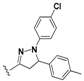
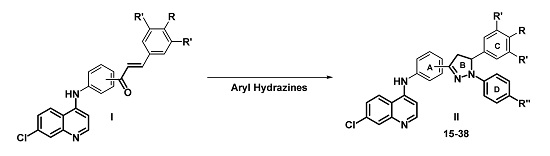
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of the Precursors 1–12
Using the same previously reported method [
15], precursors
1–
12 were obtained.
3.2.2. General Procedure for the Preparation of Compounds 15–20
A mixture of 4-(7-chloroquinolin-4-yl) amino chalcone 1–6 (0.11 mmol), phenylhydrazine 13 (0.33 mmol) in glacial acetic acid (10 mL) was submitted to microwave irradiation for 12 min at 250 W and 120 °C. Once the reaction mixture was cooled to room temperature, the resulting solution was neutralized with concentrated ammonium hydroxide. Then, crushed ice was added to the solution and a solid was precipitated, collected by vacuum filtration, washed thoroughly with water, dried, and recrystallized from ethanol.
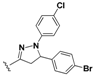
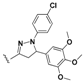
N-(4-(5-(4-Bromophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)-7-chloroquinolin-4-amine (15). Yellow solid; 90% yield; mp: 97–99 °C. FTIR (KBr) υ(cm−1): 3350 (NH), 3054 (=C-H), 1599 and 1576 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.12 (dd, J = 17.4, 6.1 Hz, 1H, H-4′a), 3.92 (dd, J = 17.4, 12.1 Hz, 1H, H-4′b), 5.49 (dd, J = 12.1, 6.1 Hz, 1H, H-5′), 6.72 (t, J = 7.6 Hz, 1H, Ar-H), 6.99 (d, J = 7.6 Hz, 2H, Ar-H), 7.07 (d, J = 5.4 Hz, 1H, H-3), 7.12–7.19 (m, 2H, Ar-H), 7.26 (d, J = 8.4 Hz, 2H, Ar-H), 7.41 (d, J = 8.7 Hz, 2H, Ar-H), 7.54 (d, J = 8.4 Hz, 2H, Ar-H), 7.59 (dd, J = 9.1, 2.3 Hz, 1H, H-6), 7.77 (d, J = 8.7 Hz, 2H, Ar-H), 7.92 (d, J = 2.3 Hz, 1H, H-8), 8.42 (d, J = 9.1 Hz, 1H, H-5), 8.51 (d, J = 5.4 Hz, 1H, H-2), 9.24 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.3 (CH2), 63.0, 100.0 (C), 103.3, 113.4, 119.1 (C), 119.2, 120.9 (C), 122.2, 125.0, 125.7, 127.4, 127.9 (C), 128.0, 128.7, 129.4, 132.4, 131.9 (C), 134.6 (C), 142.4 (C), 144.6 (C), 147.6 (C), 149.5 (C), 152.0. MS (70 eV) m/z (%): 552 (100, M+), 397 (17), 368 (34), 313 (32), 271 (35), 236 (47), 123 (34), 98 (47), 91 (67), 83 (54), 57 (81), 44 (67). Anal. Calcd. For C30H22BrClN4: C, 65.05; H, 4.00; N, 10.12. Found: C, 65.20; H, 3.98; N, 10.15.
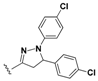
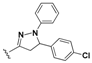
7-Chloro-N-(4-(5-(4-chlorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (16). Yellow solid; 92% yield; mp: 121–124 °C. FTIR (KBr) υ(cm−1): 3263 (NH), 3056 (=C-H), 1597 and 1572 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.09 (dd, J = 17.4, 6.1 Hz, 1H, H-4′a), 3.90 (dd, J = 17.4, 11.9 Hz, 1H, H-4′b), 5.48 (dd, J = 11.9, 6.1 Hz, 1H, H-5′), 6.71 (t, J = 7.5 Hz, 1H, Ar-H), 6.98 (d, J = 7.5 Hz, 2H, Ar-H), 7.06 (d, J = 5.1 Hz, 1H, H-3), 7.15 (t, J = 7.5 Hz, 2H, Ar-H), 7.31 (d, J = 8.5 Hz, 2H, Ar-H), 7.36–7.44 (m, 4H, Ar-H), 7.57 (dd, J = 9.0, 2.1 Hz, 1H, H-6), 7.76 (d, J = 8.5 Hz, 2H, Ar-H), 7.91 (d, J = 2.1 Hz, 1H, H-8), 8.41 (d, J = 9.0 Hz, 1H, H-5), 8.50 (d, J = 5.1 Hz, 1H, H-2), 9.25 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 42.9 (CH2), 62.4, 63.1 (C), 102.8, 112.9, 118.6, 121.6, 124.5, 125.2, 126.9, 127.3 (C), 127.6, 127.9, 128.9, 129.0, 131.9 (C), 134.1 (C), 140.9 (C), 141.5 (C), 144.2 (C), 147.1 (C), 147.2 (C), 149.5 (C), 152.0. MS (70 eV) m/z (%): 508 (100, M+), 397 (19), 368 (9), 279 (23), 254 (15), 243 (14), 91 (47), 77 (17). Anal. Calcd. For C30H22Cl2N4: C, 70.73; H, 4.35; N, 11.00. Found: C, 70.79; H, 4.37; N, 10.89.
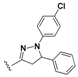
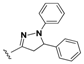
7-Chloro-N-(4-(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (17). Yellow solid; 83% yield; mp: 109–111 °C. FTIR (KBr) υ(cm−1): 3270 (NH), 3060 (=C-H), 1598 and 1573 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.11 (dd, J = 17.4, 6.2 Hz, 1H, H-4′a), 3.93 (dd, J = 17.4, 12.2 Hz, 1H, H-4′b), 5.48 (dd, J = 12.2, 6.2 Hz, 1H, H-5′), 6.65–6.73 (m, 2H, Ar-H), 7.00 (d, J = 7.8 Hz, 2H, Ar-H), 7.06–7.18 (m, 3H, Ar-H and H-3), 7.23–7.38 (m, 4H, Ar-H) 7.42 (d, J = 8.6 Hz, 2H, Ar-H), 7.60 (dd, J = 9.1, 2.2 Hz, 1H, H-6), 7.78 (d, J = 8.6 Hz, 2H, Ar-H), 7.92 (d, J = 2.2 Hz, 1H, H-8), 8.43 (d, J = 9.1 Hz, 1H, H-5), 8.52 (d, J = 5.3 Hz, 1H, H-2), 9.27 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.6 (CH2), 63.6, 112.5, 113.3, 118.8, 122.1, 125.1, 125.6, 126.4, 127.4, 127.9, 128.2, 129.2, 129.4, 134.5 (C), 141.4 (C), 143.1 (C), 144.8 (C), 147.5 (C), 147.8 (C), 150.0 (C), 150.1 (C), 152.5, 169.5 (C). MS (70 eV) m/z (%): 474 (100, M+), 397 (19), 279 (15), 121 (35), 105 (68), 91 (42), 77 (52), 57 (33), 43 (31). Anal. Calcd. For C30H23ClN4: C, 75.86; H, 4.88; N, 11.80. Found: C, 75.81; H, 4.65; N, 11.73.
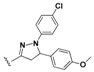
7-Chloro-N-(4-(5-(4-methoxyphenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (
18) [
27]. Yellow solid; 77% yield; mp: 111–112 °C. FTIR (KBr) υ(cm
−1): 3312 (NH), 3040 (=C-H), 1597 and 1572 (C=N and C=C).
1H-NMR (400 MHz, DMSO-
d6) δ ppm 3.06 (dd,
J = 17.3, 6.2 Hz, 1H, H-4′a), 3.70 (s, 3H, OCH
3), 3.87 (dd,
J = 17.3, 12.0 Hz, 1H, H-4′b), 5.40 (dd,
J = 12.0, 6.2 Hz, 1H, H-5′), 6.69 (t,
J = 7.3 Hz, 1H, Ar-H), 6.88 (d,
J = 8.8 Hz, 2H, Ar-H), 7.00 (d,
J = 8.0 Hz, 2H, Ar-H), 7.06 (d,
J = 5.3 Hz, 1H, H-3), 7.10–7.17 (m, 2H, Ar-H), 7.20 (d,
J = 8.5 Hz, 2H, Ar-H), 7.40 (d,
J = 8.5 Hz, 2H, Ar-H), 7.57 (dd,
J = 9.0, 1.9 Hz, 1H, H-6), 7.76 (d,
J = 8.5 Hz, 2H, Ar-H), 7.91 (d,
J = 1.9 Hz, 1H, H-8), 8.40 (d,
J = 9.0 Hz, 1H, H-5), 8.49 (d,
J = 5.3 Hz, 1H, H-2), 9.25 (br, 1H, NH).
13C-NMR (100 MHz, DMSO-
d6) δ ppm 43.5 (CH
2), 55.5 (OCH
3), 63.1, 103.1, 113.4, 114.5, 114.8, 118.9 (C), 119.0, 122.3, 124.8, 125.8, 127.3, 127.6, 128.2 (C), 129.4, 134.8 (C), 134.9 (C), 141.0 (C), 144.7 (C), 147.4 (C), 148.1 (C), 149.5 (C), 152.3, 158.9 (C). MS (70 eV)
m/
z (%): 504 (100, M
+), 399 (10), 279 (18), 121 (18), 91 (34), 77 (13), 57 (10). Anal. Calcd. For C
31H
25ClN
4O: C, 73.73; H, 4.99; N, 11.09. Found: C, 73.42; H, 4.87; N, 11.07.
7-Chloro-N-(4-(1-phenyl-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (19). Yellow solid; 76% yield; mp: 99–102 °C. FTIR (KBr) υ(cm−1): 3240 (NH), 3056 (=C-H), 1598 and 1575 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.15 (dd, J = 17.4, 7.3 Hz, 1H, H-4′a), 3.63 (s, 3H, OCH3), 3.71 (s, 6H, 2 × OCH3), 3.91 (dd, J = 17.4, 12.1 Hz, 1H, H-4′b), 5.34 (dd, J = 12.1, 7.3 Hz, 1H, H-5′), 6.64 (s, 2H, Ar-H), 6.74 (t, J = 7.2 Hz, 1H, Ar-H), 7.02–7.10 (m, 3H, Ar-H and H-3), 7.15–7.21 (m, 2H, Ar-H), 7.42 (d, J = 8.8 Hz, 2H, Ar-H), 7.60 (dd, J = 9.1, 2.2 Hz, 1H, H-6), 7.79 (d, J = 8.8 Hz, 2H, Ar-H), 7.92 (d, J = 2.2 Hz, 1H, H-8), 8.43 (d, J = 9.1 Hz, 1H, H-5), 8.52 (d, J = 5.3 Hz, 1H, H-2), 9.25 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.7 (CH2), 56.4 (2 × OCH3), 60.5 (OCH3), 64.4, 103.5, 113.6, 119.2, 120.2 (C), 122.1, 125.1, 125.7, 127.5, 127.9 (C), 128.2 (C), 128.8 (C), 129.4, 132.2, 133.3 (C), 134.6 (C), 137.1, 139.0 (C), 141.4 (C), 145.4 (C), 147.9 (C), 152.4, 153.8 (C). MS (70 eV) m/z (%): 564 (100, M+), 397 (35), 279 (18), 243 (12), 91 (32), 77 (12). Anal. Calcd. For C33H29ClN4O3: C, 70.14; H, 5.17; N, 9.92. Found: C, 70.09; H, 5.11; N, 9.82.
7-Chloro-N-(4-(1-phenyl-5-(p-tolyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (20). Yellow solid; 86% yield; mp: 89–92 °C. FTIR (KBr) υ(cm−1): 3230 (NH), 3052 (=C-H), 1599 and 1577 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 2.25 (s, 3H, CH3), 3.07 (dd, J = 17.3, 6.3 Hz, 1H, H-4′a), 3.89 (dd, J = 17.3, 12.2 Hz, 1H, H-4′b), 5.41 (dd, J = 12.2, 6.3 Hz, 1H, H-5′), 6.66–6.72 (m, 1H, Ar-H), 7.00 (d, J = 7.8 Hz, 2H, Ar-H), 7.07 (d, J = 5.3 Hz, 1H, H-3), 7.11–7.15 (m, 4H, Ar-H), 7.17–7.21 (m, 2H, Ar-H), 7.41 (d, J = 8.6 Hz, 2H, Ar-H), 7.58 (dd, J = 9.0, 2.2 Hz, 1H, H-6), 7.77 (d, J = 8.6 Hz, 2H, Ar-H), 7.92 (d, J = 2.2 Hz, 1H, H-8), 8.43 (d, J = 9.0 Hz, 1H, H-5), 8.51 (d, J = 5.3 Hz, 1H, H-2), 9.24 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 21.2 (CH3), 43.6 (CH2), 63.5, 103.3, 112.6, 113.4, 118.9, 122.1, 125.3, 126.3, 127.3, 128.0 (C), 128.2, 129.2, 130.0, 134.5 (C), 137.0 (C), 140.1 (C), 141.4 (C), 144.5 (C), 147.5 (C), 147.7 (C), 149.9 (C), 150.2 (C), 152.5. MS (70 eV) m/z (%): 488 (11, M+), 368 (31), 236 (47), 210 (61), 150 (60), 108 (100), 97 (49), 83 (60), 69 (56), 43 (39). Anal. Calcd. For C31H25ClN4: C, 76.14; H, 5.15; N, 11.46. Found: C, 76.12; H, 5.09; N, 11.36.
3.2.3. General Procedure for the Preparation of Compounds 21–26
A mixture of the corresponding chalcone 1–6 (0.11 mmol), 4-chlorophenylhydrazine hydrochloride 14 (0.22 mmol), BF3·OEt2 (0.2 mL, molar excess) as catalyst, and EtOH (8 mL) was heated under reflux for 7 h. After this time, the product was observed as a precipitate and was filtered and washed three times with the EtOH/H2O (1:0.5) mixture. No further purification was required.
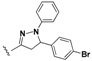
N-(4-(5-(4-Bromophenyl)-1-(4-chlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)-7-chloroquinolin-4-amine (21). Yellow solid; 80% yield; mp: >300 °C. FTIR (KBr) υ(cm−1): 3410 (NH), 3020 (=C-H), 1613 and 1589 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.18 (dd, J = 17.5, 5.8 Hz, 1H, H-4′a), 3.97 (dd, J = 17.5, 12.1 Hz, 1H, H-4′b), 5.59 (dd, J = 12.1, 5.8 Hz, 1H, H-5′), 6.94 (d, J = 6.7 Hz, 1H, H-3), 7.00 (d, J = 8.9 Hz, 2H, Ar-H), 7.21 (d, J = 8.9 Hz, 2H, Ar-H), 7.30 (d, J = 8.3 Hz, 2H, Ar-H), 7.42 (d, J = 8.3 Hz, 2H, Ar-H), 7.54 (d, J = 8.4 Hz, 2H, Ar-H), 7.84 (dd, J = 9.2, 1.3 Hz, 1H, H-6), 7.89 (d, J = 8.4 Hz, 2H, Ar-H), 8.16 (d, J = 1.3 Hz, 1H, H-8), 8.55 (d, J = 6.7 Hz, 1H, H-2), 8.86 (d, J = 9.2 Hz, 1H, H-5), 11.07 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.3 (CH2), 62.9, 101.5, 114.9, 117.0 (C), 120.9, 123.0 (C), 125.2, 126.5, 127.6, 127.7, 128.4, 129.3, 129.6, 130.8 (C), 132.6 (C), 138.3 (C), 138.5 (C), 141.1 (C), 141.4 (C), 143.2 (C), 145.2, 148.0 (C), 154.0 (C). MS (70 eV) m/z (%): 586 (55, M+), 368 (24), 346 (28), 313 (33), 236 (31), 125 (37), 97 (53), 71 (59), 57 (100), 43 (94). Anal. Calcd. For C30H21BrCl2N4: C, 61.25; H, 3.60; N, 9.52. Found: C, 61.15; H, 3.78; N, 9.63.
N-(4-(1,5-bis(4-Chlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)-7-chloroquinolin-4-amine (22). Yellow solid; 87% yield; mp: >300 °C. FTIR (KBr) υ(cm−1): 3418 (NH), 3025 (=C-H), 1614 and 1569 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.19 (dd, J = 17.6, 5.7 Hz, 1H, H-4′a), 3.97 (dd, J = 17.6, 12.2 Hz, 1H, H-4′b), 5.59 (dd, J = 12.2, 5.7 Hz, 1H, H-5′), 6.95 (d, J = 6.8 Hz, 1H, H-3), 7.01 (d, J = 8.8 Hz, 2H, Ar-H), 7.21–7.26 (m, 4H, Ar-H), 7.53–7.57 (m, 4H, Ar-H), 7.83–7.96 (m, 3H, Ar-H and H-6), 8.11 (d, J = 1.4 Hz, 1H, H-8), 8.56 (d, J = 6.8 Hz, 1H, H-2), 8.80 (d, J = 9.2 Hz, 1H, H-5), 11.00 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.3 (CH2), 63.0, 101.3, 114.9, 116.8 (C), 120.4, 121.2 (C), 123.0 (C), 125.5, 126.4, 127.8, 127.9, 128.7, 129.3, 131.1 (C), 132.5, 138.1 (C), 138.8 (C), 140.2 (C), 141.7 (C), 143.1 (C), 144.7, 147.9 (C), 154.6 (C). MS (70 eV) m/z (%): 542 (100, M+), 431 (15), 368 (13), 279 (20), 125 (14). Anal. Calcd. For C30H21Cl3N4: C, 66.25; H, 3.89; N, 10.30. Found: C, 66.40; H, 4.00; N, 10.52.
7-Chloro-N-(4-(1-(4-chlorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (23). Yellow solid; 73% yield; mp: >300 °C. FTIR (KBr) υ(cm−1): 3429 (NH), 3021 (=C-H), 1613 and 1591 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.18 (dd, J = 17.5, 6.0 Hz, 1H, H-4′a), 3.98 (dd, J = 17.5, 12.3 Hz, 1H, H-4′b), 5.56 (dd, J = 12.3, 6.0 Hz, 1H, H-5′), 6.94 (d, J = 6.8 Hz, 1H, H-3), 7.01 (d, J = 8.9 Hz, 2H, Ar-H), 7.20 (d, J = 8.9 Hz, 2H, Ar-H), 7.24–7.32 (m, 3H, Ar-H), 7.35 (d, J = 7.2 Hz, 2H, Ar-H), 7.55 (d, J = 8.4 Hz, 2H, Ar-H), 7.85 (dd, J = 9.0, 1.9 Hz, 1H, H-6), 7.91 (d, J = 8.4 Hz, 2H, Ar-H), 8.16 (d, J = 1.9 Hz, 1H, H-8), 8.55 (d, J = 6.8 Hz, 1H, H-2), 8.90 (d, J = 9.0 Hz, 1H, H-5), 11.17 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.6 (CH2), 63.7, 101.6, 114.9, 117.1 (C), 121.3, 122.8 (C), 125.0, 126.3, 126.5, 127.5, 127.6, 128.1, 129.2, 129.6, 130.7 (C), 136.4 (C), 138.1 (C), 138.6 (C), 142.5 (C), 143.4 (C), 145.5, 147.9 (C), 153.6 (C). MS (70 eV) m/z (%): 508 (100, M+), 431 (16), 369 (12), 279 (20), 254 (17), 125 (35), 90 (14). Anal. Calcd. For C30H22Cl2N4: C, 70.73; H, 4.35; N, 11.00. Found: C, 70.69; H, 4.42; N, 10.95.
7-Chloro-N-(4-(1-(4-chlorophenyl)-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (24). Yellow solid; 67% yield; mp: >300 °C. FTIR (KBr) υ(cm−1): 3421 (NH), 2679 (=C-H), 1616 and 1597 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.15 (dd, J = 17.6, 5.8 Hz, 1H, H-4′a), 3.72 (s, 3H, OCH3), 3.94 (dd, J = 17.6, 12.1 Hz, 1H, H-4′b), 5.51 (dd, J = 12.1, 5.8 Hz, 1H, H-5′), 6.86–6.97 (m, 3H, Ar-H and H-3), 7.02 (d, J = 8.8 Hz, 2H, Ar-H), 7.18–7.21 (m, 4H, Ar-H), 7.54 (d, J = 8.4 Hz, 2H, Ar-H), 7.82–7.95 (m, 3H, Ar-H and H-6), 8.14 (d, J = 1.6 Hz, 1H, H-8), 8.55 (d, J = 6.8 Hz, 1H, H-2), 8.83 (d, J = 9.0 Hz, 1H, H-5), 11.06 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.5 (CH2), 55.6 (OCH3), 63.2, 101.4, 114.9, 115.0, 116.9 (C), 120.6, 122.7 (C), 125.4, 126.4, 127.6, 127.7, 127.8, 129.2, 131.2 (C), 134.3 (C), 138.1 (C), 138.6 (C), 140.6 (C), 143.3 (C), 145.0, 147.8 (C), 154.3 (C), 159.1 (C). MS (70 eV) m/z (%): 538 (100, M+). Anal. Calcd. For C31H24Cl2N4O: C, 69.02; H, 4.48; N, 10.39. Found: C, 69.08; H, 4.44; N, 10.51.
7-Chloro-N-(4-(1-(4-chlorophenyl)-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (25). Yellow solid; 63% yield; mp: 218–220 °C. FTIR (KBr) υ(cm−1): 3434 (NH), 2969 (=C-H) and 1595 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.22 (dd, J = 17.6, 6.9 Hz, 1H, H-4′a), 3.64 (s, 3H, OCH3), 3.72 (s, 6H, 2 × OCH3), 3.96 (dd, J = 17.6, 12.1 Hz, 1H, H-4′b), 5.42 (dd, J = 12.1, 6.9 Hz, 1H, H-5′), 6.95 (d, J = 7.0 Hz, 1H, H-3), 7.05 (d, J = 9.0 Hz, 2H, Ar-H), 7.21–7.29 (m, 4H, Ar-H), 7.55 (d, J = 8.6 Hz, 2H, Ar-H), 7.86 (dd, J = 9.0, 1.8 Hz, 1H, H-6), 7.91 (d, J = 8.6 Hz, 2H, Ar-H), 8.15 (d, J = 1.8 Hz, 1H, H-8), 8.55 (d, J = 7.0 Hz, 1H, H-2), 8.85 (d, J = 9.0 Hz, 1H, H-5), 11.08 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.7 (CH2), 56.4 (2 × OCH3), 60.5 (OCH3), 64.4, 101.3, 103.4, 114.8, 115.1, 116.9 (C), 122.8 (C), 123.0 (C), 125.3, 126.5, 127.0, 127.7, 129.2, 131.1 (C), 137.2 (C), 138.2 (C), 140.2 (C), 141.0 (C), 143.8 (C), 144.7, 145.4 (C), 148.2 (C), 153.9 (C). MS (70 eV) m/z (%): 598 (7, M+), 420 (100), 294 (43), 281 (21), 166 (20), 125 (39). Anal. Calcd. For C33H28Cl2N4O3: C, 66.11; H, 4.71; N, 9.35. Found: C, 66.13; H, 4.94; N, 9.36.
7-Chloro-N-(4-(1-(4-chlorophenyl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (26). Yellow solid; 70% yield; mp: 296–298 °C. FTIR (KBr) υ(cm−1): 3424 (NH), 2974 (=C-H), 1614 and 1590 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 2.26 (s, 3H, CH3), 3.15 (dd, J = 17.6, 5.9 Hz, 1H, H-4′a), 3.95 (dd, J = 17.6, 12.3 Hz, 1H, H-4′b), 5.51 (dd, J = 12.3, 5.9 Hz, 1H, H-5′), 6.94 (d, J = 6.8 Hz, 1H, H-3), 7.01 (d, J = 9.2 Hz, 2H, Ar-H), 7.12–7.24 (m, 6H, Ar-H), 7.55 (d, J = 8.6 Hz, 2H, Ar-H), 7.85 (dd, J = 9.1, 2.0 Hz, 1H, H-6), 7.90 (d, J = 8.6 Hz, 2H, Ar-H), 8.18 (d, J = 2.0 Hz, 1H, H-8), 8.55 (d, J = 6.8 Hz, 1H, H-2), 8.89 (d, J = 9.1 Hz, 1H, H-5), 11.05 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 21.2 (CH3), 43.5 (CH2), 63.5, 101.3, 114.9, 116.9 (C), 120.3, 122.8 (C), 125.4, 126.3, 126.6, 127.6, 127.7, 129.2, 130.1, 131.2 (C), 137.3 (C), 138.1 (C), 138.6 (C), 139.5 (C), 140.4 (C), 143.3 (C), 144.5, 147.8 (C), 154.4 (C). MS (70 eV) m/z (%): 522 (100, M+), 279 (20), 243 (13), 125 (38). Anal. Calcd. For C31H24Cl2N4: C, 71.13; H, 4.62; N, 10.70. Found: C, 71.09; H, 4.56; N, 10.71.
3.2.4. General Procedure for the Preparation of Compounds 27–32
A mixture of chalcone 7–12 (0.11 mmol), phenylhydrazine 13 (0.22 mmol), glacial acetic acid (0.8 mL, molar excess) and EtOH (8 mL) was heated under reflux for 3 h until complete consumption of the chalcone (monitored by TLC). The product was observed as a precipitate and was filtered and washed with EtOH/H2O (1:0.5) mixture. No further purification was required.
N-(3-(5-(4-Bromophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)-7-chloroquinolin-4-amine (27). Yellow solid; 83% yield; mp: 228–231 °C. FTIR (KBr) υ(cm−1): 3354 (NH), 3053 (=C-H), 1602 and 1569 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.13 (dd, J = 17.4, 6.1 Hz, 1H, H-4′a), 3.93 (dd, J = 17.4, 12.1 Hz, 1H, H-4′b), 5.52 (dd, J = 12.1, 6.1 Hz, 1H, H-5′), 6.73 (t, J = 7.6 Hz, 1H, Ar-H), 6.98–7.03 (m, 3H, Ar-H and H-3), 7.16 (t, J = 7.6 Hz, 2H, Ar-H), 7.25 (d, J = 8.3 Hz, 2H, Ar-H), 7.39–7.41 (m, 1H, Ar-H), 7.47 (d, J = 4.5 Hz, 2H, Ar-H), 7.54 (d, J = 8.3 Hz, 2H, Ar-H), 7.59 (dd, J = 9.0, 1.7 Hz, 1H, H-6), 7.76 (s, 1H, Ar-H), 7.91 (d, J = 1.7 Hz, 1H, H-8), 8.44 (d, J = 9.0 Hz, 1H, H-5), 8.49 (d, J = 5.0 Hz, 1H, H-2), 9.19 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.2 (CH2), 63.0, 79.7 (C), 102.6, 113.5, 119.4, 119.9, 121.0 (C), 122.0, 123.1, 124.9, 125.6, 128.2, 128.7, 129.5, 130.2, 132.4, 134.0 (C), 134.5 (C), 135.9 (C), 141.1 (C), 144.4 (C), 147.5 (C), 148.3 (C), 150.1 (C), 152.5. MS (70 eV) m/z (%): 552 (100, M+), 397 (62), 280 (40), 218 (24), 91 (33), 77 (45). Anal. Calcd. For C30H22BrClN4: C, 65.05; H, 4.00; N, 10.12. Found: C, 65.05; H, 4.11; N, 10.25.
7-Chloro-N-(3-(5-(4-chlorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (28). Yellow solid; 81% yield; mp: 187–188 °C. FTIR (KBr) υ(cm−1): 3263 (NH), 3056 (=C-H), 1597 and 1569 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.13 (dd, J = 17.6, 6.0 Hz, 1H, H-4′a), 3.94 (dd, J = 17.6, 12.1 Hz, 1H, H-4′b), 5.55 (dd, J = 12.1, 6.0 Hz, 1H, H-5′), 6.73 (t, J = 7.2 Hz, 1H, Ar-H), 6.97–6.99 (m, 3H, Ar-H and H-3), 7.12–7.20 (m, 2H, Ar-H), 7.32 (d, J = 8.3 Hz, 2H, Ar-H), 7.37–7.43 (m, 3H, Ar-H), 7.48 (d, J = 4.8 Hz, 2H, Ar-H), 7.59 (dd, J = 8.8, 1.6 Hz, 1H, H-6), 7.77 (s, 1H, Ar-H), 7.91 (d, J = 1.6 Hz, 1H, H-8), 8.44 (d, J = 8.8 Hz, 1H, H-5), 8.50 (d, J = 5.3 Hz, 1H, H-2), 9.19 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 42.7 (CH2), 62.5, 76.5 (C), 102.1, 113.0, 118.9, 119.4, 121.5 (C), 122.6, 124.5, 125.1, 127.7, 127.9, 129.0, 129.8, 132.8, 133.5 (C), 134.0 (C), 135.9 (C), 140.6 (C), 143.9 (C), 147.8 (C), 148.5, 148.9 (C), 149.6 (C), 152.1. MS (70 eV) m/z (%): 508 (100, M+), 397 (45), 296 (36), 281 (28), 218 (59), 43 (51). Anal. Calcd. For C30H22Cl2N4: C, 70.73; H, 4.35; N, 11.00. Found: C, 70.77; H, 4.39; N, 10.91.
7-Chloro-N-(3-(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (29). Yellow solid; 73% yield; mp: 202–203 °C. FTIR (KBr) υ(cm−1): 3342 (NH), 3051 (=C-H), 1598 and 1567 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.12 (dd, J = 17.5, 6.3 Hz, 1H, H-4′a), 3.94 (dd, J = 17.5, 12.2 Hz, 1H, H-4′b), 5.50 (dd, J = 12.2, 6.3 Hz, 1H, H-5′), 6.71 (t, J = 7.3 Hz, 1H, Ar-H), 6.97–7.03 (m, 3H, Ar-H and H-3), 7.10–7.17 (m, 2H, Ar-H), 7.23–7.41 (m, 6H, Ar-H), 7.45–7.49 (m, 2H, Ar-H), 7.59 (dd, J = 9.0, 2.0 Hz, 1H, H-6), 7.78 (s, 1H, Ar-H), 7.91 (d, J = 2.0 Hz, 1H, H-8), 8.45 (d, J = 9.0 Hz, 1H, H-5), 8.49 (d, J = 5.3 Hz, 1H, H-2), 9.19 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.0 (CH2), 63.2, 99.5 (C), 102.1, 113.0, 118.4 (C), 119.4, 121.5, 122.6, 124.5, 125.1, 125.3, 125.8, 127.6, 128.9, 129.0, 129.1, 129.7, 133.6 (C), 134.0 (C), 142.4 (C), 144.1 (C), 146.8 (C), 147.9 (C), 149.5 (C), 152.0. MS (70 eV) m/z (%): 474 (100, M+), 397 (77), 280 (21), 218 (17), 91 (35), 77 (40). Anal. Calcd. For C30H23ClN4: C, 75.86; H, 4.88; N, 11.80. Found: C, 75.78; H, 4.92; N, 11.85.
7-Chloro-N-(3-(5-(4-methoxyphenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (30). Yellow solid; 68% yield; mp: 189–191 °C. FTIR (KBr) υ(cm−1): 3274 (NH), 3057 (=C-H), 1620 and 1599 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6): δ ppm = 3.09 (dd, J = 17.3, 6.2 Hz, 1H, H-4′a), 3.71 (s, 3H, OCH3), 3.90 (dd, J = 17.3, 12.2 Hz, 1H, H-4′b), 5.45 (dd, J = 12.2, 6.2 Hz, 1H, H-5′), 6.71 (t, J = 7.3 Hz, 1H, Ar-H), 6.89 (d, J = 8.7 Hz, 2H, Ar-H), 6.97–7.03 (m, 3H, Ar-H and H-3), 7.11–7.17 (m, 2H, Ar-H), 7.21 (d, J = 8.7 Hz, 2H, Ar-H), 7.39–7.41 (m, 1H, Ar-H), 7.45–7.48 (m, 2H, Ar-H), 7.57–7.60 (m, 1H, Ar-H), 7.77 (s, 1H, Ar-H), 7.91 (d, J = 2.0 Hz, 1H, H-8), 8.45 (d, J = 9.0 Hz, 1H, H-5), 8.49 (d, J = 5.3 Hz, 1H, H-2), 9.19 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ ppm = 43.0 (CH2), 55.0 (OCH3), 62.8, 102.1, 113.1, 114.3, 118.7, 119.3, 121.5, 122.5, 124.4, 125.0, 127.1, 127.7, 128.8, 129.7, 133.7 (C), 134.0 (C), 134.3 (C), 135.9 (C), 140.6 (C), 144.1 (C), 146.8 (C), 147.8 (C), 149.6 (C), 152.1, 158.5 (C). MS (70 eV) m/z (%): 504 (100, M+), 397 (57), 280 (25), 218 (21), 77 (29). Anal. Calcd. For C31H25ClN4O: C, 73.73; H, 4.99; N, 11.09. Found: C, 73.67; H, 4.89; N, 11.16.
7-Chloro-N-(3-(1-phenyl-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (31). Yellow solid; 65% yield; mp: 183–186 °C. FTIR (KBr) υ(cm−1): 3285 (NH), 3028 (=C-H), 1665 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.16 (dd, J = 17.5, 7.3 Hz, 1H, H-4′a), 3.72 (s, 3H, OCH3), 3.86 (s, 6H, 2 × OCH3), It is not observed (dd, 1H, H-4′b), 5.37 (dd, J = 12.2, 7.3 Hz, 1H, H-5′), 6.72–6.77 (m, 1H, Ar-H), 6.98–7.07 (m, 3H, Ar-H), 7.10–7.21 (m, 4H, Ar-H), 7.36–7.42 (m, 1H, Ar-H), 7.45–7.51 (m, 2H, Ar-H), 7.59 (dd, J = 9.1, 2.2 Hz, 1H, H-6), 7.78 (s, 1H, Ar-H), 7.92 (d, J = 2.2 Hz, 1H, H-8), 8.40–8.54 (m, 2H, H-5 and H-2), 9.62 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.6 (CH2), 56.3 (2 x OCH3), 60.4 (OCH3), 64.4, 100.0 (C), 102.5, 113.7, 119.3 (C), 119.4, 119.8, 121.0 (C), 122.0, 123.1, 125.0, 125.6, 128.1, 129.4, 129.5, 130.3, 134.1 (C), 134.5 (C), 137.0 (C), 138.8 (C), 145.1 (C), 147.7 (C), 148.3 (C), 150.1 (C), 152.5. MS (70 eV) m/z (%): 564 (100, M+), 397 (65), 279 (14), 91 (37), 77 (14). Anal. Calcd. For C33H29ClN4O3: C, 70.14; H, 5.17; N, 9.92. Found: C, 69.97; H, 5.25; N, 10.03.
7-Chloro-N-(3-(1-phenyl-5-(p-tolyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (32). Yellow solid; 72% yield; mp: 210–212 °C. FTIR (KBr) υ(cm−1): 3187 (NH), 3058 (=C-H), 1617 and 1594 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 2.30 (s, 3H, CH3), 3.11 (dd, J = 17.4, 6.2 Hz, 1H, H-4′a), 3.91 (dd, J = 17.4, 12.2 Hz, 1H, H-4′b), 5.48 (dd, J = 12.2, 6.2 Hz, 1H, H-5′), 6.75 (t, J = 7.1 Hz, 1H, Ar-H), 6.83 (d, J = 7.0 Hz, 1H, H-3), 7.16–7.24 (m, 2H, Ar-H), 7.24–7.29 (m, 2H, Ar-H), 7.41 (d, J = 7.9 Hz, 2H, Ar-H), 7.57 (d, J = 7.9 Hz, 2H, Ar-H), 7.77–7.82 (m, 1H, Ar-H), 7.85 (d, J = 8.3 Hz, 2H, Ar-H), 7.87 (dd, J = 9.2, 2.2 Hz, 1H, H-6), 7.90 (s, 1H, Ar-H), 8.21 (d, J = 2.2 Hz, 1H, H-8), 8.52 (d, J = 7.0 Hz, 1H, H-2), 8.92 (d, J = 9.2 Hz, 1H, H-5), 9.19 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 13.4 (CH3), 43.0 (CH2), 63.0, 100.9, 113.4, 114.5, 119.6, 119.7, 121.5, 122.4, 124.7, 125.0, 126.8, 127.8, 129.4, 130.4, 133.7 (C), 135.9 (C), 137.6 (C), 138.9 (C), 139.6 (C), 139.8 (C), 141.7 (C), 143.8, 144.1 (C), 146.3 (C), 155.5 (C). MS (70 eV) m/z (%): 488 (78, M+), 490 (100), 397 (52), 280 (23), 218 (19), 77 (14). Anal. Calcd. For C31H25ClN4: C, 76.14; H, 5.15; N, 11.46. Found: C, 76.10; H, 5.15; N, 11.52.
3.2.5. General Procedure for the Preparation of Compounds 33–38
A mixture of the corresponding 3-(7-chloroquinolin-4-yl)amino chalcone 7–12 (0.11 mmol), 4-chlorophenylhydrazine hydrochloride 14 (0.22 mmol), glacial acetic acid (0.2 mL, molar excess), and EtOH (8 mL) was heated under reflux for 8 h, until the reaction is complete and the product precipitated. Afterward, the solid product was filtered, washed with water, and recrystallized from ethanol.
N-(3-(5-(4-Bromophenyl)-1-(4-chlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)-7-chloroquinolin-4-amine (33). Yellow solid; 75% yield; mp: 201–203 °C. FTIR (KBr) υ(cm−1): 3166 (NH), 3049 (=C-H), 1618 and 1595 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.18 (dd, J = 17.6, 6.0 Hz, 1H, H-4′a), 3.96 (dd, J = 17.6, 12.4 Hz, 1H, H-4′b), 5.59 (dd, J = 12.4, 6.0 Hz, 1H, H-5′), 6.86 (d, J = 6.9 Hz, 1H, H-3), 6.99 (d, J = 8.8 Hz, 2H, Ar-H), 7.17–7.26 (m, 4H, Ar-H), 7.51 (d, J = 7.8 Hz, 1H, Ar-H), 7.55 (d, J = 8.3 Hz, 2H, Ar-H), 7.62 (t, J = 7.8 Hz, 1H, Ar-H), 7.74 (d, J = 7.8 Hz, 1H, Ar-H), 7.85–7.92 (m, 2H, Ar-H and H-6), 8.21 (d, J = 2.0 Hz, 1H, H-8), 8.53 (d, J = 6.9 Hz, 1H, H-2), 8.91 (d, J = 9.0 Hz, 1H, H-5), 11.28 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 42.8 (CH2), 62.4, 100.5, 114.5, 116.0 (C), 119.3, 122.4, 122.6 (C), 124.9, 125.6, 126.2, 127.4, 127.8, 128.8, 129.1, 130.3, 132.1 (C), 133.8 (C), 137.5 (C), 138.4 (C), 139.2 (C), 140.7 (C), 142.5 (C), 143.5, 147.3 (C), 154.8 (C). MS (70 eV) m/z (%): 588 (100), 586 (84, M+), 431 (52), 279 (32), 243 (26), 218 (20), 125 (68), 111 (25), 90 (34). Anal. Calcd. For C30H21BrCl2N4: C, 61.25; H, 3.60; N, 9.52. Found: C, 61.32; H, 3.76; N, 10.01.
N-(3-(1,5-bis(4-Chlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)-7-chloroquinolin-4-amine (34). Yellow solid; 71% yield; mp: 206–207 °C. FTIR (KBr) υ(cm−1): 3160 (NH), 3049 (=C-H), 1617 and 1588 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.17 (dd, J = 17.6, 6.0 Hz, 1H, H-4′a), 3.96 (dd, J = 17.6, 12.2 Hz, 1H, H-4′b), 5.60 (dd, J = 12.2, 6.0 Hz, 1H, H-5′), 6.85 (d, J = 7.0 Hz, 1H, H-3), 6.98 (d, J = 9.0 Hz, 2H, Ar-H), 7.19 (d, J = 9.0 Hz, 2H, Ar-H), 7.29 (d, J = 8.5 Hz, 2H, Ar-H), 7.41 (d, J = 8.5 Hz, 2H, Ar-H), 7.50 (dd, J = 8.0, 0.9 Hz, 1H, Ar-H), 7.61 (t, J = 8.0 Hz, 1H, Ar-H), 7.73 (d, J = 8.0 Hz, 1H, Ar-H), 7.84–7.91 (m, 2H, Ar-H and H-6), 8.21 (d, J = 2.0 Hz, 1H, H-8), 8.53 (d, J = 7.0 Hz, 1H, H-2), 8.93 (d, J = 9.0 Hz, 1H, H-5), 11.30 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 41.2 (CH2), 49.8, 100.0 (C), 100.6, 116.5 (C), 119.8, 120.4 (C), 125.2, 126.6, 128.0, 128.1, 130.5, 130.7, 131.0, 131.2, 131.4, 132.4, 137.6 (C), 137.7 (C), 138.1 (C), 138.9 (C), 139.1 (C), 139.6 (C), 144.1, 155.4 (C), 198.2 (C). MS (70 eV) m/z (%): 542 (100, M+), 431 (49), 279 (26), 125 (18), 90 (13). Anal. Calcd. For C30H21Cl3N4: C, 66.25; H, 3.89; N, 10.30. Found: C, 66.27; H, 3.95; N, 10.36.
7-Chloro-N-(3-(1-(4-chlorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (35). Yellow solid; 61% yield; mp: 245–246 °C. FTIR (KBr) υ(cm−1): 3354 (NH), 3056 (=C-H), 1615 and 1591 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.17 (dd, J = 17.6, 6.1 Hz, 1H, H-4′a), 3.97 (dd, J = 17.6, 12.3 Hz, 1H, H-4′b), 5.58 (dd, J = 12.3, 6.1 Hz, 1H, H-5′), 6.88 (d, J = 6.9 Hz, 1H, H-3), 7.00 (d, J = 9.0 Hz, 2H, Ar-H), 7.19 (d, J = 9.0 Hz, 2H, Ar-H), 7.28 (m, 3H, Ar-H), 7.33–7.39 (m, 2H, Ar-H), 7.50 (dd, J = 8.0, 0.8 Hz, 1H, Ar-H), 7.61 (t, J = 7.8 Hz, 1H, Ar-H), 7.72 (d, J = 7.8 Hz, 1H, Ar-H), 7.86 (dd, J = 9.1, 2.0 Hz, 1H, H-6), 7.90 (s, 1H, Ar-H), 8.18 (d, J = 2.0 Hz, 1H, H-8), 8.54 (d, J = 6.9 Hz, 1H, H-2), 8.89 (d, J = 9.1 Hz, 1H, H-5), 11.14 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.5 (CH2), 63.6, 101.1, 114.9, 116.7 (C), 120.5, 122.6, 122.9 (C), 125.1, 125.8, 126.3, 126.6, 127.7, 128.1, 129.2, 129.6, 130.8, 134.3 (C), 138.3 (C), 138.5 (C), 140.5 (C), 142.3 (C), 143.2 (C), 144.8, 147.7 (C), 154.7 (C). MS (70 eV) m/z (%): 508 (100, M+), 431 (28), 279 (16), 236 (24), 125 (33), 111 (26), 97 (19), 83 (22), 57 (35), 43 (27). Anal. Calcd. For C30H22Cl2N4: C, 70.73; H, 4.35; N, 11.00. Found: C, 70.64; H, 4.38; N, 11.12.
7-Chloro-N-(3-(1-(4-chlorophenyl)-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (36). Yellow solid; 47% yield; mp: 216–218 °C. FTIR (KBr) υ(cm−1): 3189 (NH), 3060 (=C-H), 1617 and 1594 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.20 (dd, J = 17.6, 7.0 Hz, 1H, H-4′a), 3.71 (s, 3H, OCH3), 3.95 (dd, J = 17.6, 12.3 Hz, 1H, H-4′b), 5.43 (dd, J = 12.3, 7.0 Hz, 1H, H-5′), 6.89 (d, J = 7.0 Hz, 1H, H-3), 6.98 (d, J = 9.0 Hz, 2H, Ar-H), 7.04 (d, J = 9.0 Hz, 2H, Ar-H), 7.22 (d, J = 8.8 Hz, 2H, Ar-H), 7.35 (d, J = 8.8 Hz, 2H, Ar-H), 7.51 (d, J = 8.0 Hz, 1H, Ar-H), 7.64 (t, J = 8.0 Hz, 1H, Ar-H), 7.76 (d, J = 8.0 Hz, 1H, Ar-H), 7.85 - 7.92 (m, 2H, Ar-H and H-6), 8.14 (d, J = 2.0 Hz, 1H, H-8), 8.53 (d, J = 7.0 Hz, 1H, H-2), 8.85 (d, J = 9.0 Hz, 1H, H-5), 11.20 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.4 (CH2), 55.0 (OCH3), 63.8, 100.5, 114.3, 116.1 (C), 119.6, 121.8, 122.4 (C), 124.3, 124.4, 125.9, 127.3, 128.7, 129.2, 129.6, 130.0, 134.3 (C), 137.1 (C), 138.3 (C), 138.5 (C), 139.5 (C), 140.4 (C), 140.9 (C), 143.8, 144.7 (C), 154.7 (C). MS (70 eV) m/z (%): 538 (1, M+), 419 (100), 280 (22), 243 (18), 218 (54), 126 (24), 99 (24). Anal. Calcd. For C31H24Cl2N4O: C, 69.02; H, 4.48; N, 10.39. Found: C, 69.11; H, 4.55; N, 10.41.
7-Chloro-N-(3-(1-(4-chlorophenyl)-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (37). Yellow solid; 53% yield; mp: 200–201 °C. FTIR (KBr) υ(cm−1): 3204 (NH), 3053 (=C-H), 1615 and 1589 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 3.08–3.19 (m, 1H, H-4′a), 3.71 (s, 3H, OCH3), 3.79 (s, 6H, 2 × OCH3), 3.92 (dd, J = 17.7, 12.2 Hz, 1H, H-4′b), 5.51 (dd, J = 12.2, 6.0 Hz, 1H, H-5′), 6.82–6.93 (m, 1H, H-3), 6.99 (m, 2H, Ar-H), 7.17–7.20 (m, 4H, Ar-H), 7.51 (d, J = 7.8 Hz, 1H, Ar-H), 7.57–7.72 (m, 1H, Ar-H), 7.83–7.93 (m, 3H, Ar-H), 8.15 (d, J = 9.2 Hz, 1H, H-6), 8.54 (d, J = 6.8 Hz, 1H, H-2), 8.85 (d, J = 9.2 Hz, 1H, H-5), 11.19 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 43.5 (CH2), 56.4 (2 × OCH3), 60.5 (OCH3), 64.3, 100.0 (C), 101.0, 103.4, 114.1, 114.5, 115.1, 116.4 (C), 119.7, 122.8, 123.2 (C), 126.4, 128.1, 129.0, 129.2, 134.4 (C), 136.4 (C), 137.1 (C), 137.2 (C), 138.1 (C), 139.4 (C), 143.9, 148.1 (C), 153.8 (C), 155.5 (C). MS (70 eV) m/z (%): 598 (100, M+), 431 (59), 279 (16), 125 (39), 90 (10). Anal. Calcd. For C33H28Cl2N4O3: C, 66.11; H, 4.71; N, 9.35. Found: C, 66.15; H, 4.78; N, 9.45.
7-Chloro-N-(3-(1-(4-chlorophenyl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazol-3-yl)phenyl)quinolin-4-amine (38). Yellow solid; 60% yield; mp: 207–208 °C. FTIR (KBr) υ(cm−1): 3174 (NH), 3050 (=C-H), 1618 and 1590 (C=N and C=C). 1H-NMR (400 MHz, DMSO-d6) δ ppm 2.25 (s, 3H, CH3), 3.13 (dd, J = 17.6, 6.0 Hz, 1H, H-4′a), 3.94 (dd, J = 17.6, 12.3 Hz, 1H, H-4′b), 5.52 (dd, J = 12.3, 6.0 Hz, 1H, H-5′), 6.86 (d, J = 6.9 Hz, 1H, H-3), 6.99 (d, J = 8.8 Hz, 2H, Ar-H), 7.11–7.21 (m, 6H, Ar-H), 7.50 (d, J = 7.9 Hz, 1H, Ar-H), 7.62 (t, J = 7.9 Hz, 1H, Ar-H), 7.73 (d, J = 7.9 Hz, 1H, Ar-H), 7.84–7.92 (m, 2H, Ar-H and H-6), 8.20 (s, 1H, H-8), 8.53 (d, J = 6.9 Hz, 1H, H-2), 8.91 (d, J = 9.0 Hz, 1H, H-5), 11.27 (br s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ ppm 20.7 (CH3), 43.0 (CH2), 63.0, 100.5, 114.5, 116.0 (C), 119.3, 122.3, 122.4 (C), 124.9, 125.5, 125.8, 126.2, 127.4, 128.7, 129.6, 130.4, 134.0 (C), 136.8 (C), 137.5 (C), 138.4 (C), 138.9 (C), 139.1 (C), 142.7 (C), 143.5, 147.2 (C), 154.9 (C). MS (70 eV) m/z (%): 522 (100, M+), 431 (24), 279 (16), 125 (30), 91 (12), 44 (14). Anal. Calcd. For C31H24Cl2N4: C, 71.13; H, 4.62; N, 10.70. Found: C, 71.18; H, 4.74; N, 10.80.