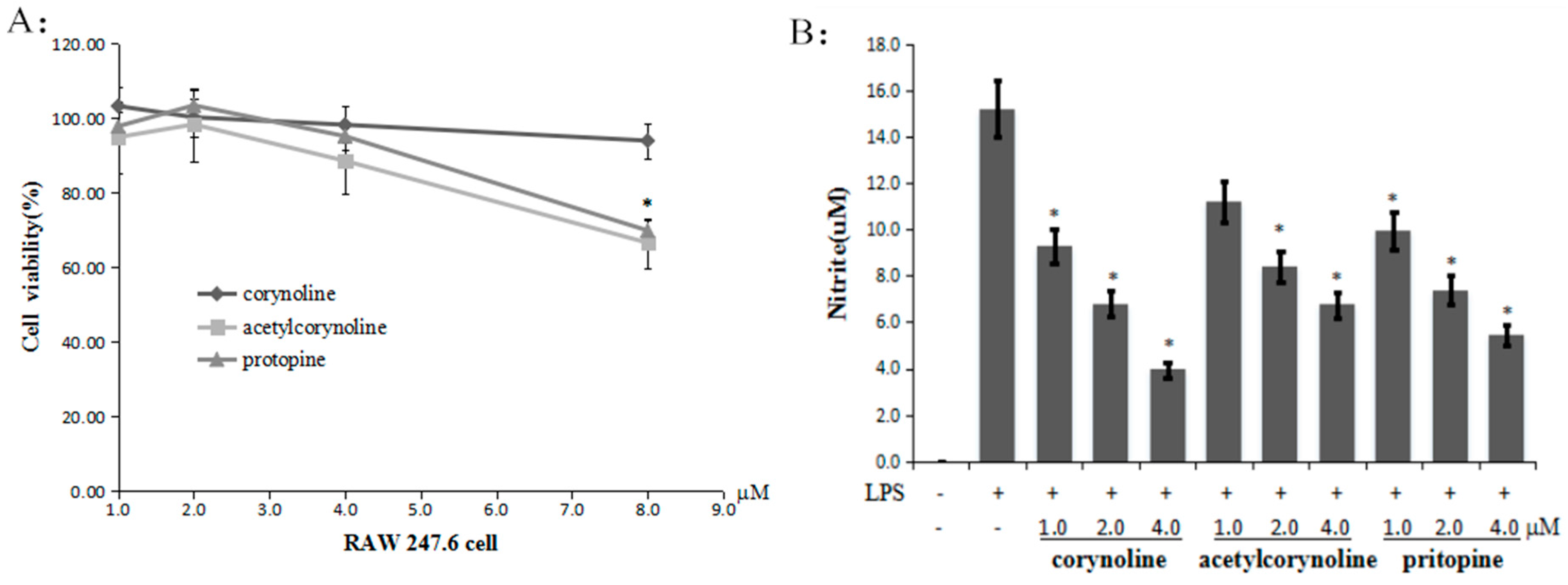
2.2. Corynoline, Acetylcorynoline and Protopine Inhibit NO Production in LPS-Induced RAW264.7 Cells
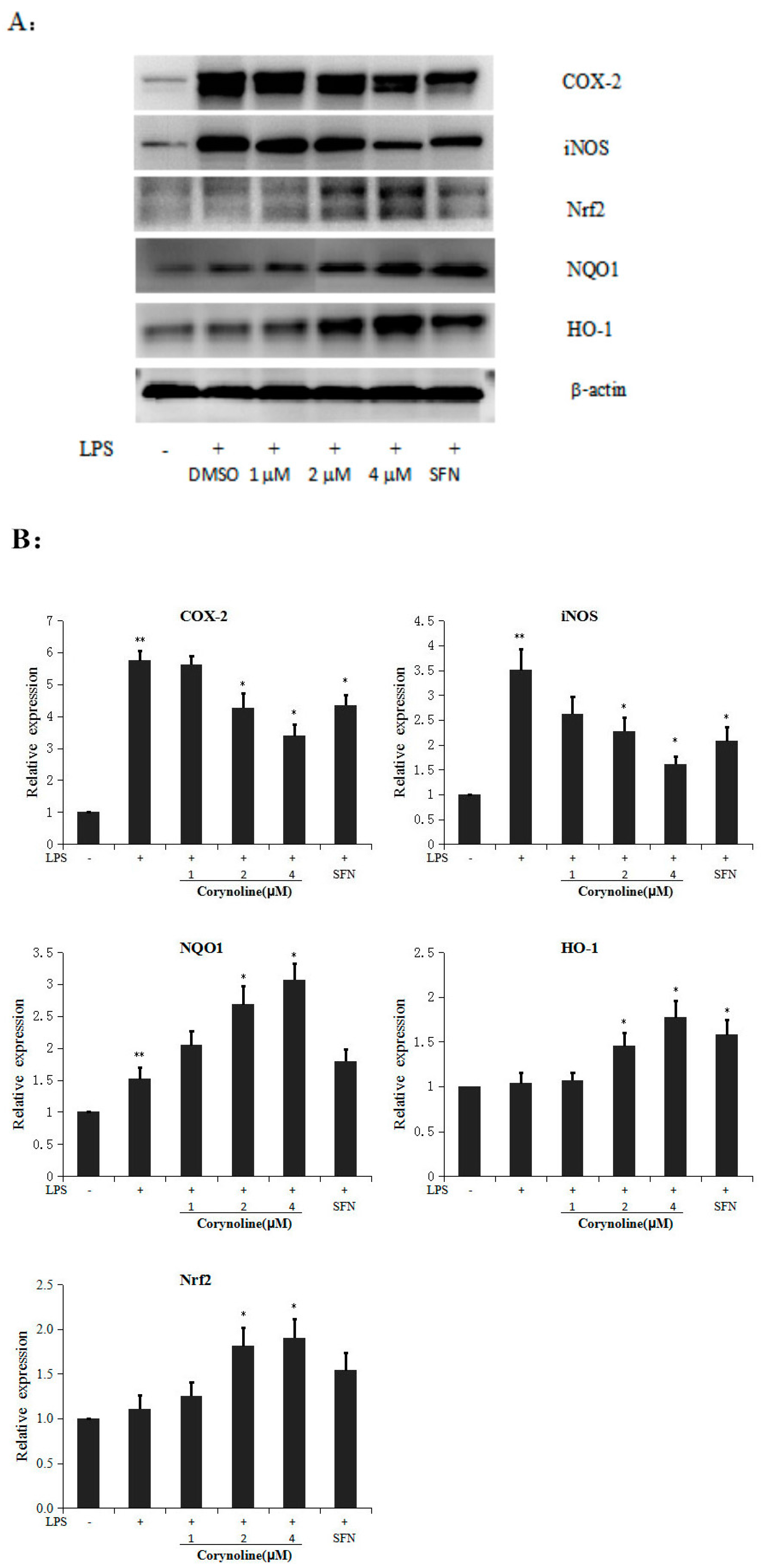
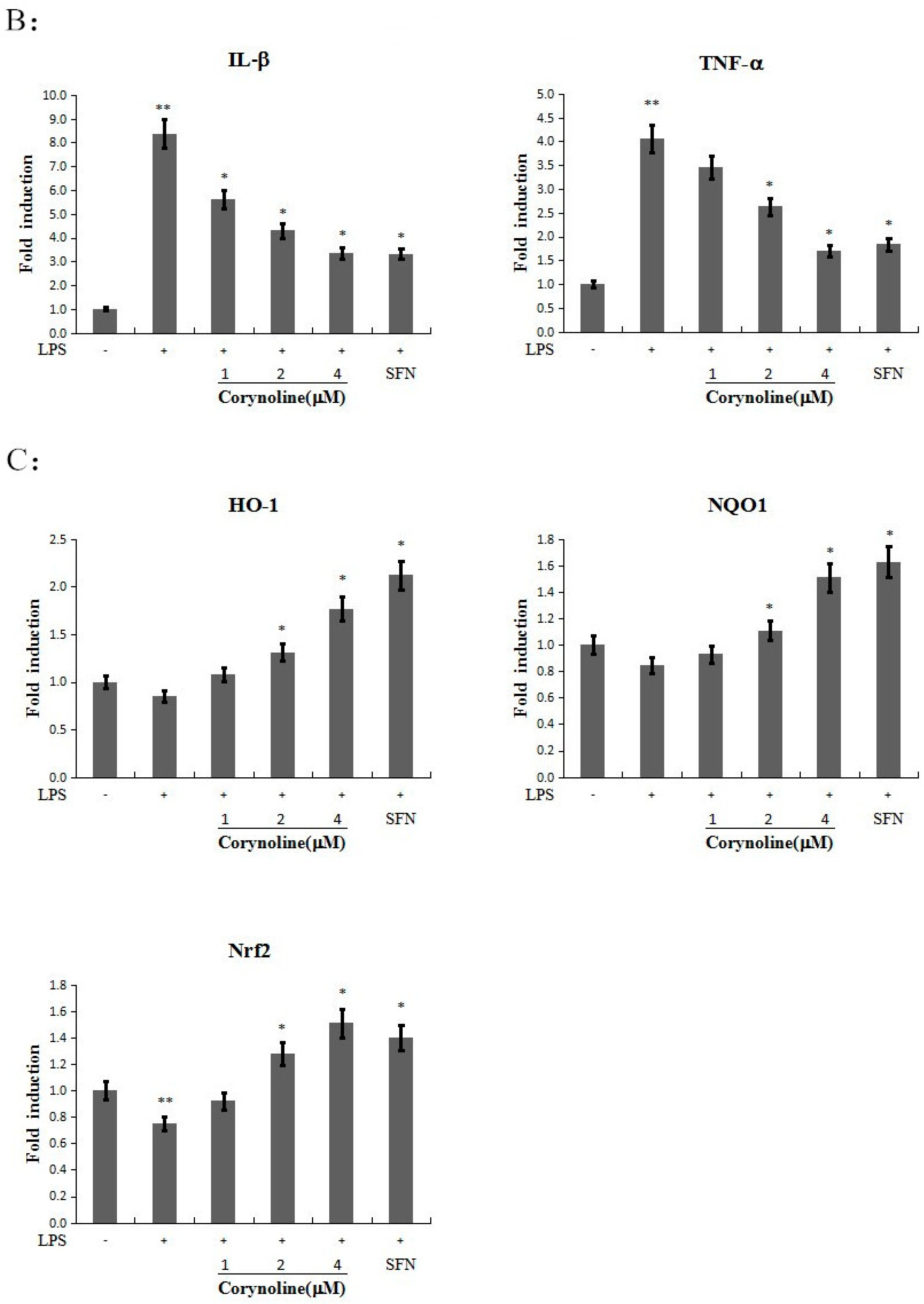
2.3. Corynoline Up-Regulates the Expression of Nrf2, HO-1 and NQO1 at Both the mRNA and Protein Levels in LPS-Induced RAW264.7 Cells
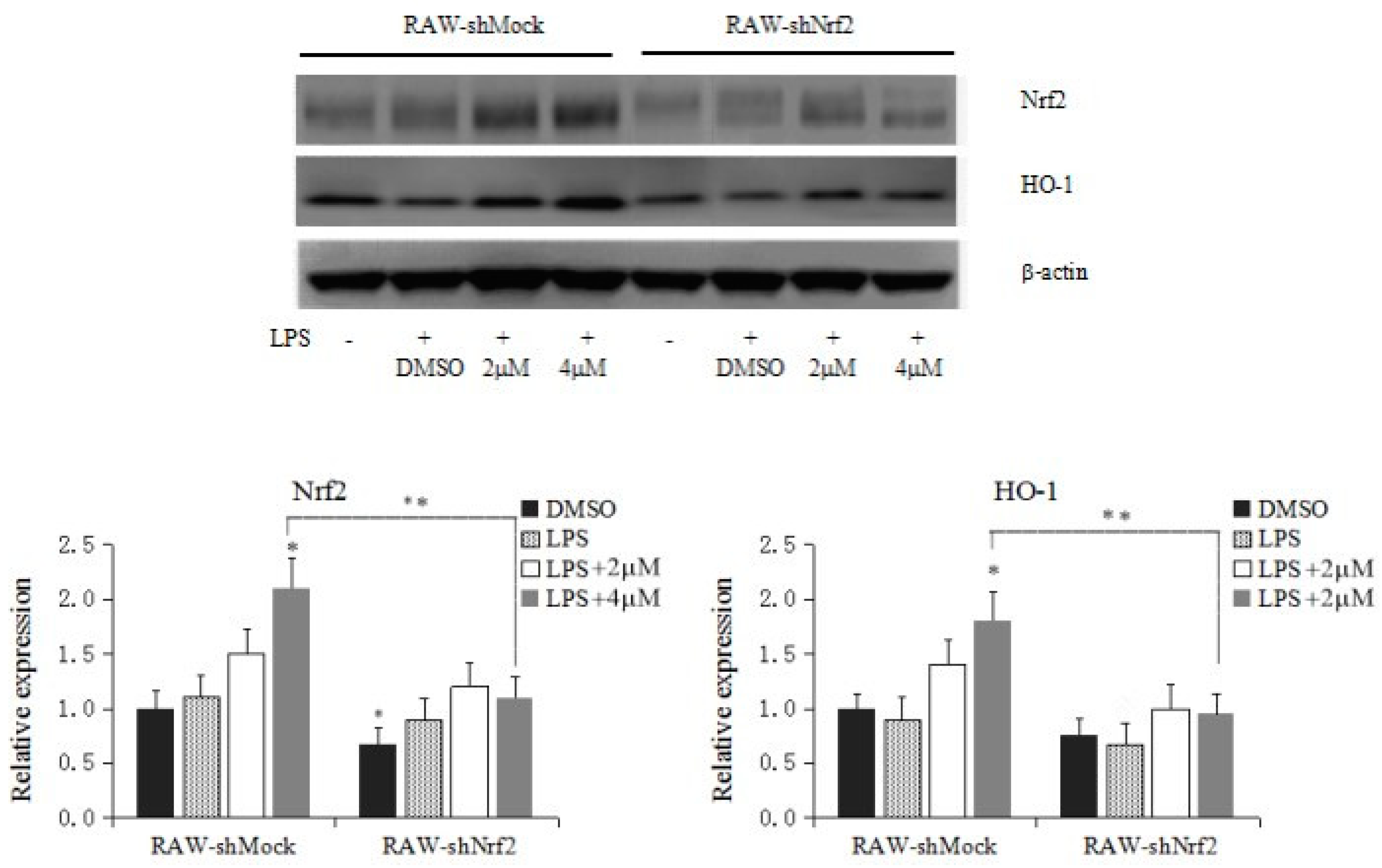
2.4. Knockdown of Nrf2 Decreases Corynoline-Induced Protein Expression of Nrf2 and Nrf2 Target Enzymes
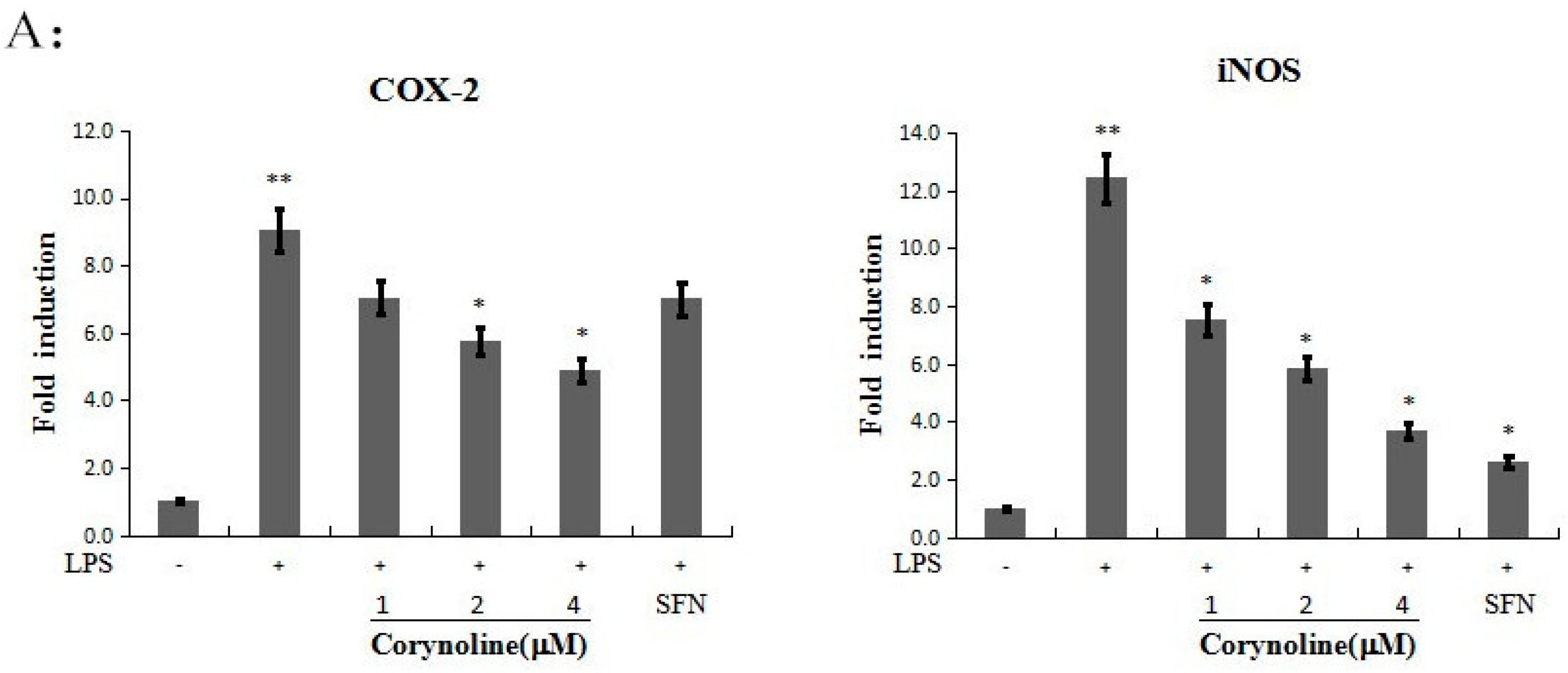
2.5. Corynoline Down-Regulates the Expression of iNOS and COX-2 at the mRNA and Protein Levels
2.6. Corynoline Down-Regulates the Expression of IL-1β and TNF-α mRNA and Protein in LPS-Induced RAW264.7 Cells
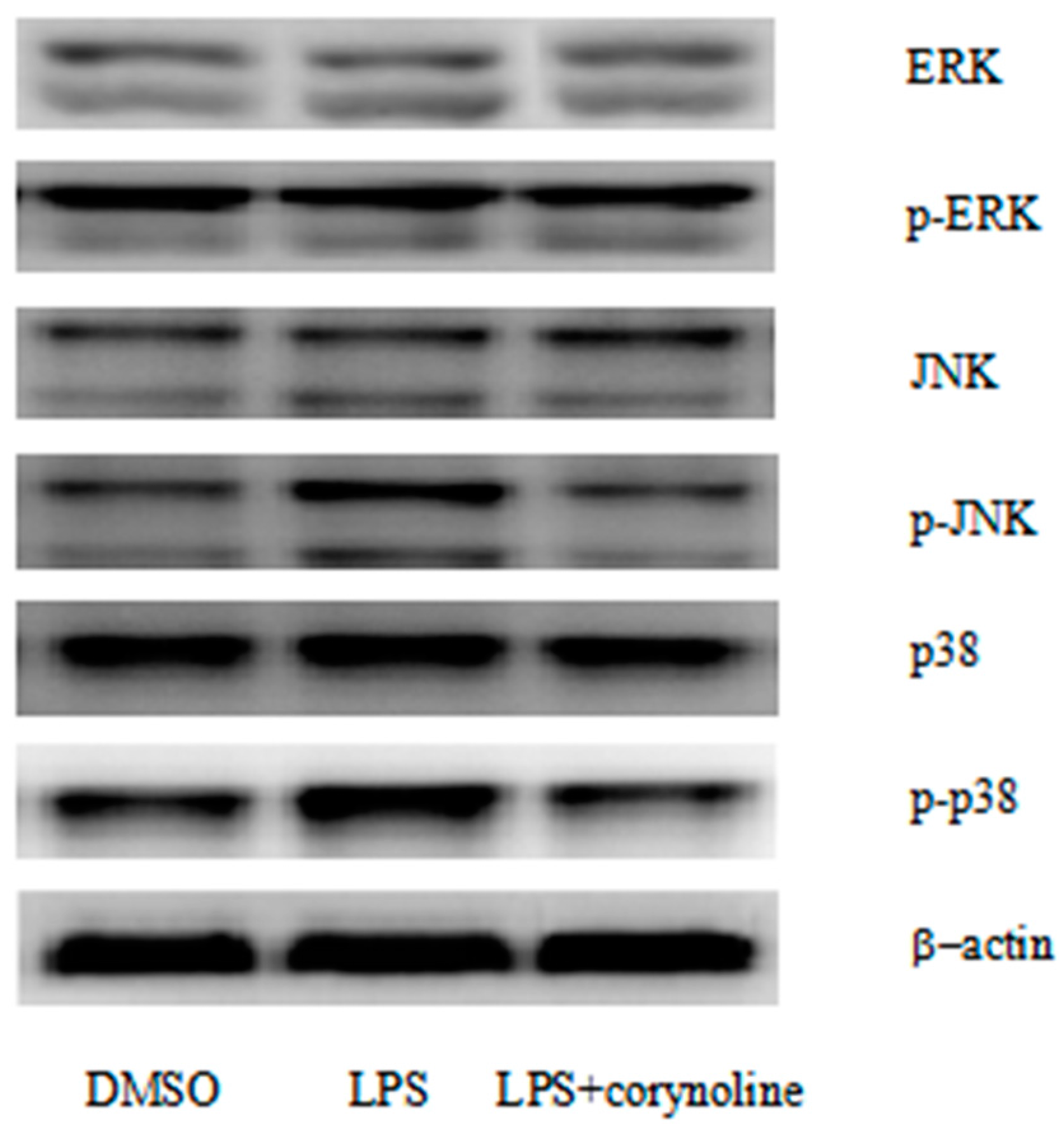
2.7. Corynoline Inhibits the Phosphorylation of p38 and JNK in LPS-Induced RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
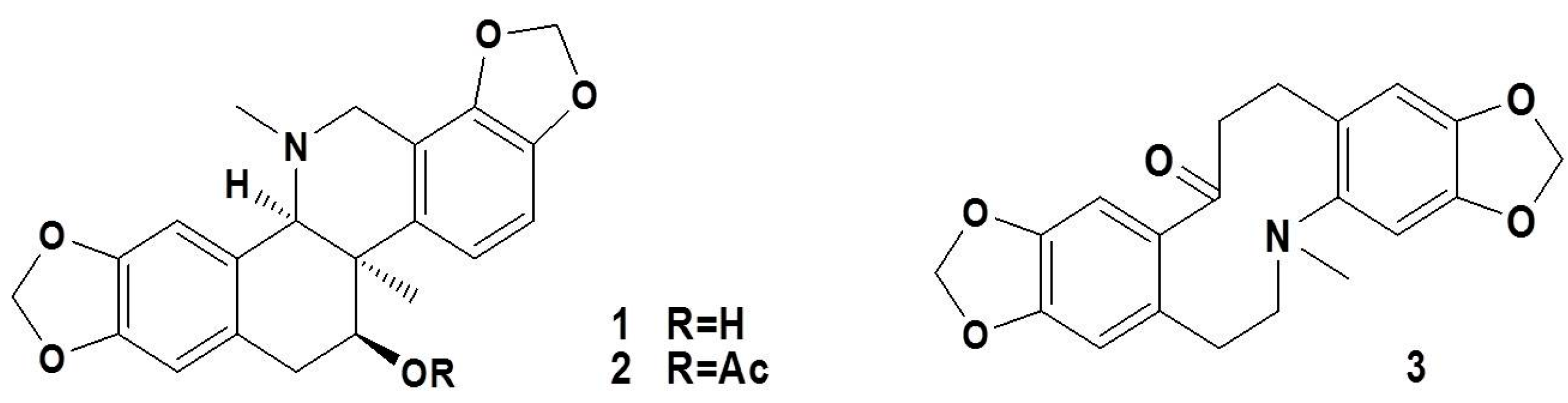
4.2. Extraction and Isolation of Alkaloids
4.3. Cell Culture and Treatments
4.4. Cell Viability Assay
4.5. Evaluation of ARE Reporter Gene Activity by Luciferase Assay
4.6. Evaluation of the Increase in NO Production Using the Nitrite Assay
4.7. Protein Lysate Preparation and Western Blotting
4.8. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.9. Cytokine Measurements
4.10. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. Cancer: Inflammation by remote control. Nature 2005, 435, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.M.; Varghese, S.; Xu, H.; Alexander, H.R. Interleukin-1 and cancer progression: The emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J. Transl. Med. 2006, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int. J. Med. Microbiol. 2007, 297, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW264.7 macrophages. J. Biol. Chem. 2009, 381, 112–117. [Google Scholar]
- Chun, K.S.; Surh, Y.J. Signal transduction pathways regulating cyclooxygenase-2 expression: Potential molecular targets for chemoprevention. Biochem. Pharmacol. 2004, 68, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, H.; Pautz, A.; Linker, K.; Schwarz, P.M. Regulation of the expression of inducible nitric oxide synthase. Eur. J. Pharmacol. 2004, 500, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.J.; Shin, J.S.; Choi, J.H.; Back, N.I.; Chung, H.G.; Lee, K.T. Quaternary alkaloid, pseudocoptisine isolated from tubers of Corydalis turtschaninovi inhibits LPS-induced nitric oxide, PGE(2), and pro-inflammatory cytokines production via the down-regulation of NF-kappaB in RAW264.7 murine macrophage cells. Int. Immunopharmacol. 2009, 9, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Lenon, G.B.; Li, C.G.; Xue, C.C.; Thien, F.C.; Story, D.F. Inhibition of release of vasoactive and inflammatory mediators in airway and vascular tissues and macrophages by a chinese herbal medicine formula for allergic rhinitis. Evid. Based Complement. Altern. Med. 2007, 4, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, K.R.; Su, Z.Y.; Boyanapalli, S.S.; Barman, D.N.; Huang, M.T.; Chen, L.; Magesh, S.; Hu, L.; Kong, A.N. In vitro and in vivo anti-inflammatory effects of a novel 4,6-bis ((E)-4-hydroxy-3-methoxystyryl)-1-phenethylpyrimidine-2(1H)-thione. Chem. Res. Toxicol. 2014, 27, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S.; Jeong, W.S.; Kong, A.N. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat. Res. 2004, 555, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Lee, J.H.; Khor, T.O.; Wu, T.Y.; Li, G.X.; Chan, J.; Yang, C.S.; Kong, A.N. Nrf2 knockout enhances intestinal tumorigenesis in Apcmin/+ mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation. Mol. Carcinog. 2014, 53, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Iqbal, A.J.; Maione, F. Natural anti-inflammatory products/compounds: Hopes and reality. Mediat. Inflamm. 2015, 2015, 374239–374241. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Dar, G.H.; Koul, S.T.; Naqshi, A.R.; Khuroo, A.A.; Malik, A.H. A new species of Corydalis DC. (Fumariaceae) from Kashmir, North-west Himalaya, India. Taiwania 2011, 56, 305–308. [Google Scholar]
- Muhammad, N.; Shrestha, R.L.; Adhikari, A.; Wadood, A.; Khan, H.; Khan, A.Z.; Maione, F.; Mascolo, N.; de Feo, V. First evidence of the analgesic activity of govaniadine, an alkaloid isolated from Corydalis govaniana Wall. Nat. Prod. Res. 2015, 29, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Xie, Z.; Cai, T.; Wu, P.; Xue, P.; Chen, X. Preparative isolation of alkaloids from Corydalis bungeana Turcz. by high-speed counter-current chromatography using stepwise elution. J. Sep. Sci. 2011, 34, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.H.; Wang, Y.C.; Liu, S.P.; Chu, C.L.; Tsai, R.T.; Ho, Y.C. Acetylcorynoline impairs the maturation of mouse bone marrow-derived dendritic cells via suppression of IκB kinase and mitogen-activated protein kinase activities. PLoS ONE 2013, 8, 58398–58408. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China (2015 Edition); Chemical Industry Press: Beijing, China, 2015; p. 201. [Google Scholar]
- Dong, Z.B.; Zhang, Y.H.; Zhao, B.J.; Li, C.; Tian, G.; Niu, B.; Qi, H.; Feng, L.; Shao, J.G. Screening for anti-inflammatory components from Corydalis bungeana Turcz. based on macrophage binding combined with HPLC. Complement. Altern. Med. 2015, 15, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K. Inhibitory effect of corynoline isolated from the aerial parts of Corydalis incisa on the acetylcholinesterase. Arch. Pharm. Res. 2002, 25, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.U.; Baek, N.I.; Kim, S.H.; Yang, J.H.; Eun, J.S.; Shin, T.Y.; Lim, J.P.; Lee, J.H.; Jeon, H.; Yun, M.Y. Cytotoxic isoquinoline alkaloids from the aerial parts of Corydalis incisa. Arch. Pharm. Res. 2007, 30, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. The role of nitric oxide synthases in lung inflammation. Curr. Opin. Investig. Drugs 2007, 8, 899–909. [Google Scholar] [PubMed]
- He, Z.B.; Chen, P.; Peng, Z.Y.; Jin, L.Y. Effect of Corynoline Isolated from Corydalis bungeana Turcz on Lipopolysaccharides-Induced Sepsis In vivo and In vitro. Trop. J. Pharm. Res. 2014, 13, 81–86. [Google Scholar] [CrossRef]
- Kamigauchi, M.; Noda, Y.; Nishijo, J.; Iwasaki, K.; Tobetto, K.; In, Y.; Tomoo, K.; Ishida, T. Cell adhesion inhibitory activity of (d)-corynoline, a hexahydrobenzo[c]phenanthridine-type alkaloid, and its structure-activity relationship, studied by X-ray crystal structure analysis and molecular docking study. Bioorg. Med. Chem. 2005, 13, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.; Scadding, G.K. Update on the use of nitric oxide as a noninvasive measure of airways inflammation. Rhinology 2009, 47, 115–120. [Google Scholar] [PubMed]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, V.; Magnelli, L.; Gallo, O. Cox-2, iNOS and p53 as play-makers of tumor angiogenesis (review). Int. J. Mol. Med. 1998, 2, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [PubMed]
- Jin, C.Y.; Lee, J.D.; Park, C.; Choi, Y.H.; Kim, G.Y. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta. Pharmacol. Sin. 2007, 28, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Barry, S.P.; Roth, R.J.; Wu, J.J.; Jones, E.A.; Bennett, A.M.; Flavell, R.A. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Figueiredo, C.P.; Pandolfo, P.; Duarte, F.S.; Prediger, R.D.; Passos, G.F.; Calixto, J.B. The role of TNF-alpha signaling pathway on COX-2 upregulation and cognitive decline induced by beta-amyloid peptide. Behav. Brain Res. 2010, 209, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Sun, Y.T.; Chen, J.J.; Chiu, K.T. TNF-α-induced cyclooxygenase-2 expression in human lung epithelial cells: Involvement of the phospholipase C-γ2, protein kinase C-α, tyrosine kinase, NF-κB-inducing kinase, and I-κB kinase 1/2 pathway. J. Immunol. 2000, 165, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.S.; Kim, S.H.; Song, Y.S.; Surh, Y.J. Celecoxib inhibits phorbol ester-induced expression of COX-2 and activation of AP-1 and p38 MAP kinase in mouse skin. Carcinogenesis 2004, 25, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Kong, A.N. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Khor, T.O.; Saw, C.L.; Loh, S.C.; Chen, A.I.; Li, S.S.; Park, J.H.; Cai, L.; Kong, A.N. Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wu, R.T.; Wu, T.; Khor, T.O.; Wang, H.; Kong, A.N. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol. 2008, 76, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Boyanapalli, S.S.; Paredes-Gonzalez, X.; Fuentes, F.; Zhang, C.; Guo, Y.; Pung, D.; Saw, C.L.; Kong, A.N. Nrf2 knockout attenuates the anti-inflammatory effects of phenethyl isothiocyanate and curcumin. Chem. Res. Toxicol. 2014, 27, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; Chen, X.L.; Mackman, N.; Ogborne, R.M.; O’Connell, M.A. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J. Immunol. 2005, 175, 4408–4415. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, M.G.; Lin, G.X.; Ma, Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2 x Keap1 x Cul3 complex and recruiting Nrf2 x Maf to the antioxidant response element enhancer. J. Biol. Chem. 2006, 281, 23620–23631. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; MacEwan, D.J.; O’Connell, M.A. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. 2008, 181, 6730–6737. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Cao, J.; Sacerdoti, D.; Li, X.; Drummond, G. Heme oxygenase: The key to renal function regulation. Am. J. Physiol.-Renal. 2009, 297, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Al-Huseini, L.M.; Aw Yeang, H.X.; Sethu, S.; Alhumeed, N.; Hamdam, J.M.; Tingle, Y.; Djouhri, L.; Kitteringham, N.; Park, B.K.; Goldring, C.E. Nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) modulates dendritic cell immune function through regulation of p38 MAPK-cAMP-responsive element binding protein/activating transcription factor 1 signaling. J. Biol. Chem. 2013, 288, 22281–22288. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Jun, M.; Jeong, W.S. Procyanidins from wild grape (Vitis amurensis) seeds regulate ARE-mediated enzyme expression via Nrf2 coupled with p38 and PI3K/Akt pathway in HepG2 cells. Int. J. Mol. Sci. 2012, 13, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chen, C.; Mo, Y.Y.; Hebbar, V.; Owuor, E.D.; Tan, T.H.; Kong, A.N. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, C.J.; Zhong, M.L.; Jiang, X.C.; Liu, G.F. Chemical Constituents from Corydalis bungeana Turcz. Nat. Prod. Res. Dev. 2013, 25, 1665–1668. [Google Scholar]
- Sample Availability: Samples of the compounds of corynoline, acetylcorynoline and protopine are available from the authors.

| Gene | Forward | Reward |
|---|---|---|
| GAPDH | 5′-TGC TCG AGA TGT CAT GAA GG-3′ | 5′-TGG CGC TCA TCG TAG GCT TT-3′ |
| COX-2 | 5′-TCC TCC TGG AAC ATG GAC TC-3′ | 5′-TGA TGG TGG CTG TTT TGG TA-3′ |
| iNOS | 5′-GTG GTG ACA AGC ACA TTT GG-3′ | 5′-GGC TGG ACT TTT CAC TCT GC-3′ |
| HO-1 | 5′-GCT CGA ATG AAC ACT CTG GAG AT-3′ | 5′-TCC AGA GAG AAA GGA AAC ACA GG-3′ |
| NQO1 | 5′-CAG AAA TGA CAT CAC AGG TGA GC-3′ | 5′-CTA AGA CCT GGA AGC CAC AGA AA-3′ |
| Nrf2 | 5′-GGC AGA GAC ATT CCC ATT TGT AG-3′ | 5′-TCG CCA AAA TCT GTG TTT AAG GT-3′ |
| TNF-α | 5′-ACG GCA TGG ATC TCA AAG AC-3′ | 5′-GGT CAC TGT CCC AGC TT-3′ |
| IL-1β | 5-′GAG TGT GGA TCC CAA GCA AT-3′ | 5′-CTC AGT GCA GGC TAT GCT TT-3′ |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zhang, C.; Wang, Z.; Tang, Z.; Kuang, H.; Kong, A.-N.T. Corynoline Isolated from Corydalis bungeana Turcz. Exhibits Anti-Inflammatory Effects via Modulation of Nfr2 and MAPKs. Molecules 2016, 21, 975. https://doi.org/10.3390/molecules21080975
Yang C, Zhang C, Wang Z, Tang Z, Kuang H, Kong A-NT. Corynoline Isolated from Corydalis bungeana Turcz. Exhibits Anti-Inflammatory Effects via Modulation of Nfr2 and MAPKs. Molecules. 2016; 21(8):975. https://doi.org/10.3390/molecules21080975
Chicago/Turabian StyleYang, Chunjuan, Chengyue Zhang, Zhibin Wang, Zhenqiu Tang, Haixue Kuang, and Ah-Ng Tony Kong. 2016. "Corynoline Isolated from Corydalis bungeana Turcz. Exhibits Anti-Inflammatory Effects via Modulation of Nfr2 and MAPKs" Molecules 21, no. 8: 975. https://doi.org/10.3390/molecules21080975
APA StyleYang, C., Zhang, C., Wang, Z., Tang, Z., Kuang, H., & Kong, A.-N. T. (2016). Corynoline Isolated from Corydalis bungeana Turcz. Exhibits Anti-Inflammatory Effects via Modulation of Nfr2 and MAPKs. Molecules, 21(8), 975. https://doi.org/10.3390/molecules21080975






