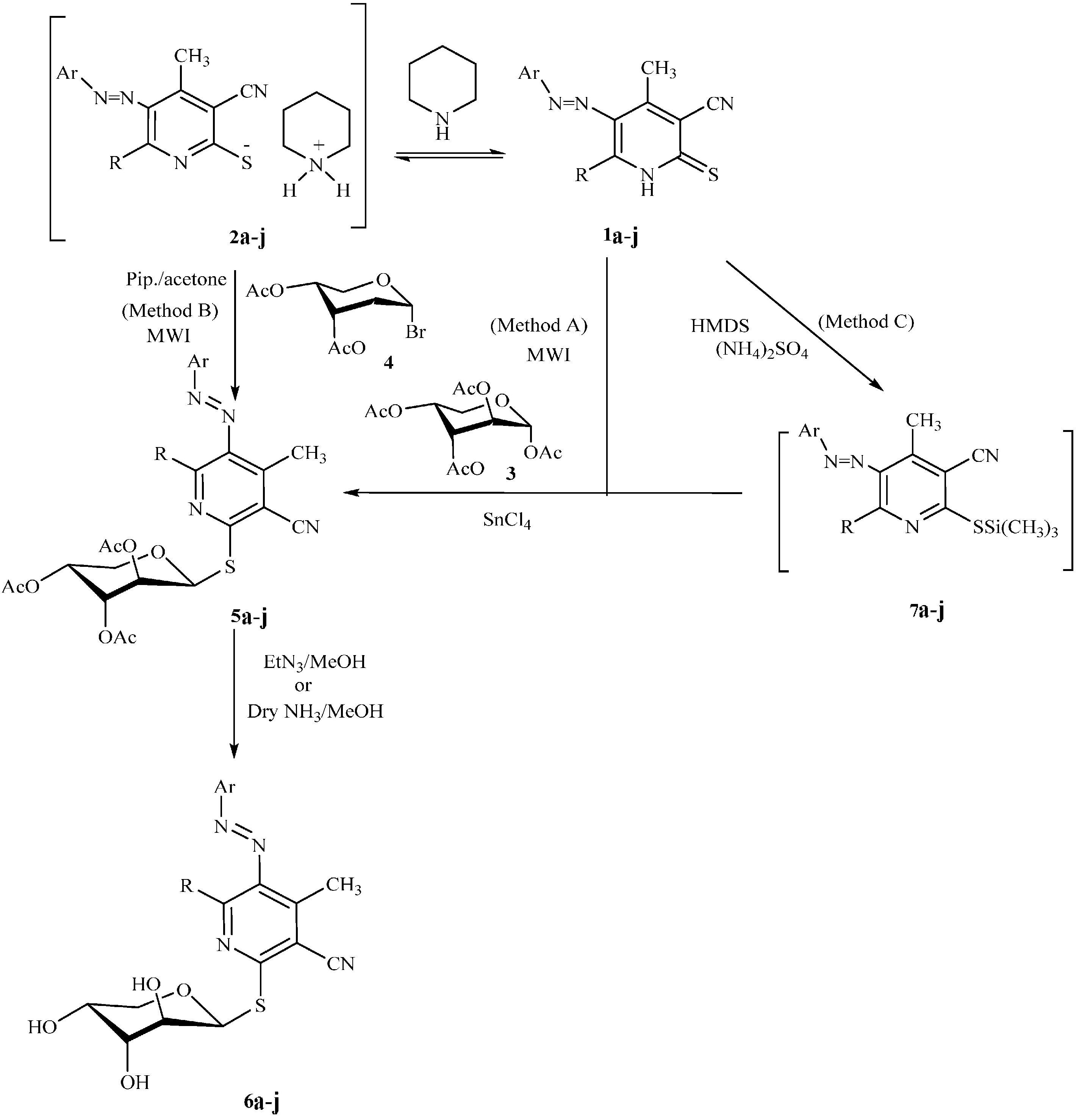
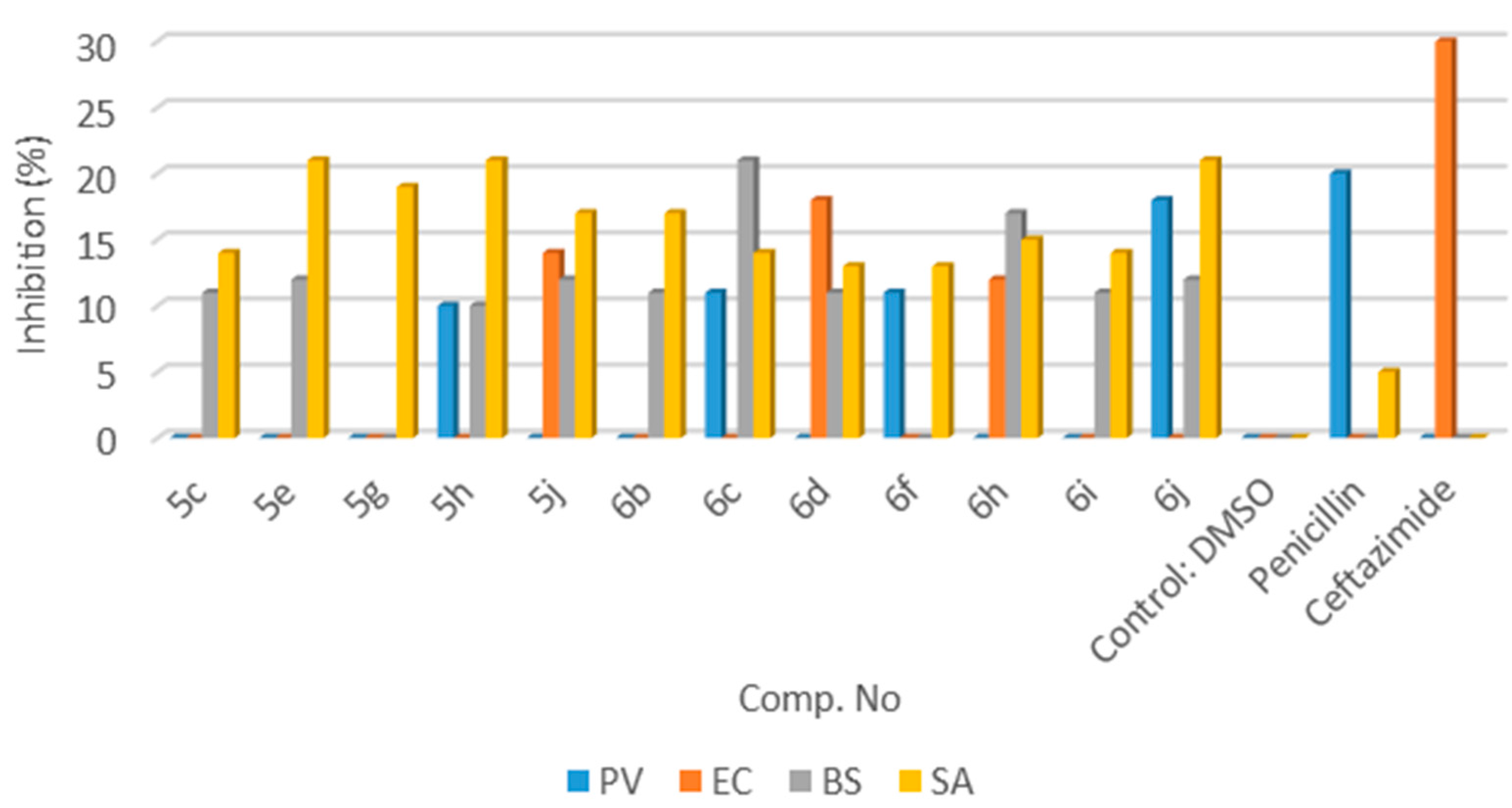
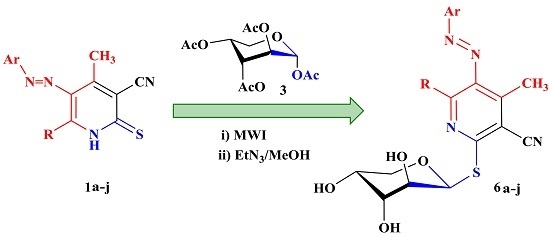
3.2.2. Product Characterization
3-Cyano-4,6-dimethyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-5-phenylazopyridine (5a): MP 132 °C; IR (KBr, cm−1) υ 2224 (CN); COSY; 1H-NMR (CDCl3) δ = 2.04, 2.11 and 2.19 (3 s, 9H, 3CH3CO), 2.27 (s, 3H, CH3), 2.59 (s, 3H,CH3), 3.74, 3.82 (dd, 1H, H-5a″, J = 3.9, 8.5 Hz), 4.17, 4.28 (dd, 1H, H-5b″, J = 3.9, 8.5 Hz), 5.33–5.37 (m, 3H, H-2″, H-3″ and H-4″), 6.46 (d, 1H, H-1″, JH-1″–H-2″ = 2.3 Hz), 7.34–7.92 (m, 5H, Ar-H); 13C-NMR (CDCl3) δ = 18.1 (CH3), 21.0, 21.1 and 21.2 (3CH3CO), 23.4 (CH3), 59.7 (C-5″), 65.7 (C-4″), 68.1 (C-3″), 69.0 (C-2″), 92.5 (C-1″), 97.3 (C-3), 113.9 (CN), 122.5–155.1 (Ar-C), 159.1 (C-2), 168.9, 169.6 and 170.4 (3CO); LC-MS (ionization method): m/z 527 [M + H]+; Anal. Calcd. for C25H26N4O7S: C, 57.03; H, 4.98; N, 10.64%. Found: C, 57.10; H, 5.01; N, 10.73%.
3-Cyano-4,6-dimethyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-5-(4′-bromophenylazo)pyridine (5b): MP 141 °C; IR (KBr, cm−1) υ 2228 (CN); COSY; NOESY; gHMBC; 1H-NMR (CDCl3) δ = 2.07, 2.15 and 2.25 (3s, 9H, 3CH3CO), 2.55 (s, 3H,CH3), 2.59 (s, 3H,CH3), 3.72, 3.79 (dd, 1H, H-5a, J = 4.6, 8.5 Hz), 4.10, 4.15 (dd, 1H, H-5b, J = 4.6, 8.5 Hz), 5.20–5.31 (m, 3H, H-2″, H-3″, H-4″ ); 6.41 (d, 1H, H-1″, JH-1″–H-2″ = 2.6 Hz), 7.58 (d, 2H, Ar-H, J = 8.7 Hz), 7.76 (d, 2H, Ar-H, J = 8.7 Hz); 13C-NMR (CDCl3) δ = 17.8 (CH3), 21.0, 21.5 and 22.0 (3CH3CO), 23.3 (CH3), 59.5 (C-5″), 65.1 (C-4″), 67.5 (C-3″), 68.8 (C-2″), 92.7 (C-1″), 96.9 (C-3), 113.8 (CN), 123.9–155.2 (Ar-C), 161.1 (C-2), 168.9, 169.8 and 170.2 (3CO); LC-MS (ionization method): m/z 605 [M + 1]; Anal. Calcd. for C25H25BrN4O7S: C, 49.59; H, 4.16; N, 9.25%. Found: C, 49.66; H, 4.05; N, 9.31%.
3-Cyano-4,6-dimethyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-5-(4′-chlorophenylazo)pyridine (5c): MP 129 °C; IR (KBr, cm−1) υ 2228 (CN); COSY; NOESY; gHMBC; 1H-NMR (CDCl3) δ = 2.10, 2.15 and 2.19 (3s, 9H, 3CH3CO), 2.55 (s, 3H,CH3), 2.59 (s, 3H,CH3), 3.74, 3.82 (dd, 1H, H-5a, J = 3.8, 8.4 Hz), 4.12, 4.18 (dd, 1H, H-5b, J = 3.8, 8.4 Hz), 5.31–5.39 (m, 3H, H-2″, H-3″ and H-4″), 6.42 (d, 1H, H-1″, JH-1″–H-2″ = 2.7 Hz), 7.51(d, 2H, Ar-H, J = 4.8 Hz); 7.81 (d, 2H, Ar-H, J = 4.8 Hz); 13C-NMR (CDCl3) δ = 18.2 (CH3), 21.1, 21.15 and 22.0 (3CH3CO), 23.4 (CH3), 60.2 (C-5″), 66.1 (C-4″), 68.1 (C-3″), 68.7 (C-2″), 92.7 (C-1″), 96.8 (C-3), 113.8 (CN), 124.5–155.6 (Ar-C), 160.8 (C-2), 169.6, 170.2 and 170.9 (3CO); LC-MS (ionization method): m/z 546 [M + 1]; Anal. Calcd. for C25H25ClN4O7S: C, 53.52; H, 4.49; N, 9.99%. Found: C, 53.77; H, 4.39; N, 10.06%.
3-Cyano-4,6-dimethyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-5-(4′-methylphenylazo)pyridine (5d): MP 134 °C; IR (KBr, cm−1) υ 2230 (CN); COSY; 1H-NMR (CDCl3) δ = 2.07, 2.15 and 2.19 (3s, 9H, 3CH3CO), 2.42 (s, 3H, CH3), 2.51 (s, 3H, CH3), 2.55 (s, 3H, CH3), 3.74, 3.77 (dd, 1H, H-5a″, J = 2.3, 8.0 Hz), 4.12, 4.17 (dd, 1H, H-5b″, J = 2.3, 8.0 Hz), 5.36–5.41 (m, 3H, H-2″, H-3″ and H-4″), 6.40 (d, 1H, H-1″, JH-1″–H-2″ = 2.7 Hz), 7.31 (d, 2H, Ar-H, J = 8.1 Hz); 7.80 (d, 2H, Ar-H, J = 8.1 Hz); 13C-NMR (CDCl3) δ = 17.8 (CH3), 21.1, 21.3 and 21.8 (3CH3CO), 23.1 (CH3), 29.7 (CH3), 59.5 (C-5″), 65.7 (C-4″), 67.8 (C-3″), 68.8 (C-2″), 92.4 (C-1″), 96.8 (C-3), 114.1 (CN), 122.3–155.4 (Ar-C), 159.9 (C-2), 168.9, 170.1 and 170.8 (3CO); LC-MS (ionization method): m/z 540 [M]; Anal. Calcd. for C26H28N4O7S: C, 57.77; H, 5.22; N, 10.36%. Found: C, 57.81; H, 5.12; N, 10.20%.
3-Cyano-4,6-dimethyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-5-(4′-methoxyphenylazo)pyridine (5e): MP 142 °C; IR (KBr, cm−1) υ 2227 (CN); COSY; 1H-NMR (CDCl3) δ = 2.05, 2.15 and 2.19 (3s, 9H, 3CH3CO), 2.55 (s, 3H, CH3), 2.59 (s, 3H, CH3), 3.52 (s, 3H, OCH3), 3.68, 3.71 (dd, 1H, H-5a″, J = 2.5, 8.0 Hz), 4.11, 4.16 (dd, 1H, H-5b″, J = 2.5, 8.0 Hz), 5.35–5.41 (m, 3H, H-2″, H-3″ and H-4″), 6.41 (d, 1H, H-1″, JH-1″–H-2″ = 2.7 Hz), 7.31 (d, 2H, Ar-H, J = 8.0 Hz); 7.80 (d, 2H, Ar-H, J = 8.0 Hz); 13C-NMR (CDCl3) δ = 21.0, 21.2 and 21.9 (3CH3CO), 22.8 (CH3), 29.7 (CH3), 40.3 (OCH3), 59.8 (C-5″), 65.8 (C-4″), 67.8 (C-3″), 69.1 (C-2″), 92.6 (C-1″), 97.3 (C-3), 114.0 (CN), 123.1–155.4 (Ar-C), 160.3 (C-2), 169.5, 170.4 and 170.9 (3CO); LC-MS (ionization method): m/z 557 [M + 1]; Anal. Calcd. for C26H28N4O8S: C, 56.11; H, 5.07; N, 10.07%. Found: C, 56.32; H, 5.19; N, 10.23%.
3-Cyano-4-methyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-5-phenylazo-6-phenylpyridine (5f): MP 177 °C; IR (KBr, cm−1) υ 2234 (CN); COSY; 1H-NMR (CDCl3) δ = 2.14, 2.21 and 2.25 (3s, 9H, 3CH3CO), 2.64 (s, 3H, CH3), 3.81, 3.86 (dd, 1H, H-5a″, J = 4.1, 8.5 Hz), 4.19, 4.25 (dd, 1H, H-5b″, J = 4.1, 8.5 Hz), 5.32–5.38 (m, 3H, H-2″, H-3″ and H-4″), 6.39 (d, 1H, H-1″, JH-1″–H-2″ = 2.1 Hz), 7.21–7.76 (m, 10H, Ar-H); 13C-NMR (CDCl3) δ = 19.1 (CH3), 21.3, 21.4 and 21.5 (3CH3CO), 60.3 (C-5″), 65.7 (C-4″), 68.3 (C-3″), 69.1 (C-2″), 92.5 (C-1″), 98.3 (C-3), 114.3 (CN), 123.3–147.4 (Ar-C), 152.3 (C-2), 169.2, 169.9 and 170.4 (3CO); LC-MS (ionization method): m/z 589 [M + 1]; Anal. Calcd. for C30H28N4O7S: C, 61.21; H, 4.79; N, 9.52%. Found: C, 61.51; H, 4.83; N, 9.74%.
3-Cyano-4-methyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-6-phenyl-5-(4′-bromophenylazo)-pyridine (5g): MP 183 °C; IR (KBr, cm−1) υ 2227 (CN); COSY; 1H-NMR (CDCl3) δ = 2.15, 2.21 and 2.27 (3s, 9H, 3CH3CO), 2.66 (s, 3H, CH3), 3.79, 3.85 (dd, 1H, H-5a″, J = 4.2, 8.1 Hz,), 4.23, 4.29 (dd, 1H, H-5b″, J = 4.2, 8.1 Hz), 5.35–5.39 (m, 3H, H-2″, H-3″ and H-4″), 6.55 (d, 1H, H-1″, JH-1″–H-2″ = 2.0 Hz), 7.28–7.91 (m, 9H, Ar-H); 13C-NMR (CDCl3) δ =18.9 (CH3), 21.2, 21.3 and 21.4 (3CH3CO), 59.8 (C-5"), 65.8 (C-4″), 67.7 (C-3″), 68.9 (C-2″), 93.2 (C-1″), 98.4 (C-3), 114.1 (CN), 124.3–154.9 (Ar-C), 160.0 (C-2), 168.9, 169.7 and 170.8 (3CO); LC-MS (ionization method): m/z 667 [M + 1]; Anal. Calcd. for C30H27BrN4O7S: C, 53.98; H, 4.08; N, 8.39%. Found: C, 54.03; H, 4.19; N, 8.52%.
3-Cyano-4-methyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-6-phenyl-5-(4′-chlorophenylazo)-pyridine (5h): MP 172 °C; IR (KBr, cm−1) υ 2231 (CN); COSY, NOESY, gHMBC, gHMQC; 1H-NMR (CDCl3) δ = 2.16, 2.25 and 2.29 (3s, 9H, 3CH3CO), 2.65 (s, 3H,CH3), 3.80, 3.86 (dd, 1H, H-5a″, J = 4.0, 8.1 Hz), 4.20, 4.30 (dd, 1 H, H-5b″, J = 4.0, 8.1 Hz), 5.39–5.42 (m, 3H, H-2″, H-3″ and H-4″), 6.54 (d, 1 H, H-1″, JH-1″–H-2″ = 1.9 Hz), 7.25–7.76 (m, 9H, Ar-H); 13C-NMR (CDCl3) δ = 19.1 (CH3), 21.2, 21.3 and 21.4 (3CH3CO), 60.1 (C-5″), 65.8 (C-4″), 67.8 (C-3″), 69.0 (C-2″), 93.1 (C-1″), 98.1 (C-3), 113.9 (CN), 123.4–155.1 (Ar-C), 159.8 (C-2), 169.3, 170.2 and 170.6 (3CO); LC-MS (ionization method): m/z 623 [M + 1]; Anal. Calcd. for C30H27ClN4O7S: C, 57.83; H, 4.37; N, 8.99%. Found: C, 57.58; H, 4.61; N, 9.20%.
3-Cyano-4-methyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-6-phenyl-5-(4′-methylphenylazo)-pyridine (5i): MP 159 °C; IR (KBr, cm−1) υ 2231 (CN); COSY; 1H-NMR (CDCl3) δ = 2.15, 2.23 and 2.27 (3s, 9H, 3CH3CO), 2.47 (s, 3H, CH3), 2.63 (s, 3H, CH3), 3.78, 3.86 (dd, 1H, H-5a″, J = 4.1, 8.2 Hz,), 4.22, 4.35 (dd, 1H, H-5b″, J = 4.1, 8.2 Hz), 5.35–5.39 (m, 3 H, H-2″, H-3″ and H-4″), 6.53 (d, 1 H, H-1″, JH-1″–H-2″ = 2.2 Hz), 7.30–7.81 (m, 9H, Ar-H); 13C-NMR (CDCl3) δ = 18.6 (CH3), 21.2, 21.3 and 21.4 (3 CH3CO), 22.0 (CH3), 60.1 (C-5″), 65.9 (C-4″), 68.2 (C-3″), 68.9 (C-2″), 93.1 (C-1″), 98.1 (C-3), 114.1 (CN), 122.5–154.0 (Ar-C), 159.7 (C-2), 168.5, 169.9 and 170.4 (3CO); LC-MS (ionization method): m/z 602 [M]; Anal. Calcd. for C31H30N4O7S: C, 61.78; H, 5.02; N, 9.30%. Found: C, 61.91; H, 5.20; N, 9.18%.
3-Cyano-4-methyl-2-(2″,3″,4″-tri-O-acetyl-β-d-arabinopyranosylthio)-6-phenyl-5-(4′-methoxyphenylazo)-pyridine (5j): MP 149 °C; IR (KBr, cm−1) υ 2229 (CN); COSY; 1H-NMR (CDCl3) δ = 2.15, 2.28 and 2.30 (3s, 9H, 3CH3CO), 2.61 (s, 3H, CH3), 3.65 (s, 3H, OCH3), 3.77, 3.87 (dd, 1H, H-5a″, J = 4.1, 8.1 Hz,), 4.23, 4.35 (dd, 1H, H-5b″, J = 4.1, 8.1 Hz), 5.37–5.41 (m, 3 H, H-2″, H-3″ and H-4″), 6.51 (d, 1 H, H-1″, JH-1″–H-2″ = 2.2 Hz), 7.25–7.80 (m, 9H, Ar-H); 13C-NMR (CDCl3) δ = 18.7 (CH3), 21.2, 21.3 and 21.4 (3CH3CO), 45.7 (OCH3), 60.2 (C-5″), 65.5 (C-4″), 67.9 (C-3″), 68.9 (C-2″), 93.2 (C-1″), 98.2 (C-3), 114.1 (CN), 122.4–154.2 (Ar-C), 159.9 (C-2), 169.1, 169.8 and 170.4 (3CO); LC-MS (ionization method): m/z 619 [M + 1]; Anal. Calcd. for C31H30N4O8S: C, 60.18; H, 4.89; N, 9.06%. Found: C, 60.25; H, 5.10; N, 9.11%.
3.2.4. Product Characterization
2-(β-d-Arabinopyranosylthio)-3-cyano-4,6-dimethyl-5-phenylazopyridine (6a): MP 225 °C; IR (KBr, cm−1) υ 3431 (OH), 2237 (CN); COSY; 1H-NMR (DMSO-d6) δ 2.57 (s, 3H, CH3), 2.63 (s, 3H, CH3), 3.57–3.94 (m, 5H, H-2″, H-3″, H-4″ and 2H-5″), 4.84–5.43 (3OH, exchangeable with D2O), 6.04 (d,1H, H-1″, JH-1″–H-2″ = 6.5 Hz,), 7.50–7.99 (m, 5H, Ar-H); 13C-NMR (DMSO-d6) δ = 18.8 (CH3), 23.2 (CH3), 65.9 (C-5″), 67.9 (C-4″), 70.4 (C-3″), 72.8 (C-2″), 96.3 (C-1″), 97.1 (C-3), 114.3 (CN), 121.5–154.8 (Ar-C), 160.8 (C-2); LC-MS (ionization method): m/z 401 [M + 1]+; Anal. Calcd. for C19H20N4O4S: C, 56.99; H, 5.03; N, 13.99%. Found: C, 57.17; H, 5.21; N, 14.21%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4,6-dimethyl-5-(4′-bromophenylazo)pyridine (6b): MP 234 °C; IR (KBr, cm−1) υ 3417 (OH), 2228 (CN); COSY; NOESY; gHMBC; 1H-NMR (DMSO-d6) δ = 2.62 (s, 3H, CH3), 2.64 (s, 3H, CH3), 3.47–3.88 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.83–5.40 (3OH, exchangeable with D2O), 6.03 (d, H-1″, JH-1″–H-2″ = 6.9 Hz), 7.69–7.85 (m, 4H, Ar-H); 13C-NMR (DMSO-d6) δ = 18.1 (CH3), 23.2 (CH3), 65.7 (C-5″), 67.3 (C-4″), 69.4 (C-3″), 72.5 (C-2″), 96.3 (C-1″), 97.6 (C-3), 114.4 (CN), 123.4–155.3 (Ar-C), 161.3 (C-2); LC-MS (ionization method): m/z 479 [M + 1] Anal. Calcd. for C19H19BrN4O4S: C, 47.61; H, 4.00; N, 11.69%. Found: C, 47.76; H, 4.04; N, 11.87%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4,6-dimethyl-5-(4′-chlorophenylazo)pyridine (6c): MP 241 °C; IR (KBr, cm−1) υ 3382 (OH), 2228 (CN); COSY; NOESY; gHMBC; 1H-NMR (DMSO-d6) δ = 2.63 (s, 3H, CH3), 2.65 (s, 3H, CH3), 3.40–3.89 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.81–5.42 (3OH, exchangeable with D2O), 6.10 (d, ,1H, H-1″, JH-1″–H-2″ = 6.9 Hz), 7.73 (d, 2H, Ar-H, J = 8.9 Hz), 7.93 (d, 2H, Ar-H, J = 8.9 Hz); 13C-NMR (DMSO-d6) δ = 18.2 (CH3), 23.3 (CH3), 65.7 (C-5″), 67.8 (C-4″), 70.1 (C-3″), 72.8 (C-2″), 96.6 (C-1″), 97.5 (C-3), 114.2 (CN), 124.0–155.1 (Ar-C), 161.8 (C-2); LC-MS (ionization method): m/z 435 [M + 1]+; Anal. Calcd. for C19H19ClN4O4S: C, 52.47; H, 4.40; N, 12.88%. Found: C, 52.51; H, 4.72; N, 13.03%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4,6-dimethyl-5-(4′-methylphenylazo)pyridine (6d): MP 210 °C; IR (KBr, cm−1) υ 3388 (OH), 2230 (CN); COSY; NOESY; ROESY; gHMBC; gHMQC; 1H-NMR (DMSO-d6) δ = 2.45 (s, 3H, CH3), 2.63 (s, 3H, CH3), 2.64 (s, 3H, CH3), 3.64–3.92 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.79–5.43 (3OH, exchangeable with D2O), 6.03 (d, 1H, H-1″, JH-1″–H-2″ = 6.9 Hz), 7.53 (d, 2H, Ar-H, J = 8.6 Hz), 7.91 (d, 2H, Ar-H, J = 8.6 Hz); 13C-NMR (DMSO-d6) δ = 18.3 (CH3), 21.3 (CH3), 22.4 (CH3), 66.4 (C-5″), 67.7 (C-4″), 70.8 (C-3″), 73.3 (C-2″), 97.0 (C-1″), 98.4 (C-3), 114.2 (CN), 123.3–155.7 (Ar-C), 162.0 (C-2); LC-MS (ionization method): m/z 414 [M]; Anal. Calcd. for C20H22N4O4S: C, 57.96; H, 5.35; N, 13.52%. Found C, 58.20; H, 5.22; N, 13.78%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4,6-dimethyl-5-(4′-methoxyphenylazo)pyridine (6e): MP 231 °C; IR (KBr, cm−1) υ 3381(OH), 2231 (CN); COSY; NOESY; ROESY; gHMBC; gHMQC; 1H-NMR (DMSO-d6) δ = 2.60 (s, 3H, CH3), 2.64 (s, 3H, CH3), 3.42 (s, 3H, OCH3), 3.57–3.93 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.69–5.40 (3OH, exchangeable with D2O), 6.04 (d, 1H, H-1″, JH-1″–H-2″ = 6.9 Hz), 7.61 (d, 2H, Ar-H, J = 8.6 Hz), 7.97 (d, 2H, Ar-H, J = 8.6 Hz); 13C-NMR (DMSO-d6) δ = 21.4 (CH3), 21.5 (CH3), 46.2 (OCH3), 66.5 (C-5″), 67.8 (C-4″), 70.5 (C-3″), 73.2 (C-2″), 96.9 (C-1″), 98.1 (C-3), 114.3 (CN), 123.3–155.7 (Ar-C), 161.6 (C-2); LC-MS (ionization method): m/z 431 [M + 1]; Anal. Calcd. for C20H22N4O5S: C, 55.80; H, 5.15; N, 13.02%. Found C, 55.95; H, 5.81; N, 13.31%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4-methyl-6-phenyl-5-phenylazopyridine (6f): MP 184 °C; IR (KBr, cm−1) υ 3437 (OH), 2227 (CN); COSY; 1H-NMR (DMSO-d6) δ = 2.60 (s, 3H, CH3), 3.34–4.32 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.94–5.40 (3OH, exchangeable with D2O), 6.04 (d, 1H, H-1″, JH-1″–H-2″= 6.7 Hz), 7.33–7.81 (m, 10 H, Ar-H); 13C-NMR (DMSO-d6) δ = 18.2 (CH3), 66.1 (C-5″), 67.4 (C-4″), 70.8 (C-3″), 72.8 (C-2″), 97.8 (C-1″), 97.7 (C-3), 114.3 (CN), 121.8–154.1 (Ar-C), 161.4 (C-2); LC-MS (ionization method): m/z 463 [M + 1]; Anal. Calcd. for C24H22N4O4S: C, 62.32; H, 4.79; N, 12.11%. Found: C, 62.52; H, 4.92; N, 12.40%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4-methyl-6-phenyl-5-(4′-bromophenylazo)pyridine (6g): MP 172 °C; IR (KBr, cm−1) υ 3423 (OH), 2230 (CN); COSY; 1H-NMR (DMSO-d6) δ = 2.56 (s, 3 H, CH3), 3.28–3.94 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.85–5.44 (3OH, exchangeable with D2O), 6.05 (d, 1H, H-1″, JH-1″–H-2″ = 6.6 Hz), 7.47–7.91 (m, 9H, Ar-H); 13C-NMR (DMSO-d6) δ = 18.4 (CH3), 65.8 (C-5″), 67.5 (C-4″), 70.3 (C-3″), 72.7 (C-2″), 97.5 (C-1″), 98.1 (C-3), 114.1 (CN), 123.8–154.2 (Ar-C), 161.4 (C-2); LC-MS (ionization method): m/z 541 [M + 1]; Anal. Calcd. for C24H21BrN4O4S: C, 53.24; H, 3.91; N, 10.35%. Found: C, 53.33; H, 4.01; N, 10.56%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4-methyl-6-phenyl-5-(4′-chlorophenyl-azo)pyridine (6h): MP 183 °C; IR (KBr, cm−1) υ 3422 (OH), 2231 (CN); COSY; 1H-NMR (DMSO-d6) δ = 2.54 (s, 3H, CH3), 3.32–3.92 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.83–5.47 (3OH, exchangeable with D2O), 6.05 (d, 1H, H-1″, JH-1″–H-2″ = 6.5 Hz), 7.42–7.78 (m, 9H, Ar-H); 13C-NMR (DMSO-d6) δ = 18.7 (CH3), 65.5 (C-5″), 67.3 (C-4″), 70.3 (C-3″), 72.7 (C-2″), 97.4 (C-1″), 97.7 (C-3), 114.2 (CN), 122.8–154.3 (Ar-C), 161.1 (C-2); LC-MS (ionization method): m/z 497 [M + 1]; Anal. Calcd. for C24H21ClN4O4S: C, 58.00; H, 4.26; N, 11.27%. Found: C, 58.21; H, 4.43; N, 11.43%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4-methyl-6-phenyl-5-(4′-methylphenylazo)pyridine (6i): MP 182 °C; IR (KBr, cm−1) υ 3431 (OH), 2234 (CN); COSY; 1H-NMR (DMSO-d6) δ = 2.41 (s, 3H, CH3), 2.53 (s, 3H, CH3), 3.38–3.90 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.47–5.39 (3OH, exchangeable with D2O), 6.04 (d, 1H, H-1″, JH-1″–H-2″ = 6.4 Hz), 7.31–7.65 (m, 9H, Ar-H); 13C-NMR (DMSO-d6) δ = 18.2 (CH3), 21.7 (CH3), 65.9 (C-5″), 67.3 (C-4″), 70.4 (C-3″), 71.9 (C-2″), 97.4 (C-1″), 97.9 (C-3), 114.3 (CN), 122.0–153.6 (Ar-C), 161.1 (C-2); LC-MS (ionization method): m/z 476 [M]; Anal. Calcd. for C25H24N4O4S: C, 63.01; H, 5.08; N, 11.76%. Found: C, 62.89; H, 5.22; N, 11.89%.
2-(β-d-Arabinopyranosylthio)-3-cyano-4-methyl-6-phenyl-5-(4′-methoxyphenylazo)pyridine (6j): MP 194 °C; IR (KBr, cm−1) υ 3421 (OH), 2229 (CN); COSY; 1H-NMR (DMSO-d6) δ = 2.50 (s, 3H, CH3), 3.34–3.84 (m, 5H, H-2″, H-3″, H-4″, H-5a″ and H-5b″), 4.45 (s, 3H, OCH3), 4.79–5.41 (3OH, exchangeable with D2O), 6.00 (d, 1H, H-1″, JH-1″–H-2″ = 6.6 Hz), 7.21–7.80 (m, 9H, Ar-H); 13C-NMR (DMSO-d6) δ = 18.4 (CH3), 45.2 (OCH3), 66.1 (C-5″), 67.2 (C-4″), 70.1 (C-3″), 72.4 (C-2″), 97.6 (C-1″), 98.1 (C-3), 113.9 (CN), 122.0–153.6 (Ar-C), 161.2 (C-2); LC-MS (ionization method): m/z 493 [M + 1]; Anal. Calcd. for C25H24N4O5S: C, 60.96; H, 4.91; N, 11.38%. Found: C, 61.07; H, 5.01; N, 11.17%.