Abstract
A high-yield one-pot two-step synthesis of 2-aminoimidazoles (2-AI), exploiting an under-air heterocyclodehydration process between α-chloroketones and guanidine derivatives, and using deep eutectic solvents (DESs) as nonconventional, “green” and “innocent” reaction media, has been accomplished successfully. The combination of either glycerol or urea with choline chloride (ChCl) proved to be effective for decreasing the reaction time to about 4–6 h in contrast to the 10–12 h usually required for the same reaction run in toxic and volatile organic solvents and under an argon atmosphere. In addition, the use of the ChCl–urea as a DES also enables the direct isolation of triaryl-substituted 2-AI derivatives by means of a simple work-up procedure consisting in filtration and crystallization, and allows the recycle of the DES mixture. A plausible mechanism highlighting the potential role played by hydrogen bonding catalysis has also been illustrated.
1. Introduction
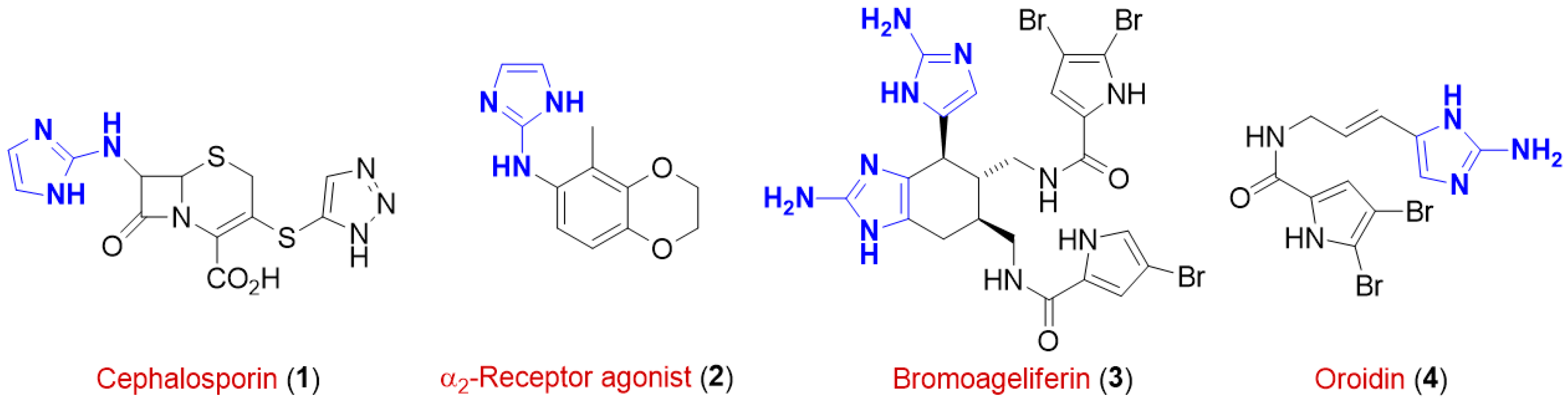
The American Chemical Society′s (ACS) Green Chemistry Institute Pharmaceutical Roundtable (GCIPR), founded in 2005 with the aim of catalyzing the integration of green chemistry and engineering into the pharmaceutical industry, strives for the need to replace conventional hazardous volatile organic solvents (VOCs) in favor of safe, green and biorenewable reaction media that are not based on crude petroleum. Solvents are, indeed, still responsible for most waste generated in the chemical industries and laboratories as they account for 80%–90% of mass utilization in a typical pharmaceutical/fine chemical operational process [1]. Thus, there is a global and increasing demand for the development of renewable solvents not based on crude petroleum [2]. 2-Aminoimidazole (2-AI) derivatives are an extremely important class of substituted nitrogen-containing heterocycles whose core has been recognized as a key and essential structural element in a wide range of bioactive molecules such as the antibiotic 1 belonging to the family of cephalosporins [3], the α2-receptor agonist 2 [4], and the naturally-occurring sponge metabolites bromoageliferin 3 and oroidin 4, which are known for their anti-biofilm activity [5,6] (Figure 1). In addition, they have found wide application in coordination chemistry [7,8], in organocatalysis [9], and proven to be either valuable pharmacophores in medicinal chemistry for the development of high value-added molecules for discovery based research [10] or useful building blocks for the design of modulators of various small molecule drugs being bioisosteres of guanidine, benzamidine and triazole rings [11].
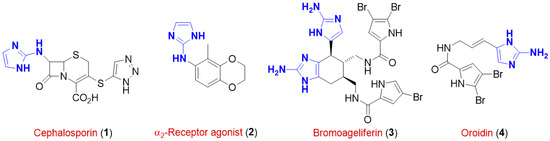
Figure 1.
Structural formulas of some pharmacologically-relevant 2-aminoimidazoles.
As such, novel synthetic approaches for the construction of 2-AI scaffolds are continuously proposed. Classical methods rely upon condensation reactions, decoration of imidazole derivatives and heterocyclic exchange reactions [10,11,12,13]. More recently, various metal-mediated cycloguanylation reactions have also been proposed [14,15,16,17,18,19]. Despite these synthetic efforts, however, the construction of 2-AIs is still performed in toxic, hazardous and expensive VOCs (e.g., THF, DMF, toluene, etc.) and reactions en route to these heterocycles are generally carried out under an inert atmosphere with strict exclusion of humidity, thereby adopting protocols of limited utility for industry users. Deep eutectic solvents (DESs) represent an emerging class of unconventional solvents that have become of growing interest both at academic and industrial levels. Compared to conventional VOCs, DESs show high thermal stability, non-flammability and practically no vapor pressure, therefore low volatility. Formation of DESs can be easily achieved by simply mixing together and gently warming at least two safe, cheap, renewable and biodegradable components, generally a hydrogen bond donor and a hydrogen bond acceptor which are capable of forming a eutectic mixture having a melting point lower than either of the individual components. Typical DES components (e.g., choline chloride (ChCl), urea, glycerol (Gly), natural carboxylic acids, amino acids and carbohydrates, polyalcohols, etc.) come from renewable sources; thus, their biodegradability is extraordinarily high, and their toxicity is non-existent or very low [20,21,22,23,24,25]. The major research effort to date has been mainly concentrated on replacement solvents for metal finishing applications [24], for biomass valorization [23], for extraction processes [26], and in polymerizations and materials science [27,28]. Emerging applications are in the fields of organometallic chemistry [29,30,31,32,33], metal-catalyzed reactions [34,35,36], and organocatalysis [37,38,39]. In this manuscript, we describe the first one-pot two-step synthesis of 2-AIs exploiting an alkylation reaction followed by a cyclocondensation process between α-chloroketones and guanidine derivatives successfully run in eutectic mixtures as “innocent” and biorenewable DES media. The whole transformation has been proven to proceed in high yield within short reaction times (4 to 6 h) compared to conventional VOCs (10 to 12 h), and could be successfully carried out under air.
2. Results and Discussion
Intrigued by the potential to synthesize substituted 2-AIs employing DESs as effective and “green” reaction media, we began our study by re-investigating the one-pot synthesis of 2-AIs published by Webber in the 1990s [12] but using the aforementioned unconventional solvents. In this seminal paper, the cyclization reaction between α-haloketones and an excess of N-acetylguanidine (at least three equivalents) proved to be effective upon stirring the reaction mixture in anhydrous DMF, under argon and at room temperature for four days, or at reflux in CH3CN for 16 h. The solvent polarity was also studied, and inferior results were obtained when the reaction was run in CHCl3, DME, or DMSO. A type III DES [21], namely ChCl–Gly (1:2), was selected as a model eutectic mixture for studying such heterocyclodehydration reaction. ChCl, also known as vitamin B4, is one of the most widely used ammonium salts in forming DESs. It is a very cheap (ca. 2 €·kg−1) and biodegradable material, and is produced on the scale of a million metric tons per year as an additive in chicken food for accelerating growth. When a solution of α-chloroketone 1a (1.0 mmol), substituted guanidine 2a (1.3 mmol) and Et3N (1.0 mmol) was vigorously stirred at 80 °C and under air in the above DES mixture, the complete disappearance of the starting ketone took place after ca. 4 h, as revealed by GC-MS analysis. Because of the high solubility of the above DES mixture in water, a very simple reaction workup was required: the desired AI 3a could indeed be isolated in 85% yield by simple dilution with equal volume mixture of water and AcOEt, followed by separation of the organic layer and removal of the solvent under reduced pressure (Table 1, entry 1) [40].

Table 1.
Synthesis of 2-aminoimidazoles 3 in a DES mixture or in other solvents a. 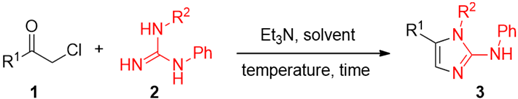

On the other hand, when performed in anhydrous THF and under an argon atmosphere, such condensation process required a longer reaction time (ca. 12 h) and the use of reflux conditions to go to completion. After partitioning the crude between AcOEt and water, product 3a could be finally isolated in 81% yield after column chromatography on silica gel (Table 1, entry 2). Further experimentation to explore the scope of the reaction established that assorted aliphatic (1b,c) and aromatic α-chloroketones with electron-donating (1d,e), electron-withdrawing (1f) and fluorine (1g) substituents, all proved to be competent partners straightforwardly providing the expected 2-AI derivatives 3b–g in very good yields (70%–83%) (Table 1, entries 3, 5, 7, 9, 11, and 13). The corresponding reactions, run in anhydrous THF and under an argon atmosphere, led to the desired products in comparable yields (71%–85%), albeit after 10–12 h reflux (Table 1, entries 4, 6, 8, 10, 12, and 14). With the aim to extending the scope of the reaction even more, it was interesting to observe that such condensation processes worked well in a relatively short reaction time (4–6 h) also by reacting α-chloroketones 1a and 1b with a different guanidine derivative (2b) in the above ChCl–Gly (1:2) DES mixture, thereby affording the corresponding 2-AIs 3h and 3i in 73% and 72% yields, respectively, (Table 1, entries 15 and 17). As for VOCs, similar yields (70%–76%) were obtained using a polar solvent such as EtOH, again to the detriment of the reaction time (12 h reflux) (Table 1, entries 16 and 18).
We were also keen to explore for this transformation another common and widely used DES, namely the ChCl–urea (1:2) eutectic mixture. At the outset of this investigation, we were rather skeptical about the successful outcome of the reaction. In fact, it has been recently reported by Shankarling and co-workers that α-bromoketones are able to react with the urea component of a DES mixture to produce 2-aminooxazole derivatives as the final products [41]. In this case, a DES is a “non-innocent” medium as it becomes an integral part of the starting materials. Remarkably, upon mixing α-chloroketone 1g with guanidine derivative 2a, and heating the reaction mixture to up to 80 °C, 2-AI 3g was found to precipitate directly from the above DES mixture during the reaction, and could be isolated by simple filtration on paper, thereby avoiding the typical organic solvent extraction procedure performed at the end of the reaction. An 1H-NMR analysis of the product 3g showed a purity of about 95%, which could be further increased to >98% after crystallization from Et2O by using hexane as a non solvent, the Et2O/hexane ratio being almost 8/2 (yield of 3g: 85%; Table 2, entry 6).

Table 2.
Preparation of 2-aminoimidazole 3a,b,d–h directly in a ChCl–urea (1:2) eutectic mixture a. 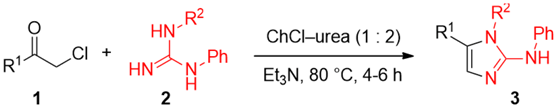

Overall, the reaction proved to be extraordinarily clean (no other by-products could be detected) and more user-friendly compared to the procedure using ChCl–Gly (1:2) as the eutectic mixture. In addition, the ChCl–urea DES could be recovered and recycled. Indeed, after filtration of 3g, the addition of 2 mL of water to the DES (reaction performed on 1.0 mmol scale of substrate; see Table 2) allowed the unreacted guanidine together with small quantities of other organic byproducts to precipitate and to be removed by filtration. Subsequent removal of water from the aqueous layer under vacuum furnished a DES mixture that could be successfully reused for an additional run without significant loss of 3g yield (80%). However, starting from the third cycle, a drop in the chemical yield was noticed: 60%. We have then explored the reactivity of other aromatic and aliphatic α-chloroketones 1a,b,d–f toward guanidines 2a,b in the above ChCl–urea medium. We were pleased to find that, again, all the planned condensation reactions proved to be clean and effective providing the desired 2-AIs 3a,b,d–f,h in good yields (65%–88%; Table 2, entries 1–5 and 7). It is worth noting that, similar to what has been shown for compound 3g, triaryl-substituted imidazoles 3a,d–f could also be successfully isolated with a purity >98% (1H-NMR) directly from the reaction mixture by simple filtration followed by crystallization (Et2O/hexane). Conversely, the isolation of the diaryl-substituted imidazoles 2-AIs 3b,h required a work-up procedure followed by a column chromatography on silica gel (See Materials and Methods).
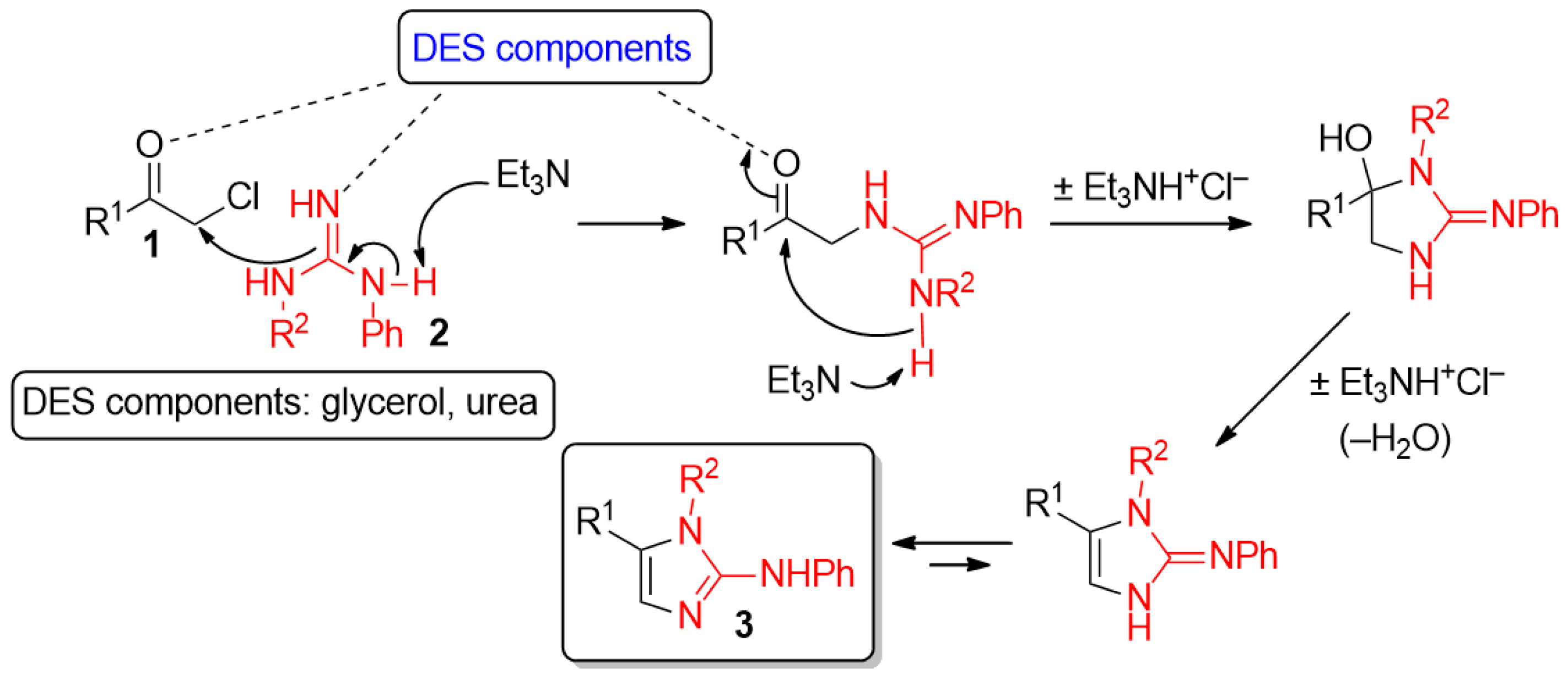
An interplay of factors may be responsible of the results obtained from the use of the ChCl–urea eutectic mixture: the higher nucleophilicity of guanidine 2a compared to that of urea (which does not compete despite being the bulk medium), the stronger intermolecular hydrogen-bonding interactions urea establishes with ChCl compared to Gly [42], which may reduce even more the solubility of the final product in such a DES mixture.
Hydrogen bond catalysis promoted by DESs components (e.g., glycerol and urea) may contribute as well to activate both carbonyl and guanidine groups, thereby exalting their electrophilic and nucleophilic character, respectively. This offers a possible explanation for the increase of reaction rate observed for such heterocyclodehydration processes in DES media compared to the longer reaction times needed in VOCs, instead. The role of a DES as a catalytic active species has also been suggested for other reactions [41,43,44,45,46,47,48,49]. A plausible and simple mechanism of formation of 2-AIs 3 from α-chloroketones 1 and guanidines 2 is depicted in Scheme 1. The tertiary base Et3N may be playing an active role in triggering as well as in driving the reaction to completion as in its absence the reaction proceeds quite sluggishly [50].
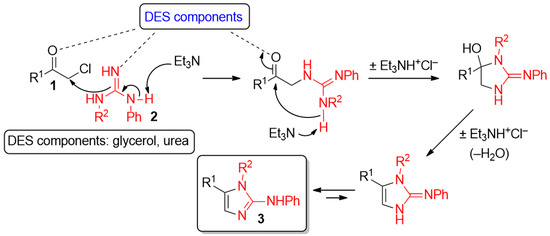
Scheme 1.
Plausible mechanism for the formation of 2-aminoimidazoles in DES mixtures.
3. Materials and Methods
3.1. General Informations
Reagents and solvents, unless otherwise specified, were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA) and used without any further purification. THF was purified by distillation from sodium/benzophenone before use. Petroleum ether refers to the 40–60 °C boiling fraction. The 1H- and the 13C-NMR spectra were recorded on a Bruker spectrometer (Bruker, Billerica, MA, USA) operating at 400.13 MHz for 1H and 100.62 MHz for 13C, with CDCl3 as the solvent and TMS as an internal standard (δ = 7.26 ppm for 1H spectra; δ = 77.0 ppm for 13C spectra). The IR spectra were recorded on a Jasco 4100 FT-IR spectrometer (Jasco, Easton, MD, USA) as CHCl3 solution. GC-MS analyses were performed with a gas-chromatograph equipped with a 5% phenylpolymethylsiloxane capillary column, 30 m, 0.25 mm i.d., and a mass-selective detector operating at 70 eV. The electrospray ionization (HRMS (ESI)) experiments were carried out in a hybrid Q-TOF mass spectrometer (Agilent, Santa Clara, CA, USA) equipped with an ion-spray ionization source. MS (+) spectra were acquired by direct infusion (5 mL∙min−1) of a solution containing the appropriate sample (10 pmol∙mL−1) dissolved in a solution 0.1% acetic acid, MeOH/H2O (50:50) at the optimum ion voltage of 4800 V. The nitrogen gas flow was set at 30 psi (pounds per square inch) and the potentials of the orifice, the focusing ring and the skimmer were kept at 30, 50 and 25 V relative to ground, respectively. TLC was performed on silica gel plates with F-254 indicator (Merck, Darmstadt, Germany); viewing was by UV light (254 nm) or phosphomolybdic acid staining solution. Chromatographic separations were performed on silica gel (63–200 mesh) using petroleum ether/AcOEt mixture as the eluent. All reactions involving air-sensitive reagents were performed under an atmosphere of nitrogen in oven-dried glassware by using syringe/septum cap techniques. The deep eutectic solvents ChCl–Gly (1:2 mol∙mol−1) and ChCl–urea (1:2 mol∙mol−1) were prepared by gently heating under stirring at 70 °C for 5 min the corresponding individual components until a clear solution was obtained. Reagent 2b was prepared as guanidinium carbonate 2b H2CO3 according to literature procedure [51]. For the preparation of compounds 3a–g in THF and 3h,i in EtOH, see Supplementary Materials.
3.2. Synthesis of 2-Aminoimidazoles 3a–g in the DES ChCl–Gly (1:2) (Table 1)
The appropriate α-chloroketone 1 (1.0 mmol), guanidine 2a (1.3 mmol) and Et3N (1 mmol) were added to the ChCl–Gly eutectic mixture (2 g) under magnetic stirring, and the mixture was then heated to 80 °C for a period of 4–6 h, until the ketone 1 disappeared, as revealed by GC-MS analysis. After this time, the mixture was cooled to room temperature and 5 mL of H2O were added. The resulting aqueous suspension was then extracted with AcOEt (3 × 10 mL). The combined organic phases were dried over Na2SO4 and concentrated in vacuo. The crude product was purified by flash-chromatography (silica gel; petroleum ether/AcOEt 80:20–95:5) to give the desired aminoimidazoles 3a–g.
3.3. Synthesis of 2-Aminoimidazoles 3h,i in the DES ChCl–Gly (1:2) (Table 1)
Guanidinium carbonate 2b H2CO3 (1.3 mmol) and KOH (1.3 mmol) were added to the ChCl–Gly eutectic mixture (2 g) under magnetic stirring, and the mixture was then heated to 80 °C for a period of 30 min, so as to liberate the free base of guanidine 2b in situ. After this time, α-chloroketone 1 and Et3N (1.3 mmol) were added, and the reaction stirred at 80 °C for 4–6 h until the ketone 1 disappeared, as revealed by GC-MS analysis. The reaction mixture was then cooled to room temperature and 5 mL of H2O were added. The resulting aqueous suspension was extracted with AcOEt (3 × 10 mL) and the combined organic phases were dried over Na2SO4 and concentrated in vacuo. The crude product was purified by flash-chromatography (silica gel; petroleum ether/AcOEt 20:80–40:60) to give the desired aminoimidazoles 3h,i.
3.4. Synthesis of 2-Aminoimidazoles 3a,b,d–g in the DES ChCl–Urea (1:2) (Table 2)
The appropriate α-chloroketone (1a,b,d–g) (1.0 mmol), guanidine 2a (1.3 mmol) and Et3N (1 mmol) were added to the ChCl–urea eutectic mixture (2 g) under magnetic stirring, and the mixture was then heated to 80 °C for a period of 4–6 h until the ketone 1 disappeared, as revealed by GC-MS analysis. After this time, the reaction mixture was cooled to room temperature and the purification of product 3a,d–g was achieved by filtration on paper. The brown solid obtained was further purified by crystallization from Et2O/hexane (ca. 8/2 ratio). The purification of compounds 3b was achieved as following: after the completion of the reaction, the mixture was cooled to room temperature and 5 mL of H2O were added. The resulting aqueous suspension was extracted with AcOEt (3 × 10 mL) and the combined organic phases were dried over Na2SO4, and concentrated in vacuo. The crude product was purified by flash-chromatography (silica gel; petroleum ether/AcOEt 20:80–40:60) to give the desired aminoimidazoles 3b.
3.5. Synthesis of 2-Aminoimidazoles 3h in the DES ChCl–Urea (1:2) (Table 2)
Guanidinium carbonate 2b H2CO3 (1.3 mmol) and KOH (1.3 mmol) were added to the ChCl–urea eutectic mixture (2 g) under magnetic stirring, and the mixture was then heated to 80 °C for a period of 30 min, so as to liberate the free base of guanidine 2b in situ. After this time, α-chloroketone 1a and Et3N (1.3 mmol) were added and the reaction stirred at 80 °C for 4 h until the ketone 1a disappeared, as revealed by GC-MS analysis. The reaction mixture was then cooled to room temperature and 5 mL of H2O were added. The resulting aqueous suspension was extracted with AcOEt (3 × 10 mL) and the combined organic phases were dried over Na2SO4 and concentrated in vacuo. The crude product was purified by flash-chromatography (silica gel; petroleum ether/AcOEt 20:80–40:60) to give the desired aminoimidazole 3h.
3.6. Characterization Data of Compounds 3a–i (Table 1)
N,1,5-Triphenyl-1H-imidazol-2-amine (3a). Pale yellow solid (264 mg, 85%), m.p. 116–118 °C. 1H-NMR (400.13 MHz, CDCl3): δ 6.10 (1 H, broad s, NH, exchanges with D2O), 6.86–6.90 (1 H, m, Ph), 7.09 (1 H, s, imidazole H), 7.22–7.46 (12 H, m, Ph), 7.83 (2 H, d, J = 7.3 Hz, Ph) ppm. 13C-NMR (100.62 MHz, CDCl3): δ 112.0, 116.2, 120.8, 124.5, 125.1, 126.5, 128.2, 128.4, 128.9, 129.9, 134.0, 136.2, 138.2, 141.1, 143.3 ppm. FT-IR (CHCl3): ν 3410 (NH), 3011, 2963, 2933, 2842, 1735, 1656, 1601, 1503, 1393 cm−1. GC/MS (70 eV): m/z (%) 311 (100) [M]+, 310 (35), 295 (33), 207 (49), 104 (20). HRMS (ESI): calcd. for C21H18N3 [M + H]+ 312.1501; found 312.1503.
5-tert-Butyl-N,1-diphenyl-1H-imidazol-2-amine (3b). Yellow oil (215 mg, 74%). 1H-NMR (400.13 MHz, CDCl3): δ 1.34 [9 H, s, (CH3)3C], 5.96 (1 H, broad s, NH, exchanges with D2O), 6.57 (1 H, s, imidazole H), 6.79–6.89 (1 H, m, Ph), 7.16–7.20 (4 H, m, Ph), 7.35–7.45 (5 H, m, Ph) ppm. 13C-NMR (100.62 MHz, CDCl3): δ 29.8, 31.8, 110.1, 115.7, 120.3, 124.8, 127.6, 128.9, 129.7, 137.0, 141.8, 142.2, 149.1 ppm. FT-IR (CHCl3): ν 3424 (NH), 3030, 2967, 2870, 1725, 1676, 1545, 1479, 1419, 1316 cm−1. GC/MS (70 eV): m/z (%) 291 (70) [M]+, 276 (100), 104 (15),77 (32). HRMS (ESI): calcd. for C19H22N3 [M + H]+ 292.1814; found 292.1813.
5-Methyl-N,1-diphenyl-1H-imidazol-2-amine (3c). Yellow oil (174 mg, 70%). 1H-NMR (400.13 MHz, CDCl3): δ 2.25 (3 H, s, CH3), 5.94 (1 H, broad s, NH, exchange with D2O), 6.58 (1 H, s, imidazole H), 6.86–6.99 (1 H, m, Ph), 7.20–7.49 (9 H, m, Ph) ppm. 13C-NMR (100.62 MHz, CDCl3): δ 13.9, 112.8, 116.2, 120.7, 125.0, 127.8, 129.0, 129.8, 134.9, 136.7, 141.6. 142.5 ppm. FT-IR (CHCl3): ν 3424 (NH), 3032, 2967, 2905, 2871, 1725, 1676, 1543, 1480, 1419, 1316 cm−1. GC/MS (70 eV): m/z (%) 249 (100) [M]+, 248 (68), 234 (40), 104 (25), 77 (38). HRMS (ESI): calcd. for C16H16N3 [M + H]+ 250.1345; found 250.1348.
N,1-Diphenyl-5-p-tolyl-1H-imidazol-2-amine (3d). Yellow solid (260 mg, 80%), m.p. 120–122 °C. 1H-NMR (400.13 MHz, CDCl3): δ 2.33 (3 H, s, CH3), 6.10 (1 H, broad s, NH, exchanges with D2O), 6.84–6.87 (1 H, s, Ph), 7.04 (1 H, s, imidazole H), 7.15–7.21 (4 H, m, Ar), 7.32–7.43 (7 H, m, Ar), 7.72 (2 H, d, J = 7.3 Hz, Ar) ppm. 13C-NMR (100.62 MHz, CDCl3): δ 21.1, 111.5, 116.1, 120.6, 124.4, 125.0, 128.0, 128.8, 129.0, 129.8, 131.2, 136.0, 136.3, 138.3, 141.2, 143.1 ppm. FT-IR (CHCl3): ν 3409 (NH), 3011, 2963, 2930, 2845, 1736, 1656, 1602, 1503, 1391 cm−1. GC/MS (70 eV): m/z (%) 325 (100) [M]+, 324 (26), 207 (50), 104 (12), 77 (23). HRMS (ESI): calcd. for C22H20N3 [M + H]+ 326.1658; found 326.1655.
5-(4-Methoxyphenyl)-N,1-diphenyl-1H-imidazol-2-amine (3e). Dark yellow solid (269 mg, 79%), m.p. 139–141 °C. 1H-NMR (400.13 MHz, CDCl3): δ 3.83 (3 H, s, OCH3), 6.11 (1 H, broad s, NH, exchanges with D2O), 6.87–6.94 (3 H, m, Ar), 7.07 (1 H, s, imidazole H), 7.23–7.52 (9 H, m, Ar), 7.76 (2 H, d, J = 7.4 Hz, Ar) ppm. 13C-NMR (100.62 MHz, CDCl3): δ = 55.2, 111.0, 113.9, 116.3, 120.8, 125.2, 125.8, 126.9, 128.2, 129.0, 130.0, 136.5, 138.2, 141.3, 143.2, 158.5 ppm. FT-IR (CHCl3): ν 3410 (NH), 3067, 3011, 2963, 1933, 2842, 1735, 1656, 1601, 1503, 1393 cm−1. GC/MS (70 eV): m/z (%) 341 (100) [M]+, 340 (20), 326 (15), 207 (48), 104 (12), 77 (25). HRMS (ESI): calcd. for C22H20N3O [M + H]+ 342.1607; found 342.1609.
5-(4-Chlorophenyl)-N,1-diphenyl-1H-imidazol-2-amine (3f). Yellow solid (276 mg, 80%), m.p. 106–108 °C. 1H-NMR (400.13 MHz, CDCl3): δ 6.06 (1 H, broad s, NH, exchanges with D2O) 6.92 (1 H, t, J = 7.3 Hz, Ar), 7.10 (1 H, s, imidazole H), 7.24–7.53 (11 H, m, Ar), 7.75 (2 H, d, J = 7.4 Hz, Ar) ppm. 13C-NMR (100.62 MHz, CDCl3): δ 112.2, 116.4, 121.1, 125.3, 125.8, 128.5(2C), 129.0, 130.1, 131.9, 132.6. 136.1, 137.3, 140.8, 143.5 ppm. FT-IR (CHCl3): ν 3425 (NH), 3067, 3010, 2929, 1682, 1598, 1545, 1497, 1484, 1451, 1385 cm−1. GC/MS (70 eV): m/z (%) 345 (100) [M]+, 344 (30), 329 (28), 207 (51), 104 (15), 77 (32). HRMS (ESI): calcd. for C21H17ClN3 [M + H]+ 346.1112; found 346.1109.
5-(4-Fluorophenyl)-N,1-diphenyl-1H-imidazol-2-amine (3g). Pale yellow solid (273 mg, 83%), m.p. 101–103 °C. 1H-NMR (400.13 MHz, CDCl3): δ 6.46 (1 H, broad s, NH, exchanges with D2O), 7.15 (1 H, t, J = 7.3 Hz, Ar), 7.26 (1 H, s, imidazole H),7.31 (2 H, t, J = 8.7 Hz, Ar), 7.49 (2 H, t, J = 7.9 Hz, Ar), 7.57–7.71 (7 H, m, Ar), 8.04–8.08 (2 H, m, Ar) ppm. 13C-NMR (100.62 MHz, CDCl3): δ 111.5, 115.0 (d, J = 21.3 Hz), 120.6, 124.9, 125.9, 125.9 (d, J = 5.3 Hz), 128.0, 128.7, 129.7, 130.2, 136.0, 137.2, 141.0, 143.2, 161.5 (d, J = 244.7 Hz) ppm. FT-IR (CHCl3): ν 3424 (NH), 3067, 3045, 3009, 2970, 1681, 1600, 1575, 1538, 1499, 1451, 1386, 1338, 1232 cm−1. GC/MS (70 eV): m/z (%) 329 (100) [M]+, 328 (29), 313 (29), 207 (35), 104 (15), 77 (25). HRMS (ESI): calcd. for C21H17FN3 [M + H]+ 330.1407; found 330.1405.
N,5-Diphenyl-1H-imidazol-2-amine (3h). Pale yellow solid (172 mg, 73%), m.p. 116–118 °C. 1H-NMR (400.13 MHz, CDCl3): δ 4.90 (2 H, broad s, 2 × NH, exchanges with D2O), 6.99 (1 H, s, imidazole H), 7.17–7.20 (1 H, m, Ph), 7.31–7.49 (7 H, m, Ph), 7.69 (2 H, d, J = 7.3 Hz, Ph) ppm. 13C-NMR (100.62 MHz, CDCl3): δ 111.2, 124.3, 124.5, 126.3, 127.7, 128.4, 129.8, 134.1, 136.9, 137.7, 148.0 ppm. FT-IR (CHCl3): ν 3464, 3067, 3037, 3009, 2962, 2930, 2855, 1616, 1600, 1504, 1454, 1354 cm−1. GC/MS (70 eV): m/z (%) 235 (100) [M]+, 234 (15), 193(12), 165 (15), 132 (75), 131 (35), 104 (30), 77 (25). HRMS (ESI): calcd. for C15H14N3 [M + H]+ 236.1188; found 236.1186.
5-tert-Butyl-N-phenyl-1H-imidazol-2-amine (3i). Pale yellow oil (155 mg, 72%). 1H-NMR (400.13 MHz, CDCl3): δ 1.27 (9 H, s, (CH3)3C), 4.40 (2 H, broad s, 2 × NH, exchanges with D2O), 6.38 (1 H, s, imidazole H),7.13–7.18 (2 H, m, Ph), 7.32–7.35 (1 H, m, Ph), 7.44–7.47 (2 H, m, Ph) ppm. 13C-NMR (100.62 MHz, CDCl3): δ 29.8, 31.4, 108.8, 124.3, 125.2, 127.2, 128.9, 137.8, 148.1 ppm. FT-IR (CHCl3): ν 3422 (NH), 3030, 2967, 2870, 1723, 1675, 1544, 1480, 1420, 1315 cm−1. GC/MS (70 eV): m/z (%) 215 (23) [M]+, 200 (100), 143 (8), 104 (10), 77 (20). HRMS (ESI): calcd. for C13H18N3 [M + H]+ 216.1501; found 216.1503.
4. Conclusions
In this paper we have described the first “green” and efficient condensation-mediated synthesis of 2-AIs successfully run in ChCl-based DESs as environmentally friendly, safe, and nonconventional reaction media. The scope of the reaction is broad in terms of the α-chloroketones employed, and both mono- and diphenyl-substituted guanidines proved to be competent partners. Compared to VOCs, shorter reaction times (4 to 6 h vs. 10 to 12 h) were required for the reaction to be completed in DESs, and the expected adducts could be isolated in very good yields (70%–80%), even with an operationally simple work-up procedure based on filtration and crystallization in the case of triaryl-substituted imidazoles prepared in a ChCl–urea eutectic mixture, which could be recycled. It is likely that some DES components (e.g., glycerol and urea) may speed up the reaction through a hydrogen bonding catalysis by activating both the carbonyl group and the guanidine derivative, thereby promoting first a nucleophilic substitution of the chloride ion, and subsequently the cyclodehydration reaction. Further investigation into the application of DESs both as reagents and catalysts in other condensation and multicomponent reactions are underway in our laboratory.
Supplementary Materials
General procedures for the synthesis of 2-AIs in THF and EtOH and the 1H- and 13C-NMR spectra of compounds 3a–i are available online at http://www.mdpi.com/1420-3049/21/7/924/s1.
Acknowledgments
The authors would like to acknowledge the Inter-University Consortium C.I.N.M.P.I.S., the University of Bari, and the University of Salento for supporting this work.
Author Contributions
M.C. carried out the experimental work; S.P., F.M.P., P.V. and L.T. analyzed data, wrote the experimental part, and participated in the discussion of the obtained results; L.T. contributed reagents/materials/analysis tools; and A.S. and V.C. conceived the experiments and wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Ashcroft, C.P.; Dunn, P.J.; Hayler, J.D.; Wells, A.S. Survey of Solvent Usage in Papers Published in Organic Process Research & Development 1997–2012. Org. Process Res. Dev. 2015, 19, 740–747. [Google Scholar]
- Dunn, P.J.; Wells, A.S.; Williams, M.T. Future Trends for Green Chemistry in the Pharmaceutical Industry. In Green Chemistry in the Pharmaceutical Industry; Dunn, P.J., Wells, A.S., Williams, M.T., Eds.; Wiley-VCH: Weinheim, Germany, 2010; pp. 333–355. [Google Scholar]
- Jung, F.; Olivier, A.; Boucherot, D. A new approach to the synthesis of amino imidazoles application to the cephalosporin series. Tetrahedron Lett. 1989, 30, 2379–2382. [Google Scholar] [CrossRef]
- Munk, S.A.; Harcourt, D.A.; Arasasingham, P.N.; Burke, J.A.; Kharlamb, A.B.; Manlapaz, C.A.; Padillo, E.U.; Roberts, D.; Runde, E.; Williams, L.; et al. Synthesis and Evaluation of 2-(Arylamino)imidazoles as α2-Adrenergic Agonists. J. Med. Chem. 1997, 40, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Kitamura, H.; Yamaguchi, K.; Fukuzawa, S.; Kamijima, C.; Yazawa, K.; Kuramoto, M.; Wang, G.Y.S.; Fujitani, Y.; Uemura, D. Development of chemical substances regulating biofilm formation. Bull. Chem. Soc. Jpn. 1997, 70, 3061–3069. [Google Scholar] [CrossRef]
- Assmann, M.; Lichte, E.; Pawlik, J.R.; Kock, M. Chemical defenses of the Caribbean sponges Agelas wiedenmayeri and Agelas conifera. Mar. Ecol. Prog. Ser. 2000, 207, 255–262. [Google Scholar] [CrossRef]
- Tamm, M.; Randoll, S.; Bannenberg, T.; Herdtweck, E. Titanium complexes with imidazolin-2-iminato ligand. Chem. Commun. 2004, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Randoll, S.; Herdtweck, E.; Kleigrewe, N.; Kehr, G.; Erker, G.; Rieger, B. Imidazolin-2-iminato titanium complexes: Synthesis, structure and use in ethylene polymerization catalysis. Dalton Trans. 2006, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Selig, P. Guanidine Organocatalysis. Synthesis 2013, 45, 703–718. [Google Scholar] [CrossRef]
- Sullivan, J.D.; Giles, R.L.; Looper, R.E. 2-Aminoimidazoles from Leucetta Sponges: Synthesis and Biology of an Important Pharmacophore. Curr. Bioact. Compd. 2009, 5, 39–78. [Google Scholar] [CrossRef]
- Žula, A.; Kikelj, D.; Ilaš, J. 2-Aminoimidazoles in medicinal chemistry. Mini Rev. Med. Chem. 2013, 13, 1921–1943. [Google Scholar] [CrossRef] [PubMed]
- Little, T.L.; Webber, S.E. A Simple and Practical Synthesis of 2-Aminoimidazoles. J. Org. Chem. 1994, 59, 7299–7305. [Google Scholar] [CrossRef]
- Lemrová, B.; Soural, M. Synthetic Strategies for Preparing Bicyclic Guanidines. Eur. J. Org. Chem. 2015, 1869–1886. [Google Scholar] [CrossRef]
- Gibbons, J.B.; Gligorich, K.M.; Welm, B.E.; Looper, R.E. Synthesis of the Reported Structures for Kealiinines B and C. Org. Lett. 2012, 14, 4734–4737. [Google Scholar] [CrossRef] [PubMed]
- Giles, R.L.; Sullivan, J.D.; Steiner, A.M.; Looper, R.E. Addition-Hydroamination Reactions of Propargyl Cyanamides: Rapid Access to Highly Substituted 2-Aminoimidazoles. Angew. Chem. Int. Ed. 2009, 48, 3116–3120. [Google Scholar] [CrossRef] [PubMed]
- Ermolat’ev, D.S.; Bariwal, J.B.; Steenackers, H.P.L.; De Keersmaecker, S.C.J.; Van der Eycken, E.V. Concise and Diversity-Oriented Route toward Polysubstituted 2-Aminoimidazole Alkaloids and Their Analogues. Angew. Chem. Int. Ed. 2010, 49, 9465–9468. [Google Scholar] [CrossRef] [PubMed]
- Zavesky, B.P.; Babij, N.R.; Wolfe, J.P. Synthesis of Substituted 2-Aminoimidazoles via Pd-Catalyzed Alkyne Carboamination Reactions. Application to the Synthesis of Preclathridine Natural Products. Org. Lett. 2014, 16, 4952–4955. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Eickhoff, J.A.; Zakarian, A. Synthesis of 2-Aminoazoles from Thioesters via α-Heterosubstituted Ketones by Copper-Mediated Cross-Coupling. J. Org. Chem. 2015, 80, 9989–9999. [Google Scholar] [CrossRef] [PubMed]
- Garlets, Z.J.; Silvi, M.; Wolfe, J.P. Synthesis of Cyclic Guanidines via Silver-Catalyzed Intramolecular Alkene Hydroamination Reactions of N-Allylguanidines. Org. Lett. 2016, 18, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem. Eur. J. 2007, 13, 6495–6501. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Vigier, K.; Chatel, G.; Jérôme, F. Contribution of Deep Eutectic Solvents for Biomass Processing: Opportunities, Challenges, and Limitations. ChemCatChem 2015, 7, 1250–1260. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 4, 612–632. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C. Deep eutectic solvents in polymerizations: A greener alternative to conventional syntheses. ChemSusChem 2014, 7, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Mallardo, V.; Rizzi, R.; Sassone, F.C.; Mansueto, R.; Perna, F.M.; Salomone, A.; Capriati, V. Regioselective desymmetrization of diaryltetrahydrofurans via directed ortho-lithiation: An unexpected help from green chemistry. Chem. Commun. 2014, 50, 8655–8658. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; García-Álvarez, J.; Hernán-Gómez, A.; Kennedy, A.R.; Hevia, E. Introducing Deep Eutectic Solvents to Polar Organometallic Chemistry: Chemoselective Addition of Organolithium and Grignard Reagents to Ketones in Air. Angew. Chem. Int. Ed. 2014, 53, 5969–5973. [Google Scholar] [CrossRef] [PubMed]
- Sassone, F.C.; Perna, F.M.; Salomone, A.; Florio, S.; Capriati, V. Unexpected lateral-lithiation-induced alkylative ring opening of tetrahydrofurans in deep eutectic solvents: Synthesis of functionalised primary alcohols. Chem. Commun. 2015, 51, 9459–9462. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, J.; Hevia, E.; Capriati, V. Reactivity of Polar Organometallic Compounds in Unconventional Reaction Media: Challenges and Opportunities. Eur. J. Org. Chem. 2015, 6779–6799. [Google Scholar] [CrossRef]
- Cicco, L.; Sblendorio, S.; Mansueto, R.; Perna, F.M.; Salomone, A.; Florio, S.; Capriati, V. Water opens the door to organolithiums and Grignard reagents: Exploring and comparing the reactivity of highly polar organometallic compounds in unconventional reaction media towards the synthesis of tetrahydrofurans. Chem. Sci. 2016, 7, 1192–1199. [Google Scholar] [CrossRef]
- Rodríguez-Àlvarez, M.J.; Vidal, C.; Díez, J.; García-Àlvarez, J. Introducing deep eutectic solvents as biorenewable media for Au(I)-catalysed cycloisomerisation of γ-alkynoic acids: An unprecedented catalytic system. Chem. Commun. 2014, 50, 12927–12929. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Merz, L.; García-Àlvarez, J. Deep eutectic solvents: Biorenewable reaction media for Au(I)-catalysed cycloisomerisations and one-pot tandem cycloisomerisation/Diels-Alder reactions. Green Chem. 2015, 17, 3870–3878. [Google Scholar] [CrossRef]
- Mancuso, R.; Maner, A.; Cicco, L.; Perna, F.M.; Capriati, V.; Gabriele, B. Synthesis of thiophenes in a deep eutectic solvent: heterocyclodehydration and iodocyclization of 1-mercapto-3-yn-2-ols in a choline chloride/glycerol medium. Tetrahedron 2016, 72, 4239–4244. [Google Scholar] [CrossRef]
- Müller, C.R.; Meiners, I.; de María, P.D. Highly enantioselective tandem enzyme-organocatalyst crossed aldol reactions with acetaldehyde in deep-eutectic-solvents. RSC Adv. 2014, 4, 46097–46101. [Google Scholar] [CrossRef]
- Massolo, E.; Palmieri, S.; Benaglia, M.; Capriati, V.; Perna, F.M. Stereoselective organocatalysed reactions in deep eutectic solvents: Highly tunable and biorenewable reaction media for sustainable organic synthesis. Green Chem. 2016, 18, 792–797. [Google Scholar] [CrossRef]
- Martínez, R.; Berbegal, L.; Guillena, G.; Ramón, D.J. Bio-renewable enantioselective aldol reaction in natural deep eutectic solvents. Green Chem. 2016, 18, 1724–1730. [Google Scholar] [CrossRef]
- By running the reaction at a lower temperature (66 °C), compound 3a could be isolated only with a slight decrease of the reaction yield (80%) after 4 h.
- Singh, B.S.; Lobo, H.R.; Pinjari, D.V.; Jarag, K.J.; Pandit, A.B.; Shankarling, G.S. Comparative material study and synthesis of 4-(4-nitrophenyl)oxazol-2-amine via sonochemical and thermal method. Ultrason. Sonochem. 2013, 20, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.L.; Painter, P.; Colina, C.M. Experimental and Computational Studies of Choline Chloride-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2014, 59, 3652–3662. [Google Scholar] [CrossRef]
- Hawkins, I.; Handy, S.T. Synthesis of aurones under neutral conditions using a deep eutectic solvent. Tetrahedron 2013, 69, 9200–9204. [Google Scholar] [CrossRef]
- Handy, S.; Lavender, K. Organic Synthesis in deep eutectic solvents: Paal-Knorr reactions. Tetrahedron Lett. 2013, 54, 4377–4379. [Google Scholar] [CrossRef]
- Nemati, F.; Hosseini, M.M.; Kiani, H. Glycerol as a green solvent for efficient, one-pot and catalyst free synthesis of 2,4,5-triaryl and 1,2,4,5-tetraaryl imidazole derivatives. J. Saudi Chem. Soc. 2013, in press. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.-T.; Ma, E.-Q.; Mo, L.-P.; Zhang, Z.-H. Superparamagnetic CuFeO2 Nanoparticles in Deep Eutectic Solvent: An Efficient and Recyclable Catalytic System for the Synthesis of Imidazo(1,2-a)pyridines. ChemCatChem 2014, 6, 2854–2859. [Google Scholar] [CrossRef]
- Hu, H.-C.; Liu, Y.-H.; Li, B.-L.; Cui, Z.-S.; Zhang, Z.-H. Deep eutectic solvent based on choline chloride and malonic acid as an efficient and reusable catalytic system for one-pot synthesis of functionalized pyrroles. RSC Adv. 2015, 5, 7720–7728. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.; Zhang, Z.-H. A General, Efficient and Green Procedure for Synthesis of Dihydropyrimidine-5-carboxamides in Low Melting Betaine Hydrochloride/Urea Mixture. Chin. J. Chem. 2016, 34, 637–645. [Google Scholar] [CrossRef]
- It is worth noting that the use of weaker bases than Et3N has proved to be not effective. For example, once the cyclocondensation of α-chloroketone 1a with guanidine derivative 2a was carried out in a ChCl–Gly eutectic mixture in the presence of 1,4-diazabicyclo(2.2.2)octane (DABCO), the expected 2-AI 3a could be isolated in 67% yield only, the remaining material being the dehalogenated starting ketone, that is acetophenone. For a basicity scale, see: Frenna, V.; Vivona, N.; Consiglio, G.; Spinelli, D. Amine basicities in benzene and in water. J. Chem. Soc. Perkin Trans. 2 1985, 1865–1868. [Google Scholar] [CrossRef]
- Shao, J.; Chen, W.; Giulianotti, M.A.; Houghten, R.A.; Yu, Y. Palladium-Catalyzed C-H Functionalization Using Guanidine as a Directing Group: Ortho Arylation and Olefination of Arylguanidines. Org. Lett. 2012, 14, 5452–5455. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 3a–i are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).