Abstract
A number of 1,2-benzothiazines have been synthesized in a three-step process. Nine chalcones 1–9 bearing methyl, fluoro, chloro and bromo substituents were chlorosulfonated with chlorosulfonic acid to generate the chalcone sulfonyl chlorides 10–18. These were converted to the dibromo compounds 19–27 through reaction with bromine in glacial acetic acid. Compounds 19–27 were reacted with ammonia, methylamine, ethylamine, aniline and benzylamine to generate a library of 45 1,2-benzothiazines 28–72. Compounds 28–72 were evaluated for their antimicrobial activity using broth microdilution techniques against two Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) and two Gram-negative bacteria (Proteus vulgaris and Salmonella typhimurium). The results demonstrated that none of the compounds showed any activity against Gram-negative bacteria P. vulgaris and S. typhimurium; however, compounds 31, 33, 38, 43, 45, 50, 53, 55, 58, 60, 63 and 68 showed activity against Gram-positive bacteria Bacillus subtilis and Staphylococcous aureus. The range of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) was 25–600 µg/mL, though some of the MIC and MBC concentrations were high, indicating weak activity. Structure activity relationship studies revealed that the compounds with a hydrogen atom or an ethyl group on the nitrogen of the thiazine ring exerted antibacterial activity against Gram-positive bacteria. The results also showed that the compounds where the benzene ring of the benzoyl moiety contained a methyl group or a chlorine or bromine atom in the para position showed higher antimicrobial activity. Similar influences were identified where either a bromine or chlorine atom was in the meta position.
1. Introduction
The emergence of drug-resistant strains of bacteria is an increasing threat to society. Many antibiotics that were formerly effective in curing bacterial infections are no longer efficient because of the evolution of resistant strains. Therefore, there is an urgent need for a new class of antibacterial agents [1]. Clinically, antibiotic resistance is one of the greatest challenges of the 21st century. Antibiotic resistance has led to the emergence of superbugs such as methicillin-resistant Staphylococcus aureus and extremely resistant tuberculosis bacteria which are impossible to treat with available medicines [2,3]. New antibacterial agents are currently being developed at a much slower pace than our growing need for such drugs. Amongst the family of heterocyclic compounds, those with nitrogen and sulfur atoms have been identified as having the most comprehensive spectrum of biological activities [4]. The biological and pharmacological properties of these compounds have been demonstrated in a number of studies [4,5].
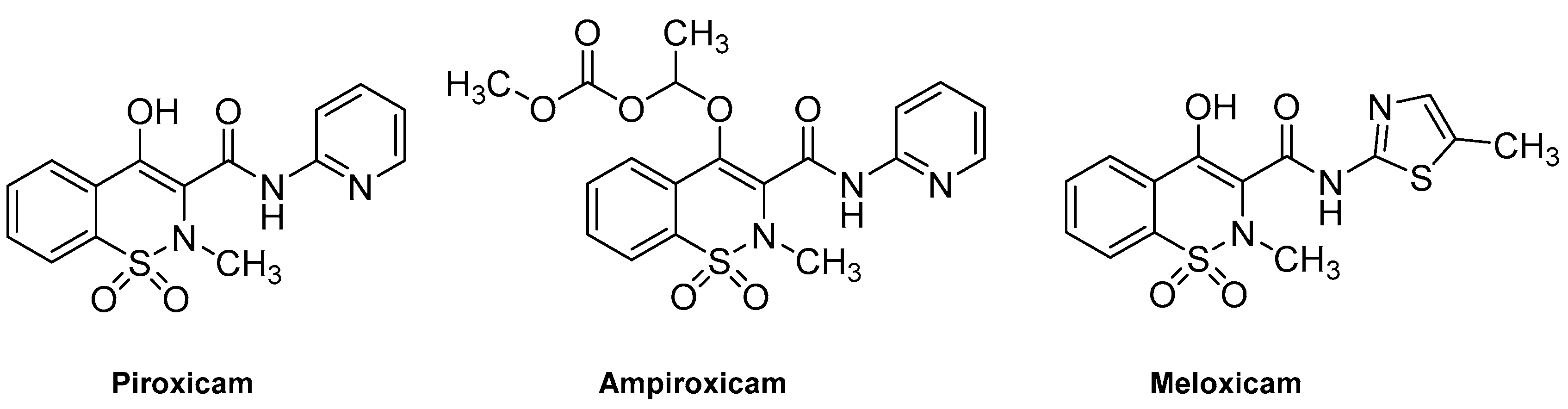
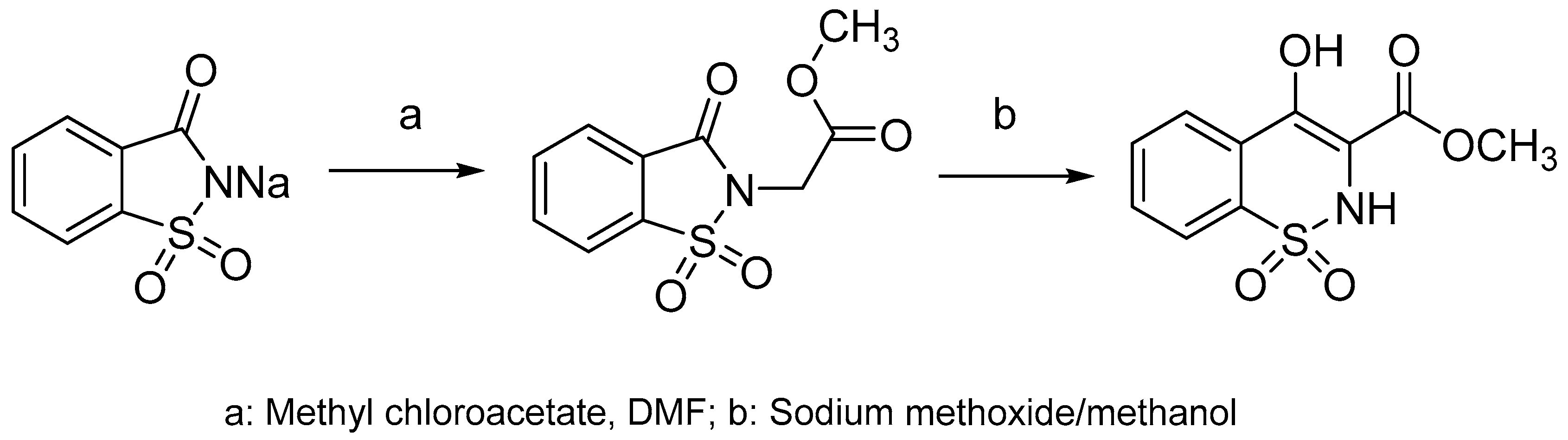
Derivatives of benzothiazine have, depending upon the substituents present, shown biological activities that range from antipsychotic to anti-inflammatory [6,7,8]. The 1,2-benzothiazines have shown various biological activities such as anti-inflammatory [9], CNS depressant [10,11], anti-depressants [12], anticancer [13] and antimicrobial [14]. They also act as potent calpain I inhibitors [15] and vasodilators [16]. Previous research has shown 1,2-benzothiazines to be potent antimicrobial agents against Gram-positive bacterial strains and fungal strains such as Aspergillus flavus, Aspergillus niger, Fusarium oxysporum [17]. In addition 1,2-benzothiazines have been seen to show marked activity against B. subtilis [17,18,19,20]. In recent years there has been a rapid growth in the literature pertaining to the benzothiazine ring system; much of this literature relates to the discovery that 3-carboxamides of 2-alkyl-4-hydroxy-2H-1,2-benzothiazine-1,1-dioxides are anti-inflammatory agents, which includes the drugs piroxicam, ampiroxicam and meloxicam (Figure 1) [21,22,23,24,25,26,27,28]. The common route to these types of compounds is via the alkoxide rearrangement of saccharin derivatives (Scheme 1) [29,30,31,32,33,34,35].
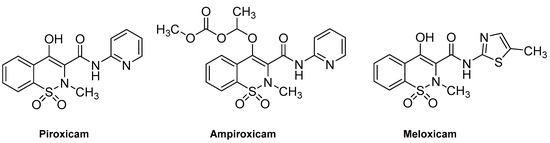
Figure 1.
Structures of 1,2-benzothiazine-based drugs.
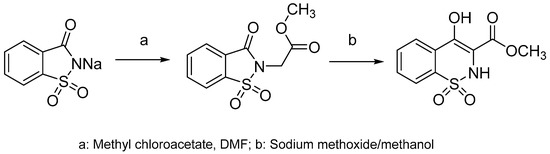
Scheme 1.
Synthesis of 1,2-benzothiazines via the alkoxide rearrangement of saccharin derivatives.
There are a number of different synthetic routes to 1,2-benzothiazines; however, the starting materials are not readily available. We recently developed a straightforward route to 1,2-benzothiazines using readily available chalcones [36]. In light of these observations, a series of new 1,2-benzothiazines were synthesized with the aim of obtaining potential antibacterial agents.
2. Results
2.1. Chemistry
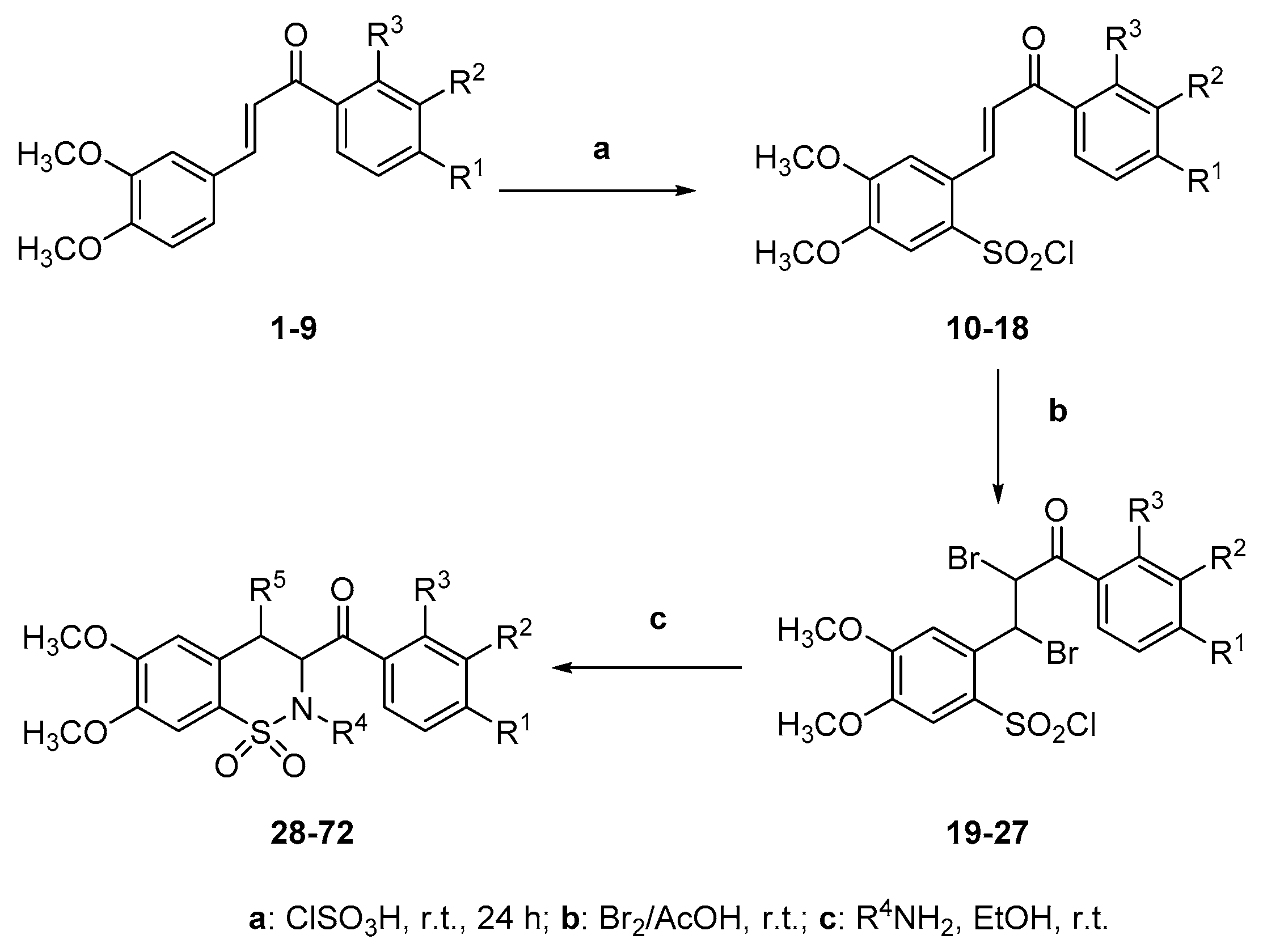
Synthetic target compounds 28–72 were synthesized in a three-step process outlined in Scheme 2 in yields of 44%–96%. The chalcones 1–9, which were synthesized by reacting different acetophenones with 3,4-dimethoxybenzaldehyde following known literature methods [37,38,39], were reacted with chlorosulfonic acid at room temperature to generate the chalcone sulfonyl chlorides 10–18. Melting points and spectral data (IR, 1H-NMR, 13C-NMR and nanoESI-MS) were consistent with literature data (see Supplementary Materials for 1H-NMR, 13C-NMR, HSMS of representative compounds). The sulfonyl chlorides 10–18 were deemed pure enough without recrystallization and reacted with bromine in glacial acetic acid at room temperature, which yielded the chalcone dibromo sulfonyl chlorides 19–27. Subsequently, the dibromo chalcone sulfonyl chlorides 19–27 were treated with ammonia and four primary amines (methylamine, ethylamine, aniline and benzylamine) to afford a benzothiazine library of 45 compounds 28–72. The structures of the newly synthesized benzothiazines 28–72 were appropriately characterized by spectral data. The IR spectra of compounds 28–72 confirmed the presence of the carbonyl signal in the region 1650–1720 cm−1 and all these compounds exhibited additional bands corresponding to the SO2 group (νmax 1340 and 1170 cm−1).
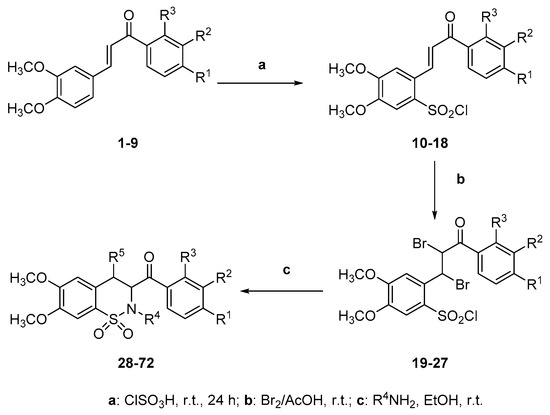
Scheme 2.
Synthetic steps for compounds 10–18, 19–27 and 1,2-benzothiazines 28–72.
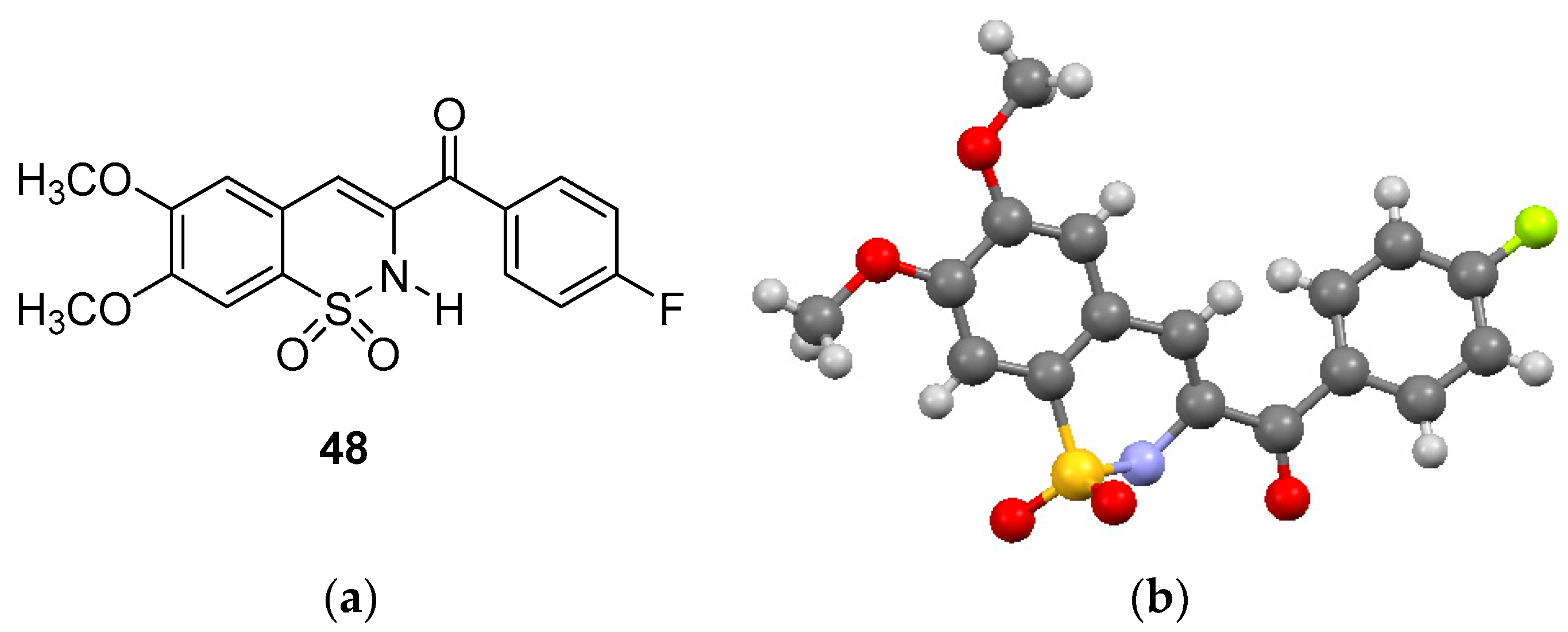
Compounds 29, 34, 39, 54, 59, 64 and 69 formed when the dibromo chalcone sulfonyl chlorides were reacted with methylamine. The 1H-NMR of these compounds showed the presence of the methylamino group in the δ 2.2–2.8 region. The methine protons all appeared as doublet-of-doublets in all the 1,2-benzothizaines, as expected, with the exception of compound 48 which did not show peaks for the two methine protons. Compound 48 showed two singlets at δ 3.93 and δ 4.01 for the two methoxy groups and three singlets in the aromatic region between δ 6.86–7.84. A broad singlet at δ 8.20 was observed for the amino proton which was exchangeable with D2O. The definitive structure of compound 48 was confirmed by spectral and X-ray analysis with the ORTEP representation of the molecular structure shown in Figure 2b.
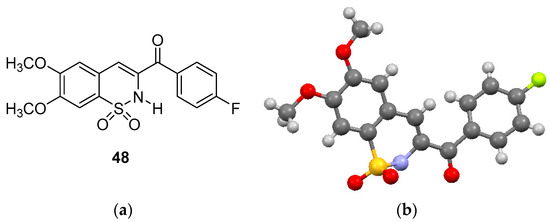
Figure 2.
(a) Structure of (6,7-dimethoxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-3-yl)(4-fluoro-phenyl)methanone and (b) ORTEP diagram of 48.
2.2. Antimicrobial Screening
The synthesized compounds 28–72 (Table 1) dissolved in DMSO were evaluated for their antimicrobial activity against bacterial strains of Bacillus subtilis ATCC 6633, Staphylococcous aureus ATCC 6538, Salmonella typhimurium ATCC 14028 (Salmonella enterica) and Proteus vulgaris ATCC 13315 by determining their minimum inhibitory concentrations (MICs) using a micro-broth dilution technique and their minimum bactericidal concentrations (MBCs). Their activities were compared with those of the known antibacterial streptomycin. There was no inhibition of growth of any of the bacteria in the presence of DMSO up to 5% concentration; however, there was a slight decrease in absorbance between concentrations of 5% to 8%, but after 8% there was a steep decrease in absorbance showing inhibition of growth.

Table 1.
The general structure of compounds 28–72 where R1, R2 and R3 refer to substituents attached to the benzoyl moiety while R4 and R5 refer to the substituents attached to the thiazine ring.
The MICs and MBCs of selected compounds are presented against both Gram-positive and Gram-negative bacterial strains in Table 2. Results of the anti-bacterial activity showed that the MICs and MBCs of compounds (31, 33, 38, 43, 45, 50, 55, 58, 60, 63 and 68) varied between the ranges of 25–600 µg/mL for B. subtilis. The MICs range of these compounds for S. aureus varied between 100–500 µg/mL whereas only compounds 53, 58 and 60 showed a bactericidal effect in the range of 200–400 µg/mL. None of the compounds showed any activity against the selected Gram-negative bacteria as MIC values were higher than 600 µg/mL.

Table 2.
MIC and MBC (μg/mL) of selected compounds and streptomycin against Gram-positive bacteria B. subtilis, S. aureus and Gram-negative bacteria P. vulgaris, S. typhimurium and their Clog p-values (For MIC and MBC values in μmol/mL see Supplementary Materials Table S1).
The MIC of most of the compounds that showed antimicrobial activity against bacterial species B. subtilis and S. aureus was quite high (400–600 μg/mL) as compared to streptomycin which has a MIC of 12.5 μg/mL for Gram-positive bacteria (Table 2), therefore showing very weak antibacterial activity as compared to streptomycin. Only compounds 33, 38, 43 and 58 showed MIC values between 25–50 μg/mL for B. subtilis (see Supplementary Materials Figures S1–S6).
3. Discussion
Prompted by the well-established antibacterial properties of benzothiazines, a series of 1,2-benzothiazine derivatives, covering different modifications of the benzothiazine scaffold, were synthesized from readily available chalcones. The title compounds were assayed in vitro for the evaluation of their antimicrobial activity against Gram-positive and Gram-negative bacteria.
The compounds with a hydrogen or an ethyl group on the nitrogen in the thiazine rings showed antibacterial properties against Gram-positive bacteria. The structure–antimicrobial activity relationship in the synthesized compounds revealed that 33, 38, 43, 53, 58, 63 and 68 inhibited the growth of Gram-positive bacteria. These compounds all contain a hydrogen atom on the nitrogen of the thiazine ring. This suggests that the amino group in the thiazine ring may be playing a role in the antibacterial property of these compounds. The results, therefore, support previous work which revealed that 4H-1,4-benzothiazines showed antibacterial activity against Gram-positive bacteria and fungi but no significant activity against Gram-negative bacteria [37]. In addition to this, compounds with an ethyl moiety on the nitrogen, 45, 50, 55 and 60, showed some inhibition of Gram-positive bacteria, though at higher concentrations.
In previous work on 1,4-benzothiazines, it was noted that the presence of an extra nitrogen and oxygen atom increases the lipophilicity of the compounds [37]. They reported that in a series of 1,2-benzothiazine-1,1-dioxides (N-methyl analogues), the compounds with greater lipophilicity possessed higher antibacterial activity. In the current work, compounds 29, 34, 39, 54, 59, 64 and 69 (N-methyl analogues) showed no activity, while 33, 38, 43, 53, 58, 63 and 68 showed weak antibacterial activity. These results are in contrast with the work of Ahmed and colleagues [37]. On the other hand, compounds 45, 50, 55 and 60 (N-ethyl analogues) showed some antibacterial activity which is in line with previous work [37].
The reaction of methylamine with the dibromo sulfonyl chlorides 19–27 unexpectedly resulted in the 4-methyamino derivatives 29, 34, 39, 54, 59, 64 and 69 which showed no antibacterial activity. However, the compounds with a bromine atom on carbon-4 in the thiazine ring displayed weak antibacterial activity, which would suggest that a bromine atom may be playing some role. This is supported by the fact that compound 48 did not show any antibacterial activity.
The presence of different substituents in the benzene ring of the benzoyl moiety also had an effect on the antibacterial activity against B. subtilis. Compounds with a chlorine or bromine atom or a methyl group in the para position showed increased antibacterial activity and a bromine atom in the meta position gave similar results. Amongst the compounds which contain no substituents in the benzene ring, only 31 showed very weak antibacterial activity (MIC of 500 μg/mL for B. subtilis and S. aureus) while compounds 28–30 and 32 showed no activity. However, compounds substituted with methyl, chloro, bromo and fluoro substituents in the meta or para position showed inhibition for B. subtilis. The ortho-substituted compounds with the same substituents inhibited S. aureus weakly.
In summary, the study conducted in this work has designed a simple synthetic route to 1,2-benzothiazines from readily available starting materials. Some of the compounds (33, 38, 43, 45, 50, 53, 55 and 58) synthesized, based on a library of 45 structures, showed some biological activity against Gram-positive bacteria B. subtilis and S. aureus, though the antibacterial activity was very weak as compared to the reference streptomycin standard. Based on our results, in vitro cytotoxicity work was not conducted for these compounds.
4. Experimental Section
4.1. General Information
All chemicals were purchased form Sigma Aldrich (St. Louis, MO, USA) and were used without any further purification. Melting points were determined using a Gallenkamp melting point apparatus (Thermo Fisher Scientific, Paisley, UK) and are uncorrected. The NMR spectra were recorded using a 600 MHz spectrometer (JEOL Co Ltd., Tokyo, Japan) with tetramethylsilane as internal standard and solvents as indicated. Chemical shifts were measured in ppm (δ) relative to TMS (0.00 ppm). Coupling constants (Ј) are reported in Hertz (Hz). The following abbreviations are used to describe the signal multiplicities: s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Accurate mass measurements were made at the National Mass Spectrometry Facility using Thermo Scientific LTQ Orbitrap XL with Advion Nanomate nanoelectrospray system (Waltham, MA, USA). Infrared spectra were recorded with a Varian spectrophotometer as KBr discs. TLC (thin layer chromatography) was performed using silica gel plates by dissolving the compound in dichloromethane and using the solvent system dichloromethane and diethyl ether. Clog P values were calculated using ChemBioDraw Ultra 13.0 (CambridgeSoft, Perkin Elmer, UK).
4.2. Synthesis of Chalcones
Chalcones 1–9 were synthesized by the well-established procedure using acetophenones and 3,4-dimethoxybenzaldehyde [38].
4.3. General Procedure for the Synthesis of Chalcone Sulfonyl Chlorides 10–18
The chalcones 1–9, (10 g; 0.032 mol) were added in portions to stirred chlorosulfonic acid (37.67 g; 0.32 mol) in an ice bath. After the addition was complete the reaction mixture was left stirring at room temperature. Progress of the reaction was monitored by thin layer chromatography (TLC). When the reaction was complete (24 h), the mixture was poured slowly over ice to remove the excess chlorosulfonic acid. The sulfonyl chloride was filtered by suction filtration and washed with cold water acetonitrile mixture. The resulting precipitate was considered pure enough to be used in subsequent reactions by TLC analysis.
4.4. General Procedure for the Synthesis of Dibromo Chalcone Sulfonyl Chlorides 19–27
The crude chalcone sulfonyl chloride 10–18 (10 g; 0.032 mol) was added to glacial acetic acid (125 mL) with stirring. The resulting mixture was stirred at room temperature and to the stirred mixture was added bromine (20.48 g; 0.13 mol) dissolved in 50 mL glacial acetic acid. The mixture was stirred until a precipitate was formed which was filtered and washed with cold glacial acetic acid.
4.5. General Procedure for the Synthesis of Benzothiazines 28–72
To a stirred solution, at room temperature compounds 19–27, (1 g) in ethanol (25 mL) were added drop-wise to a primary amine (3 mol equivalent). The mixture was warmed on a water bath for 10–20 min making sure that the solvent does not evaporate. The mixture was then stirred at room temperature for a further half hour and then ice was added until a precipitate appeared. The resulting solid was filtered and allowed to dry overnight and recrystallized from the appropriate solvent.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (28). Recrystallization solvent: ethanol; yield 68.7%; mp 203–204 °C; IR (KBr, ν cm−1) 1688.9 (C=O), 1598.8 (C-C, aromatic), 1329.6, 1146.2 (SO2). 1H-NMR (CDCl3): δ 3.87 (d, 1H), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.51(d, 1H), 7.00–8.10 (m, 7H, Ar-H). 13C-NMR (CDCl3): δ 30.00, 46.00, 54.25, 56.51, 103.00, 107.00, 112.50, 117.00, 120.00, 128.50, 134.00, 138.00, 149.00, 151.50, 192.10. HR-MS (nES) m/z calcd [M + H]+ 346.0744: observed 346.0741.
(6,7-Dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (29). Recrystallization solvent: ethanol; yield 79.5%; mp 190–192 °C. IR (KBr, ν cm−1) 1598.1 (C=O), 1545.0 (C-C, aromatic), 1383.6, 1157.1 (SO2). 1H-NMR (CDCl3): δ 2.61 (d, 3H, CH3), 2.80 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.63(d, 1H), 6.80 (s, 1H), 7.24–7.79 (m, 7H, Ar-H), 11.4 (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 9.00, 11.00, 30.10, 30.40, 32.10, 32.20, 55.10, 54.20, 101.00, 112.00, 113.00, 126.00, 126.40, 126.81, 128.37, 128.40, 132.00, 160.00, 192.00. HR-MS (nES) m/z calcd [M + H]+ 391.1322: observed 391.1322.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (30). Recrystallization solvent: ethanol; yield 93.0%; mp 200–201 °C. IR (KBr, ν cm−1) 1693.5(C=O), 1594.7 (C-C, aromatic), 1365.8, 1136.0 (SO2). 1H-NMR (CDCl3): δ 1.32 (t, 3H, CH3), 3.48 (q, 2H, CH2), 3.68 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 5.12 (d, 1H, CH), 5.39 (d, 1H, CH), 6.68–7.92 (7H, Ar-H). 13C-NMR (CDCl3): 13.20, 4594, 48.83, 56.20, 56.32, 62.85, 102.61, 108.00, 128.82, 128.90, 129.10, 130.00, 134.10, 134.57, 150.75, 152.83, 193.70. HR-MS (nES) m/z calcd [M + H]+ 454.0318: observed 454.0316.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (31). Recrystallization solvent: ethanol; yield 51.5%; mp 121–124 °C. IR (KBr, ν cm−1) 1689.1 (C=O), 1597.3 (C-C, aromatic), 1382.9, 1142.8 (SO2). 1H-NMR (CDCl3): δ 3.77 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 5.759(d, 1H, CH), 7.04 (d, 1H, CH), 7.10–8.12 (m, 12H, Ar-H). 13C-NMR (CDCl3): δ 29.07, 43.56, 56.46, 56.90, 11.25, 112.45, 121.40, 122.28, 125.30, 129.00, 129.03, 129.24, 130.10, 130.70, 134.20, 136.10, 148.20, 152.25, 190.30. HR-MS (nES) m/z calcd [M + H]+ 502.0318: observed 502.0309.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (32). Recrystallization solvent: ethanol; yield 91.8%; mp 133–134 °C. IR (KBr, ν cm−1) 1685.7 (C=O), 1589.7 (C-C, aromatic), 1359.9, 1145.8 (SO2). 1H-NMR (CDCl3): 3.90 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.38 (d, 1H, CH2), 4.63 (d, 1H, CH2), 5.00 (d, 1H, CH), 5.37 (d, 1H, CH), 7.11–7.85 (12H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 48.50, 52.00, 56.38, 60.50, 98.50, 101.50, 109.00, 122.00, 123.50, 124.00, 128.12, 128.13, 128.68, 128.90. 129.00, 134.00, 135.00, 136.5, 194.00.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (33). Recrystallization solvent: ethanol; yield 50.6%; mp 176–177 °C. IR (KBr, ν cm−1) 1681.6 (C=O), 1505.9 (C-C, aromatic), 1329.3, 1153.8 (SO2). 1H-NMR (CDCl3): δ 2.41 (s, 3H, CH3), 3.85 (d, 1H, CH), 3.92 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.50 (d, 1H, CH), 7.00–8.99 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 21.84, 29.68, 45.94, 55.50, 56.48, 104.18, 106.64, 125.00, 129.05, 129.21, 129.73, 146.00, 151.00, 154.00, 189.60.
(6,7-Dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (34). Recrystallization solvent: ethanol; yield 79.7%; mp 183–184 °C. IR (KBr, ν cm−1) 1698.9 (C=O), 1575.0 (C-C, aromatic), 1383.0, 1161.1 (SO2). 1H-NMR (CDCl3): δ 2.34 (s, 3H, CH3), 2.59 (s, 3H, CH3), 2.80 (d, 3H, CH3), 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.62 (d, 1H, CH), 6.81 (d, 1H, CH), 7.16–7.70 (m, 6H, Ar-H), 11.30, (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 21.41, 29.16, 30.89, 31.66, 56.46, 56.47, 111.86, 112.71, 126.86, 129.00, 129.05, 137.50, 142.00, 148.10, 152.00, 165.00, 188.10. HR-MS (nES) m/z calcd [M + H]+ 405.1479: observed 405.1474.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (35). Recrystallization solvent: ethanol; yield 86.0%; mp 157–158 °C. IR (KBr, ν cm−1) 1670.8 (C=O), 1605.5 (C-C, aromatic), 1303.6, 1150.4 (SO2). 1H-NMR (CDCl3): δ 1.03 (t, 3H, CH3), 2.41 (s, 3H, CH3), 3.34 (q, 2H, CH2), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.05 (d, 1H, CH), 5.37 (d, 1H, CH), 7.17–7.91 (6H, Ar-H). 13C-NMR (CDCl3): δ 12.77, 21.77, 29.68, 45.30, 47.56, 56.39, 61.73, 102.27, 109.15, 129.19, 129.20, 129.74, 130.05, 132.00, 146.00, 151.80, 153.00, 192.80. HR-MS (nES) m/z calcd [M + H]+ 468.0475: observed 468.0472.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (36). Recrystallization solvent: ethanol; yield 93.6%; mp 146–147 °C. IR (KBr, ν cm−1) 1670.1 (C=O), 1598.9 (C-C, aromatic), 1382.6, 1138.5 (SO2). 1H-NMR (CDCl3): δ 2.4 (s, 3H, CH3), 3.71 (d, 1H, CH), 3.75 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 5.75 (d, 1H, CH), 7.09–8.01 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.68, 44.00, 46.00, 56.49, 56.50, 112.00, 114.00, 122.13, 125.45, 129.14, 129.20, 129.21, 129.73, 134.05, 145.40, 148.05, 152.34, 190.10. HR-MS (nES) m/z calcd [M + H]+ 516.0475: observed 516.0469.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (37). Recrystallization solvent: ethanol; yield 94.8%; mp 151–152 °C. IR (KBr, ν cm−1) 1680.3 (C=O), 1606.9 (C-C, aromatic), 1315.7, 1149.1 (SO2). 1H-NMR (CDCl3): δ 2.36 (s, 3H, CH3), 3.89 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.36 (d, 1H, CH2), 4.62 (d, 1H, CH2), 4.98 (d, 1H), 5.35 (d, 1H), 7.12–7.75 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 21.77, 29.69, 48.07, 51.18, 56.32, 56.37, 102.24, 108.91, 127.85, 128.67, 128.92, 128.96, 129.12, 129.20, 130.10, 132.00, 135.07, 146.05, 151.10, 153.20, 192.45. HR-MS (nES) m/z calcd [M + H]+ 530.0631: observed 530.0626.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-chlorophenyl)methanone (38). Recrystallization solvent: ethanol; yield 73.1%; mp 184–185 °C. IR (KBr, ν cm−1) 1686.0 (C=O), 1589.0 (C-C, aromatic), 1334.4, 1152.3 (SO2). 1H-NMR (CDCl3): δ 3.80 (s, 1H, CH), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.50 (d, 1H, CH), 7.00–8.06 (m, 6 H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 45.85, 55.43, 56.51, 104.19, 106.64, 124.00, 128.00, 129.46, 130.49, 133.00, 141.00, 151.00, 154.00, 182.00. HR-MS (nES) m/z calcd [M − Br]+ 380.0354: observed 380.0356.
(4-Chlorophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (39). Recrystallization solvent: ethanol; yield 83.3%; mp 252–253 °C. IR (KBr, ν cm−1) 1668.1 (C=O), 1541.2 (C-C, aromatic), 1381.2, 1144.7 (SO2). 1H-NMR (CDCl3): δ 2.60 (s, 3H, CH3), 2.81 (d, 3H, NHCH3), 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.85 (broad singlet, 1H, NH (exchangeable with D2O), 5.57 (d, 1H, CH), 6.78 (d, 1H, CH), 7.24–7.71 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.60, 29.69, 32.00, 56.47, 56.49, 91.00, 111.06, 112.25, 127.00, 128.21, 128.57, 137.35, 138.56, 149.34, 152.00, 163.00, 186.25. HR-MS (nES) m/z calcd [M + H]+ 425.0932: observed 425.0931.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-chlorophenyl)methanone (40). Recrystallization solvent: ethanol; yield 64.3%; mp 185–186 °C. IR (KBr, ν cm−1) 1681.8 (C=O), 1589.9 (C-C, aromatic), 1364.5, 1136.0 (SO2). 1H-NMR (CDCl3): δ 1.26 (t, 3H, CH3), 3.58 (q, 2H, CH2), 3.58 (d, 1H, CH), 3.82 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 5.13 (d, 1H, CH), 6.78–7.90 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 13.48, 29.69, 39.54, 44.29, 56.28, 56.49, 102.47, 105.91, 127.00, 129.24, 129.67, 131.85, 134.25, 140.05, 150.00, 153.75, 197.00.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-chlorophenyl)methanone (41). Recrystallization solvent: ethanol; Yield 76.8%; mp 162–164 °C. IR (KBr, ν cm−1) 1689.4 (C=O), 1590.1 (C-C, aromatic), 1343.2, 1146.4 (SO2). 1H-NMR (CDCl3): δ 3.76 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 5.70 (d, 1H, CH), 7.10 (d, 1H, CH), 7.24–8.06 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 43.70, 46.25, 56.02, 56.20, 112.20, 113.00, 122.00, 126.05, 129.20, 129.27, 129.42, 130.41, 136.00, 141.25, 149.10, 153.70, 189.50. HR-MS (nES) m/z calcd [M + H]+ 535.9929: observed 535.9926.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-chlorophenyl)methanone (42). Recrystallization solvent: ethanol; yield 58.1%; mp 166–168 °C. IR (KBr, ν cm−1) 1682.5 (C=O), 1589.0 (C-C, aromatic), 1357.9, 1146.9 (SO2). 1H-NMR (CDCl3): δ 3.92 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.35 (d, H, CH2), 4.57 (d, 1H, CH2), 4.92 (d, 1H), 5.48 (d, 1H), 7.10–7.77 (m, 11H, Ar-H). 13C-NMR (CDCl3): 47.71, 51.71, 56.32, 56.37, 60.90, 102.28, 108.85, 128.12, 128.66, 128.90, 129.67, 129.40, 130.33, 131.25, 132.80, 135.20, 141.20, 150.72, 152.29. 191.73. HR-MS (nES) m/z calcd [M + H]+ 550.0085: observed 550.0082.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (43). Recrystallization solvent: ethanol; yield 69.8%; mp 193–194 °C. IR (KBr, ν cm−1) 1686.0 (C=O), 1585.1 (C-C, aromatic), 1333.8, 1152.1 (SO2). 1H-NMR (CDCl3): δ 3.79 (d, 1H, CH), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.50 (d, 1H, CH), 7.00–7.97 (m, 6 H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 45.00, 54.50, 56.51, 106.00, 106.05, 124.00, 127.00, 130.52, 130.60, 132.46, 133.00, 133.05, 154.00, 195.00. HR-MS (nES) m/z calcd [M − Br]+ 423.9849: observed 423.9850.
(4-Bromo-6,7-dimethoxy-2-methyl-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (44). Recrystallization solvent: ethanol; yield 78.8%; mp 189–190 °C. IR (KBr, ν cm−1) 1677.3 (C=O), 1585.3 (C-C, aromatic), 1302.6, 1150.3 (SO2). 1H-NMR (CDCl3): δ 2.85 (s, 3H, CH3), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.88 (d, 1H, CH), 5.31 (d, 1H, CH), 7.24–7.86 (m, 6 H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 37.38, 46.81, 56.45, 56.56, 102.66, 109.42128.50, 129.00, 129.50, 130.47, 132.29, 133.10, 151.70, 152.00, 192.00.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (45). Recrystallization solvent: ethanol; yield 86.3%; mp 171–172 °C. IR (KBr, ν cm−1) 1676.6 (C=O), 1583.7 (C-C, aromatic), 1306.2, 1149.4 (SO2). 1H-NMR (CDCl3): δ 1.02 (t, 3H, CH3), 3.33 (q, 2H, CH2), 3.95 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.02 (d, 1H, CH), 5.32 (d, 1H, CH), 7.11–7.87 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 45.20, 47.75, 56.90, 57.00, 62.00, 102.10, 108.80, 110.50, 112.30, 130.20, 130.45, 132.40, 132.60, 151.80, 151.80, 152.10, 192.00. HR-MS (nES) m/z calcd [M + H]+ 517.9267: observed 517.9264.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (46). Recrystallization solvent: ethanol; yield 89.5%; mp 218–219 °C. IR (KBr, ν cm−1) 1688.7 (C=O), 1585.0 (C-C, aromatic), 1382.3, 1145.6 (SO2). 1H-NMR (CDCl3): δ 3.76 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 5.70 (d, 1H, CH), 7.05 (d, 1H, CH), 7.11–7.98 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 43.45, 56.05, 56.40, 11.30, 112.10, 120.30, 120.45, 125.37, 127.35, 128.10, 128.45, 129.30, 130.10, 132.10, 132.89, 148.37, 152.35, 189.75. HR-MS (nES) m/z calcd [M + H]+ 579.9423: observed 579.9416.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (47). Recrystallization solvent: ethanol; yield 80.6%; mp 176–177 °C. IR (KBr, ν cm−1) 1685.5 (C=O), 1585.3 (C-C, aromatic), 1293.6, 1146.6 (SO2). 1H-NMR (CDCl3): δ 3.89 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.33 (d, 1H, CH2), 4.58 (d, 1H, CH2), 4.91 (d, 1H, CH), 5.28 (d, 1H, CH), 7.09–7.70 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.95, 47.75, 51.45, 56.00, 60.24, 102.00, 108.50, 128.20, 128.69, 128.94, 128.98, 130.39, 132.31, 132.70, 135.10, 135.20, 136.50, 150.50, 152.34, 192.00. HR-MS (nES) m/z calcd [M + H]+ 593.9580: observed 593.9579.
(6,7-Dimethoxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (48). Recrystallization solvent: ethanol; yield 58.0%; mp 249–250 °C. IR (KBr, ν cm−1) 1638.2 (C=O), 1597.3 (C-C, aromatic), 1363.4, 1152.7 (SO2). 1H-NMR (CDCl3): δ 3.93 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 6.86–7.84 (m, 7H, Ar-H), 8.2 (broad singlet, 1H, NH (exchangeable with D2O). HR-MS (nES) m/z calcd [M + H]+ 534.0381: observed 534.0376.
(4-Bromo-6,7-dimethoxy-2-methyl-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (49). Recrystallization solvent: ethanol; yield 85.7%; mp 195–196 °C. IR (KBr, ν cm−1) 1685.3 (C=O), 1598.0 (C-C, aromatic), 1325.1, 1146.5 (SO2). 1H-NMR (CDCl3): δ 2.10 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.84 (d, 1H, CH), 5.30 (d, 1H, CH), 7.10–8.00 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 37.25, 46.25, 56.44, 56.50, 103.56, 110.65, 116.15, 117.50, 128.00, 129.67, 131.45, 132.00, 150.50, 152.00, 192.00. HR-MS (nES) m/z calcd [M + H]+ 458.0068: observed 458.0063.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (50). Recrystallization solvent: ethanol; yield 95.3%; mp 190–191 °C. IR (KBr, ν cm−1) 1693.4 (C=O), 1598.3 (C-C, aromatic), 1345.8, 1185.0 (SO2). 1H-NMR (CDCl3): δ 3.92 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.50 (d, 1H, CH), 6.50 (d, 1H, CH), 6.85–8.10 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 16.00, 25.00, 29.70, 38.00, 43.50, 57.00, 110.05, 111.00, 117.00, 117.03, 117.05, 124.00, 131.76, 131.790, 132.00, 164.00, 192.00. HR-MS (nES) m/z calcd [M + H]+ 472.0224: observed 472.0212.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (51). Recrystallization solvent: ethanol; yield 85.2%; mp 170–171 °C. IR (KBr, ν cm−1) 1688.9 (C=O), 1598.8 (C-C, aromatic), 1382.7, 1146.8 (SO2). 1H-NMR (CDCl3): δ 3.76 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 5.71 (d, 1H, CH), 7.12 (d, 1H, CH), 7.24–8.16 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.45, 43.75, 56.34, 56.78, 11.10, 112.25, 116.10, 122.00, 122.30, 125.95, 129.30, 129.46, 130.00, 130.80, 131.30, 131.57, 132.00, 152.40, 189.75. HR-MS (nES) m/z calcd [M + H]+ 520.0224: observed 520.0218.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (52). Recrystallization solvent: ethanol; yield 87.7%; mp 173–174 °C. IR (KBr, ν cm−1) 1683.6 (C=O), 1596.9 (C-C, aromatic), 1356.5, 1147.3 (SO2). 1H-NMR (CDCl3): δ 3.90 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.38 (d, 1H, CH2), 4.60 (d, 1H, CH2), 4.98 (d, 1H, CH), 5.31 (d, 1H, CH), 7.10–7.89 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.95, 47.96, 51.87, 56.21, 56.35, 102.35, 109.00, 116.20, 116.25, 128.00, 128.67, 128.94, 130.05, 131.00, 131.50, 132.00, 135.10, 151.58, 152.00, 192.00. HR-MS (nES) m/z calcd [M + H]+ 534.0381: observed 534.0376.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (53). Recrystallization solvent: ethanol; yield 53.5%; mp 181–182 °C. IR (KBr, ν cm−1) 1686.0 (C=O), 1594.0 (C-C, aromatic), 1328.8, 1149.9 (SO2). 1H-NMR (CDCl3): δ 3.81 (d, 1H, CH), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.50 (d, 1H, CH), 7.01–8.04 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 45.95, 55.22, 56.51, 56.85, 104.19, 106.65, 127.33, 128.33, 128.84, 129.50, 130.41, 134.51, 135.49, 136.40, 151.44, 154.07, 188.26.
(3-Chlorophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (54). Recrystallization solvent: ethanol; yield 77.0%; mp 176–177 °C. IR (KBr, ν cm−1) 1600.9 (C=O), 1505.3 (C-C, aromatic), 1381.1, 1136.4 (SO2). 1H-NMR (CDCl3): δ 2.63 (s, 3H,=3), 2.82 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.11 (d, 1H, CH), 5.55 (s, 1H, CH), 6.77–7.66 (m, 6H, Ar-H), 11.28, (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 29.06, 30.25, 31.84, 56.48, 56.48, 90.98, 111.71, 112.81, 124.82, 126.97, 126.97, 129.57, 130.79, 135.25, 142.00, 149.40, 153.45, 166.00, 186.00. HR-MS (nES) m/z calcd [M + H]+ 425.0932: observed 425.0927.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (55). Recrystallization solvent: ethanol; yield 67.8%; mp 141–142 °C. IR (KBr, ν cm−1) 1688.6 (C=O), 1590.7 (C-C, aromatic), 1300.1, 1143.6 (SO2). 1H-NMR (CDCl3): δ 1.04 (t, 3H, CH3), 3.35 (q, 2H, CH2), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.02 (d, 1H, CH), 5.32 (d, 1H, CH), 7.18–7.98 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 12.65, 45.75, 47, 38, 56.43, 56.75, 61.71, 102.37, 109.07, 127.06, 129.03, 129.91, 130.33, 131.50, 132.50, 134.20, 134.70, 150.87, 152.34, 191.82. HR-MS (nES) m/z calcd [M + H]+ 487.9929: observed 487.9923.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (56). Recrystallization solvent: ethanol; yield 78.9%; mp 229–230 °C. IR (KBr, ν cm−1) 1701.3 (C=O), 1595.8 (C-C, aromatic), 1337.2, 1140.8 (SO2). 1H-NMR (CDCl3): δ 3.84 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.40 (d, 1H, CH), 5.80 (d, 1H, CH), 6.90–7.42 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 47.90, 56.37, 56.90, 63.54, 102.97, 107.62, 125.74, 126.78, 128.74, 128.90, 129.78, 130.28, 131.50, 132.00, 134.10, 134.20, 136.50, 138.00, 151.20, 153.12. HR-MS (nES) m/z calcd [M + H]+ 535.9929: observed 535.9925.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (57). Recrystallization solvent: ethanol; yield 74.4%; mp 154–155 °C. IR (KBr, ν cm−1) 1691.2 (C=O), 1591.8 (C-C, aromatic), 1335.9, 1138.2 (SO2). 1H-NMR (CDCl3): δ 3.90 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.40 (d, 1H, CH2), 4.57 (d, 1H, CH2), 4.97 (d, 1H, CH), 5.27 (d, 1H, CH), 7.10–7.85 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 47.75, 51.78, 56.35, 56.39, 60.95, 102.31, 108.85, 126.50, 127.69, 128.22, 128.70, 128.93, 130.19, 131.50, 134.17, 134.50, 134.70, 135.30, 135.50, 151.50, 152.10, 192.00. HR-MS (nES) m/z calcd [M + NH4]+ 567.0351: observed 567.0345.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (58). Recrystallization solvent: ethanol; yield 44.5%; mp 177–178 °C. IR (KBr, ν cm−1) 1685.9 (C=O), 1587.6 (C-C, aromatic), 1328.8, 1149.3 (SO2). 1H-NMR (CDCl3): δ 3.80 (d, 1H, CH), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.49 (d, 1H, CH), 7.00–8.19 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.95, 46.10, 55.10, 56.51, 104.10, 106.80, 123.50, 126.40, 127.50, 128.10, 131.35, 131.86, 136.40, 137.24, 151.75, 153.95, 188.10. HR-MS (nES) m/z calcd [M + HBr]+ 423.9849: observed 423.9850.
(3-Bromophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (59). Recrystallization solvent: ethanol; yield 76.4%; mp 178–179 °C. IR (KBr, ν cm−1) 1678.8 (C=O), 1570.4 (C-C, aromatic), 1311.2, 1136.4 (SO2). 1H-NMR (CDCl3): δ 2.63 (s, 3H, CH3), 2.82 (d, 3H, NHCH3), 3.93 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.55 (d, 1H, CH), 6.77 (d, 1H, CH), 7.21–7.84 (m, 6H, Ar-H), 11.31 (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 29.68, 29.80, 31.83, 56.49, 56.90, 91.20, 111.69, 112.20, 123.10, 125.32, 126.23, 128.10, 129.93, 133.74, 141.35, 149.45, 152.00, 159.56, 186.34. HR-MS (nES) m/z calcd [M + H]+ 469.0427: observed 469.0419.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (60). Recrystallization solvent: ethanol; yield 85.2%; mp 130–131 °C. IR (KBr, ν cm−1) 1689.3 (C=O), 1590.2 (C-C, aromatic), 1350.4, 1142.9 (SO2). 1H-NMR (CDCl3): δ 1.02 (t, 3H, CH3), 3.36 (q, 2H, CH2), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.03 (d, 1H, CH), 5.32 (d, 1H, CH), 7.17–8.14 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 12.64, 29.69, 45.74, 47.35, 56.43, 61.70, 101.70, 108.00, 122.10, 126.20, 128.20, 130.05, 131.15, 136.05, 137.25, 138.15, 150.25, 152.15, 192.15. HR-MS (nES) m/z calcd [M + H]+ 531.9423: observed 531.9421.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (61). Recrystallization solvent: ethanol; yield 80.2%; mp 232–233 °C. IR (KBr, ν cm−1) 1701.6 (C=O), 1595.3 (C-C, aromatic), 1284.2, 1141.5 (SO2). 1H-NMR (CDCl3): δ 3.85 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 5.45 (d, 1H, CH), 5.80 (d, 1H, CH), 6.80–7.94 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 51.93, 56.24, 56.38, 56.48, 62.06, 102.53, 108.04, 127.33, 127.43, 128.17, 129.18, 129.40, 130.03, 132.67, 133.84, 134.86, 138.10, 150.96, 152.97, 196.11. HR-MS (nES) m/z calcd [M + NH4]+ 596.9689: observed 596.9685.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (62). Recrystallization solvent: ethanol; Yield 96.9%; mp 167–168 °C. IR (KBr, ν cm−1) 1690.8 (C=O), 1590.8 (C-C, aromatic), 1334.6, 1137.9 (SO2). 1H-NMR (CDCl3): δ 3.90 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.40 (d, 1H, CH2), 4.56 (d, 1H, CH2), 4.97 (d, 1H, CH), 5.26 (d, 1H, CH), 7.10–7.96 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 47.72, 56.35, 56.39, 60.97, 102.31, 108.85, 122.80, 127.40, 127.75, 128.24, 128.60, 128.71, 128.93, 130.41, 131.89, 135.10, 136.20, 137.25, 151.45, 152.10, 192.23. HR-MS (nES) m/z calcd [M + NH4]+ 610.9845: observed 610.9842.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-chlorophenyl)methanone (63). Recrystallization solvent: ethanol; yield 72.8%; mp 185–186 °C. IR (KBr, ν cm−1) 1696.5 (C=O), 1677.0 (C-C, aromatic), 1337.3, 1153.7 (SO2). 1H-NMR (CDCl3): δ 3.80 (d, 1H, CH), 3.91 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.41 (d, 1H, CH), 6.97–7.53 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 47.50, 56.49, 56.51, 104.00, 106.00, 122.00, 125.00, 126.50, 130.79, 133.90, 147.50, 153.50, 160.0, 161.90, 192.00.
(2-Chlorophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (64). Recrystallization solvent: ethanol; yield 65.4%; mp 118–119 °C. IR (KBr, ν cm−1) 1602.0 (C=O), 1568.8 (C-C, aromatic), 1309.7, 1147.6 (SO2). 1H-NMR (CDCl3): δ 2.56 (s, 3H, CH3), 2.81 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.91 (d, 1H, CH), 5.26 (s, 1H, CH), 7.24–7.52 (m, 6H, Ar-H), 6.76, (broad singlet, 1H, NH. 13C-NMR (CDCl3): δ 27.80, 32.00, 56.50, 57.50, 95.00, 101.00, 101.90, 111.00, 112.00, 126.00, 127.00, 129.92, 130.00, 131.00, 132.00, 164.00, 190.00. HR-MS (nES) m/z calcd [M + H]+ 425.0932: observed 425.0928.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-chlorophenyl)methanone (65). Recrystallization solvent: ethanol; yield 48.2%; mp 129–131 °C. IR (KBr, ν cm−1) 1701.3 (C=O), 1589.9 (C-C, aromatic), 1383.4, 1141.1 (SO2). 1H-NMR (CDCl3): δ 1.31 (t, 3H, CH3), 3.44 (q, 2H, CH2), 3.82 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 5.29 (d, 1H, CH), 5.44 (d, 1H, CH), 6.86–7.74 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 45.04, 51.63, 52.98, 56.01, 61.49, 62.44, 102.36, 10258, 108.08, 108.72, 127.04, 127.09, 127.68, 130.93, 132.95, 150.85, 152.44, 152.92, 193.34. HR-MS (nES) m/z calcd [M + H]+ 487.9927: observed 487.9918.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-chlorophenyl)methanone (66). Recrystallization solvent: ethanol; yield 84.2%; mp 209–210 °C. IR (KBr, ν cm−1) 1710.0 (C=O), 1587.7 (C-C, aromatic), 1304.6, 1144.7 (SO2). 1H-NMR (CDCl3): δ 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 5.52 (d, 1H, CH), 5.80 (d, 1H, CH), 6.80–7.94 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 52.05, 56.42, 56.46, 102.45, 108.20, 126.36, 126.78, 127.14, 127.56, 127.85, 128.45, 129.56, 130.04, 132.05, 133.56, 137.34, 136.00, 150.78, 153.45, 196.67. HR-MS (nES) m/z calcd [M + H]+ 535.9929 535: observed 535.9924.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-chlorophenyl)methanone (67). Recrystallization solvent: ethanol; yield 90.8%; mp 156–157 °C. IR (KBr, ν cm−1) 1692.5 (C=O), 1592.0 (C-C, aromatic), 1342.2, 1140.2 (SO2). 1H-NMR (CDCl3): δ 3.89 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.45 (d, 1H, CH2), 4.70 (d, 1H, CH2), 5.05 (d, 1H, CH), 5.28 (d, 1H, CH), 7.01–7.42 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 50.75, 52.52, 56.38, 60.45, 102.34, 108.25, 126.50, 127.34, 128.50, 128.78, 129.04, 129.42, 130.87, 131.50, 132.23, 135.60, 135.80, 136.00, 150.70, 152.10, 194.00. HR-MS (nES) m/z calcd [M + NH4]+ 567.0351: observed 567.0342.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-bromophenyl)methanone (68). Recrystallization solvent: ethanol; yield 56.6%; mp 183–184 °C. IR (KBr, ν cm−1) 1696.7 (C=O), 1676.3 (C-C, aromatic), 1336.3, 1153.2 (SO2). 1H-NMR (CDCl3): δ 3.85 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 5.45 (d, 1H, CH), 5.78 (d, 1H, CH) 6.98–7.93 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 47.96, 56.41, 63.53, 102.97, 107.62, 124.10, 125.74, 127.21, 128.00, 129.79, 130.50, 131.65, 137.26. 138.05, 150.12, 190.45. HR-MS (nES) m/z calcd [M − Br]+ 423.9849: observed 423.9849.
(2-Bromophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (69). Recrystallization solvent: ethanol; yield 75.2%; mp 122–123 °C. IR (KBr, ν cm−1) 1737.9 (C=O), 1655.7 (C-C, aromatic), 1383.0, 1147.1 (SO2). 1H-NMR (CDCl3): δ 2.55 (s, 3H, CH3), 2.83 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.80 (d, 1H, CH), 5.16 (s, 1H, CH), 6.77–7.53 (m, 6H, Ar-H), 11.35, (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 29.31, 29.78, 31.82, 56.51, 56.58, 94.73, 11.70, 112.76, 118.00, 127.44, 128.79, 129.40, 130.21, 130.75, 133.01, 143.45, 148.12, 151.75, 164.91, 192.30. HR-MS (nES) m/z calcd [M + H]+ 469.0427: observed 469.0418.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-bromophenyl)methanone (70). Recrystallization solvent: ethanol; yield 69.3%; mp 129–130 °C. IR (KBr, ν cm−1) 1689.5 (C=O), 1590.1 (C-C, aromatic), 1350.4, 1142.8 (SO2). 1H-NMR (CDCl3): δ 1.05 (t, 3H, CH3), 3.35 (q, 2H, CH2), 3.89 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 5.03 (d, 1H, CH), 5.29 (d, 1H, CH), 7.19–8.15 (m, 6H, Ar-H). 13C-NMR (CDCl3): 12.64, 45.73, 47.34, 56.35, 56.41, 61.69, 102.35, 109.05, 123.37, 127.48, 129.89, 130.53, 131.94, 132.20, 136.00, 137.08, 150.85, 152.32, 191.72.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-bromophenyl)methanone (71). Recrystallization solvent: ethanol; yield 96.8%; mp 210–211 °C. IR (KBr, ν cm−1) 1709.5 (C=O), 1588.1 (C-C, aromatic), 1305.1, 1144.7 (SO2). 1H-NMR (CDCl3): δ 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 5.52 (d, 1H, CH), 5.79 (d, 1H, CH), 7.24–7.62 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 51.96, 56.42, 56.51, 102.45, 108.23, 119.50, 126.60, 126.85, 127.25, 127.45, 128.85, 128.95, 130.06, 132.32, 132.85, 134.23, 138.45, 151.50, 151.80, 196.10. HR-MS (nES) m/z calcd [M + H]+ 579.9423: observed 579.9423.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-bromophenyl)methanone (72). Recrystallization solvent: ethanol; yield 81.6%; mp 123–125 °C. IR (KBr, ν cm−1) 1690.6 (C=O), 1590.4 (C-C, aromatic), 1334.3, 1137.9 (SO2). 1H-NMR (CDCl3): δ 3.89 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.39 (d, 1H, CH2), 4.55 (d, 1H, CH2), 4.97 (d, 1H, CH), 5.27 (d, 1H, CH), 7.10–7.96 (m, 11H, Ar-H). 13C-NMR (CDCl3): 47.72, 51.74, 56.34, 56.38, 60.97, 102.30, 108.83, 123.80, 127.37, 128.21, 128.68, 128.89, 130.40, 131.85, 135.00, 135.60, 140.10, 150.74, 152.32, 191.62. HR-MS (nES) m/z calcd [M + H]+ 593.9580: observed 593.9578.
4.6. Antimicrobial Activity
For determining the antibacterial activities, the synthesized compounds were dissolved in DMSO (20 mg/mL). Broth dilution method in a 96 wells micro-titre plate was used to test the compounds (27–72) [6]. Mueller Hinton Broth was used as diluents in the wells of the micro-titre plate. The dilutions were prepared at the required quantities of 600, 500, 400, 200, 100, 50, 25, 12.5 µg/mL. Equal volume of bacterial culture was added to it after comparing it with 0.5 McFarland standard and incubated at 37 °C for 16 to 18 h on a rotary shaker at 150 rpm [39]. Plates were then examined for the turbidity as an indicator of growth and the absorbance was taken with the help of ELISA reader at 590 nm [40]. First well that appeared having zero absorbance was taken to be the MIC (Minimum inhibitory concentration) [8]. This was followed by an MTT assay for confirmation. Streptomycin was used as a reference control at concentrations of 50, 25, 12.5, 6.25, 3.12, 1.56, 0.78, 0.39 µg/mL. Streptomycin is a broad spectrum antibiotic which inhibits the growth of both Gram-positive and Gram-negative bacteria and has been widely used as a reference [41,42]. In order to ensure that the solvent had no effect on bacterial growth, a control test was also performed containing inoculated broth supplemented with different concentrations (1%–16%) of DMSO. All the tests were performed in triplicates.
The MBC (minimum bactericidal concentration) was determined by sub culturing the preparations that have shown no evidence of growth on Mueller Hinton Agar in neat and dilutions of 1:10 and 1:100. A colony-forming unit (CFU) count was done for the agar plates and the dilution that gave 99.9% reduction to the original inoculum was considered to be the MBC.
All the compounds were tested for their in vitro growth inhibitory activity against different bacteria. The bacterial strains used were Bacillus subtilis ATCC 6633 and Staphylococcus aureus ATCC 6538 as Gram-positive bacteria and Salmonella typhimurium ATCC 14028 (Salmonella enterica) and Proteus vulgaris ATCC 13315 as Gram-negative bacteria. Staphylococcus aureus is a clinically important pathogen as it causes various types of infections [43] and Bacillus subtilis was used as a model organism as it belongs to the same group as pathogenic Bacillus anthracis species [44]. Salmonella typhimurium is clinically important as it can cause enteritis. Proteus vulgaris has been isolated from long term care facilities and hospitals and from immunocompromised patients. The strains used in this study were obtained from culture collection of University of Hertfordshire.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/7/861/s1.
Acknowledgments
Authors are grateful to Virendra P. Shah for assistance with some of the synthetic work. The authors are grateful to the University of Hertfordshire for providing funds for Open Access.
Author Contributions
C.P., M.G. and J.P.B. planned, designed and carried out the synthetic and microbiological screening work, discussed results, wrote and reviewed the manuscript. M.S., J.F., A.P.H. and J.P.B. contributed with the collection and analysis of the spectral data. L.M. performed the crystallography studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Webb, G.F.; D’Agata, E.M.C.; Magal, P.; Ruan, S. A model of anti biotic-resistant bacterial epidemics in hospitals. Proc. Nat. Acad. Sci. USA 2005, 102, 13343–13348. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.V.; Fowler, V.G., Jr.; Skov, R.; Bruun, N.E. Future challenges and treatment of Staphylococcus aureus bacteremia with emphasis on MRSA. Future Microbiol. 2011, 6, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Yew, W.W.; Lange, C.; Leung, C.C. Treatment of tuberculosis: Update 2010. Eur. Respir. J. 2011, 37, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Varma, R.S. Greener and expeditious synthesis of bioactive heterocycles using microwave irradiation. Pure Appl. Chem. 2008, 80, 777–790. [Google Scholar] [CrossRef]
- Maheshwari, M.; Goyal, A. A review: Synthesis and medicinal importance of 1,4-benzothiazine analogs. Asian J. Pharm. Clin. Res. 2015, 8, 41–46. [Google Scholar]
- Bariş, Ö.; Güllüce, M.; Şahin, F.; Özer, H.; Kiliç, H.; Özkan, H.; Sökmen, M.; Özbek, T. Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan. (Asteraceae). Turk. J. Biol. 2006, 30, 65–73. [Google Scholar]
- Buwa, L.V.; Afolayan, A.J. Antimicrobial activity of some medicinal plants used for the treatment of tuberculosis in the Eastern Cape Province, South Africa. Afr. J. Biotechnol. 2009, 8, 6683–6687. [Google Scholar]
- Salie, F.; Eagles, P.F.K.; Leng, H.M.J. Preliminary antimicrobial screening of four South African Asteraceae species. J. Ethnopharmacol. 1996, 52, 27–33. [Google Scholar] [CrossRef]
- Weeks, P.D.; Vinick, F.J.; Breitenbach, R.; Jung, S. Efficient synthesis of 2-methyl-4-hydroxy-2H-1,2-benzothiazine 1,1-dioxides. J. Org. Chem. 1983, 48, 3601–3603. [Google Scholar] [CrossRef]
- Tani, J.; Yamada, Y.; Ochiai, T.; Ishida, R.; Inoue, I.; Oine, T. Studies on biologically-active halogenated compounds.2. Chemical modification of 6-amino-2-fluoromethyl-3-(ortho-tosyl)-4(3H)-quinazolinone and the CNS depressant activities of related compounds. Chem. Pharm. Bull. 1979, 27, 2675–2687. [Google Scholar] [CrossRef] [PubMed]
- Tani, J.; Yamada, Y.; Oine, T.; Ochiai, T.; Ishida, R.; Inoue, I. Studies on biologically-active halogenated compounds.1. Synthesis and central nervous-system despressant activity of 2-(fluoromethyl)-3-aryl-4(3H)- quinazolinone derivatives. J. Med. Chem. 1979, 22, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, K.I.; Artemenko, G.N.; Sokolova, T.V.; Avdulov, N.A.; Zagorevskii, V.A. Synthesis and pharmacological activity of benzothiazine derivatives. Pharm. Chem. J. 1982, 16, 110–113. [Google Scholar] [CrossRef]
- Niewiadomy, A.; Matysiak, J.; Karpinska, M.M. Synthesis and anticancer activity of new 2-aryl-4H-3,1-benzothiazines. Arch. Pharm. 2011, 344, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiari, Y.M.; Qaisi, A.M.; Abu Shuheil, M.Y.; El-Abadelah, M.M.; Voelter, W. Synthesis and antibacterial potency of 4-methyl-2,7-dioxo-1,2,3,4,7,10-hexahydropyrido[2,3-f]quinoxaline-8-carboxylic acid, selected [a]-fused heterocyles and acyclic precursors. Z. Naturforsch. Sect. B J. Chem. Sci. 2007, 62, 1453–1458. [Google Scholar] [CrossRef]
- Wells, G.J.; Tao, M.; Josef, K.A.; Bihovsky, R. 1,2-Benzothiazine 1,1-dioxide P-2-P-3 peptide mimetic aldehyde calpain I inhibitors. J. Med. Chem. 2001, 44, 3488–3503. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.B.; Deshmukh, S.A.; Jagtap, S.S.; Mulik, A.R. Synthesis and study of biological activity of some new 1,4-benzothiazines. Indian J. Chem. 2007, 46B, 852–859. [Google Scholar] [CrossRef]
- Martinez, A.; Gil, C. Chapter 9: Heterocycles containing nitrogen and sulfur as potent biologically active scaffolds. In Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; The Royal Society of Chemistry: London, UK, 2016; pp. 231–261. [Google Scholar]
- Mor, S.; Nagoria, S. Efficient and convenient synthesis, characterization, and antimicrobial evaluation of some new tetracyclic 1,4-benzothiazines. Synth. Commun. 2016, 46, 169–178. [Google Scholar] [CrossRef]
- Mor, S.; Pahal, P.; Narasimhan, B. Synthesis, characterization, antimicrobial activities and QSAR studies of some 10a-phenylbenzo[b]indeno[1,2-e][1,4]thiazin-11(10aH)-ones. Eur. J. Med. Chem. 2012, 53, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Rathod, A.K. A microwave-assisted synthesis of some new benzothiazines derivatives and their antimicrobial activity. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 47–49. [Google Scholar]
- Carty, T.J.; Eskra, J.D.; Lombardino, J.G.; Hoffman, W.W. Piroxicam, a potent inhibitor of prostaglandin production in cell-culture—Structure-activity-study. Prostaglandins 1980, 19, 51–59. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Choudary, J.A.; Elsegood, M.R.J.; Siddiqui, H.L.; Khan, K.M. A facile synthesis of novel biologically active 4-hydroxy-N′-(benzylidene)-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxides. Eur. J. Med. Chem. 2009, 44, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Liu, Y. 2,1-Benzothiazine 2,2-Dioxides. 4*. synthesis, structure, and analgesic properties of 4-Hydroxy-1-Methyl-2,2-Dioxo-n-(pyridin-2-yl)-1h-2λ6,1-Benzothiazine-3-Carboxamides. Chem. Heterocycl. Compd. 2014, 50, 564–572. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Sim, G.; Grinevich, L.A. The effective synthesis of N-(Arylalkyl)-1-R-4-hydroxy-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides as promising analgesics of a new chemical class. Sci. Pharm. 2015, 83, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Davidenko, A.A.; Grinevich, L.A. 4-Hydroxyquinolones-2. 247.4-Hydroxy-2-Oxo-1,2-Dihydroquinoline or 4-Hydroxy-2,2-Dioxo-1H-2λ6,1-Benzothiazine? Chem. Heterocycl. Compd. 2015, 50, 1443–1448. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Sim, G. 2,1-benzothiazine 2,2-dioxides. 3*. 4-hydroxy-1-methyl-2,2-dioxo-n-(1,3-thiazol-2-yl)-1H-2λ6,1-benzothiazine-3-carboxamides—A new group of potential analgetics. Chem. Heterocycl. Compd. 2014, 50, 103–110. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Choudary, J.A.; Ahmad, S. An efficient synthesis of 2-alkyl-4-hydroxy-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxides. Bull. Korean Chem. Soc. 2005, 26, 1771–1775. [Google Scholar] [CrossRef]
- Lombardino, J.G. The synthesis of some new 1,2-benzothiazines. Org. Prep. Proced. Int. 1980, 12, 269–274. [Google Scholar] [CrossRef]
- Bluke, Z.; Sladek, M.; Abel, U.; Kauss, V. Evaluation of an enantioselective synthesis of 6-chloro-3,4-dihydro-2H-1,2-benzothiazine-3-carboxylic acid 1,1-dioxide and its derivatives. Chem. Heterocycl. Compd. 2015, 51, 187–193. [Google Scholar] [CrossRef]
- Gannarapu, M.R.; Vasamsetti, S.B.; Punna, N.; Royya, N.K.; Pamulaparthy, S.R.; Nanubolu, J.B.; Kotamraju, S.; Banda, N. Synthesis of novel 1,2-benzothiazine 1,1-dioxide-3-ethanone oxime N-aryl acetamide ether derivatives as potent anti-inflammatory agents and inhibitors of monocyte-to-macrophage transformation. Eur. J. Med. Chem. 2014, 75, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Popov, K.; Volovnenko, T.; Volovenko, Y. Novel benzothiazine derivatives via formylation of 2,3-dihydro-4H-benzo[e][1,4]thiazin-3-on-1,1-dioxide. J. Heterocycl. Chem. 2013, 50, E217–E220. [Google Scholar] [CrossRef]
- Kacem, Y.; Ben Hassine, B. Efficient synthesis of new imidazo[1,2-b][1,2]benzothiazine 4,4-dioxide derivatives via lateral lithiation of N-mesitylenesulfonyl hydantoins. Tetrahedron Lett. 2012, 53, 5608–5610. [Google Scholar] [CrossRef]
- Kumar, N.; Drabu, S.; Mondal, S.C. NSAID’s and selectively COX-2 inhibitors as potential chemoprotective agents against cancer: 1st Cancer Update. Arab. J. Chem. 2013, 6, 1–23. [Google Scholar] [CrossRef]
- Lazer, E.S.; Miao, C.K.; Cywin, C.L.; Sorcek, R.; Wong, H.C.; Meng, Z.; Potocki, I.; Hoermann, M.; Snow, R.J.; Tschantz, M.A.; et al. Effect of structural modification of enol-carboxamide-type nonsteroidal antiinflammatory drugs on COX-2/COX-1 selectivity. J. Med. Chem. 1997, 40, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Gosetto, F.; Serritella, S.; Manfroni, G.; Tabarrini, O.; Iraci, N.; Brincat, J.P.; Carosati, E.; Villarini, M.; Kaatz, G.W.; et al. Pyrazolo[4,3-c][1,2]benzothiazines 5,5-dioxide: A promising new class of Staphylococcus aureus NorA efflux pump inhibitors. J. Med. Chem. 2012, 55, 3568–3572. [Google Scholar] [CrossRef] [PubMed]
- Bassin, J.P.; Frearson, M.J.; Al-Nawwar, K. Novel synthesis of a 2H-1,2-benzothiazine-1,1-dioxide derivative. Synth. Commun. 2000, 30, 3693–3701. [Google Scholar] [CrossRef]
- Ahmad, N.; Zia-Ur-Rehman, M.; Siddiqui, H.L.; Ullah, M.F.; Parvez, M. Microwave assisted synthesis and structure-activity relationship of 4-hydroxy-N′-[1-phenylethylidene]-2H/2-methyl-1,2-benzothiazine-3- carbohydrazide 1,1-dioxides as anti-microbial agents. Eur. J. Med. Chem. 2011, 46, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Tomar, V.; Bhattacharjee, G.; Kamaluddin; Rajakumar, S.; Srivastava, K.; Puri, S.K. Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur. J. Med. Chem. 2010, 45, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Chikhalia, K.H.; Patel, M.J.; Vashi, D.B. Design, synthesis and evaluation of novel quinolyl chalcones as antibacterial agents. Arkivoc 2008, 2008, 189–197. [Google Scholar]
- Ncube, N.S.; Afolayan, A.J.; Okoh, A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr. J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]
- Dongamanti, A.; Devulapally, M.G.; Aamate, V.K.; Gundu, S. Microwave-assisted synthesis and antimicrobial evaluation of novel pyrazolines. Chem. Heterocycl. Compd. 2015, 51, 872–882. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, H.; Cao, C.; Zhou, S.; Pang, G.; Shi, Y. Synthesis of fluorinated carbazoles via C–H arylation catalyzed by Pd/Cu bimetal system and their antibacterial activities. Bioorg. Med. Chem. 2016, 24, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Medical progress: Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, T.N.; Osman, H.; Werngren, J.; Hoffner, S.; Engman, L.; Holmgren, A. Ebselen and analogs as inhibitors of Bacillus anthracis thioredoxin reductase and bactericidal antibacterials targeting Bacillus species, Staphylococcus aureus and Mycobacterium tuberculosis. Biochim. Biophys. Acta 2016, 1860, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–9 and 28–72 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).