Location and Effects of an Antitumoral Catechin on the Structural Properties of Phosphatidylethanolamine Membranes
Abstract
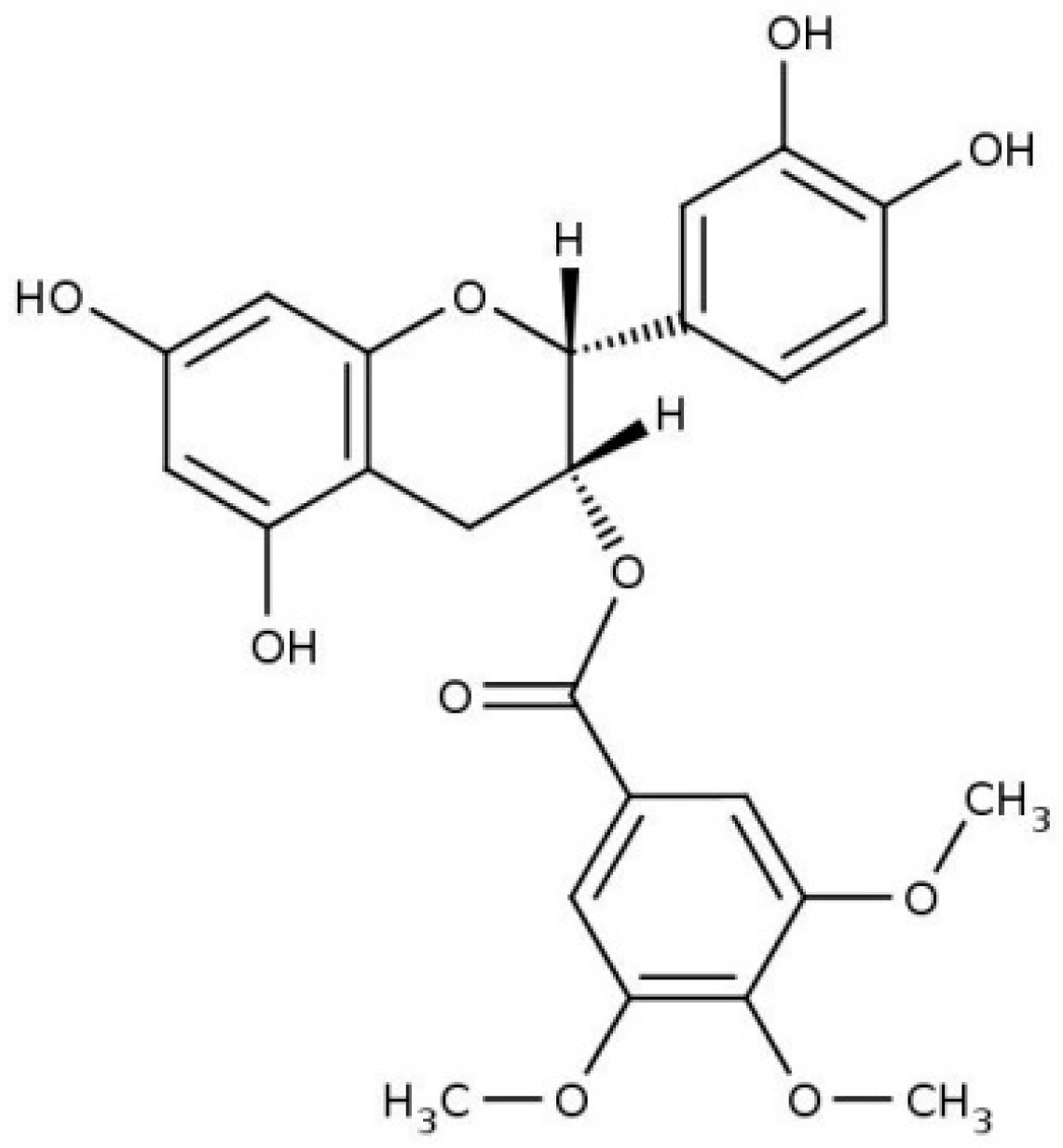
:1. Introduction
2. Results and Discussion
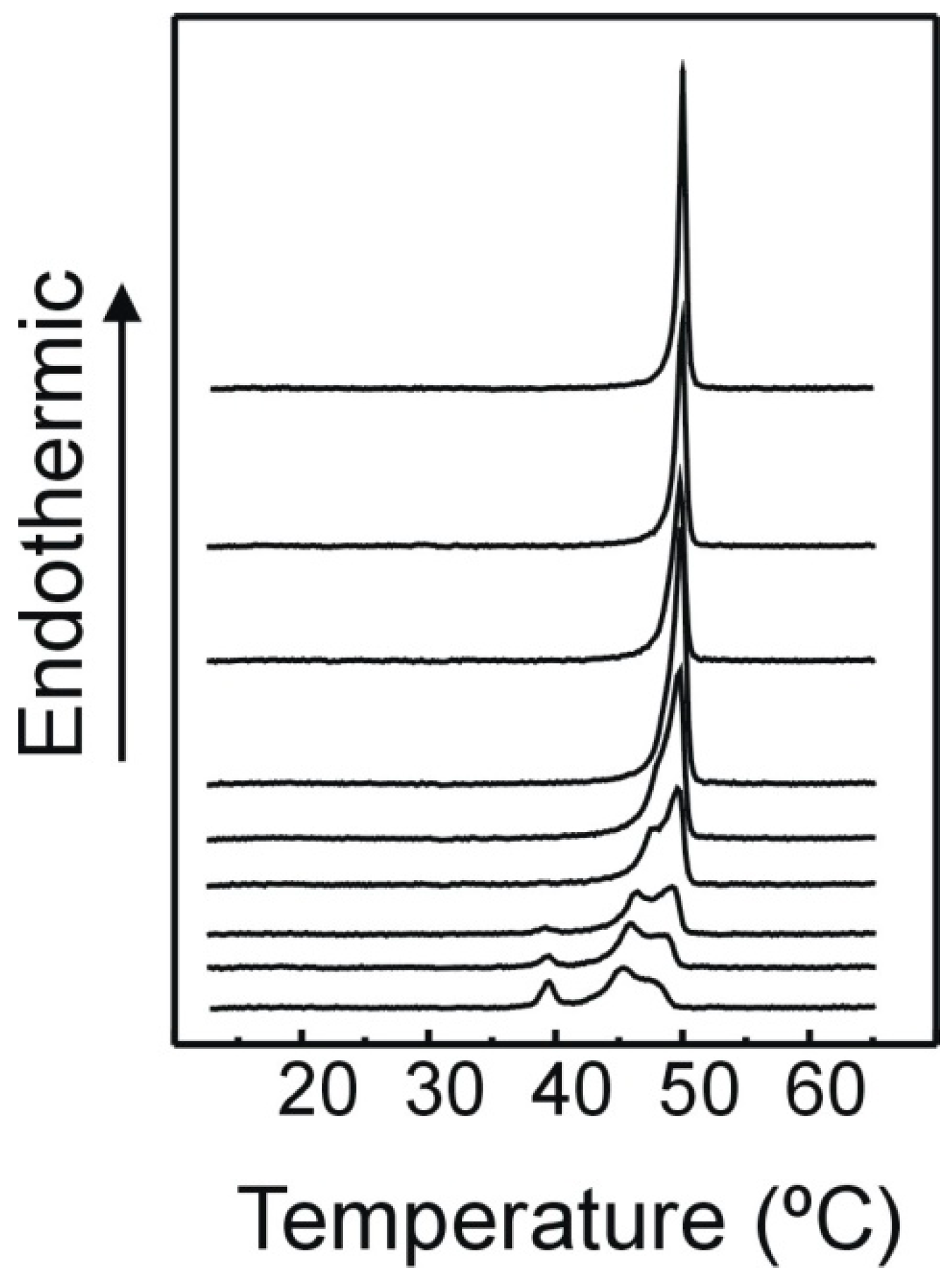
2.1. DSC
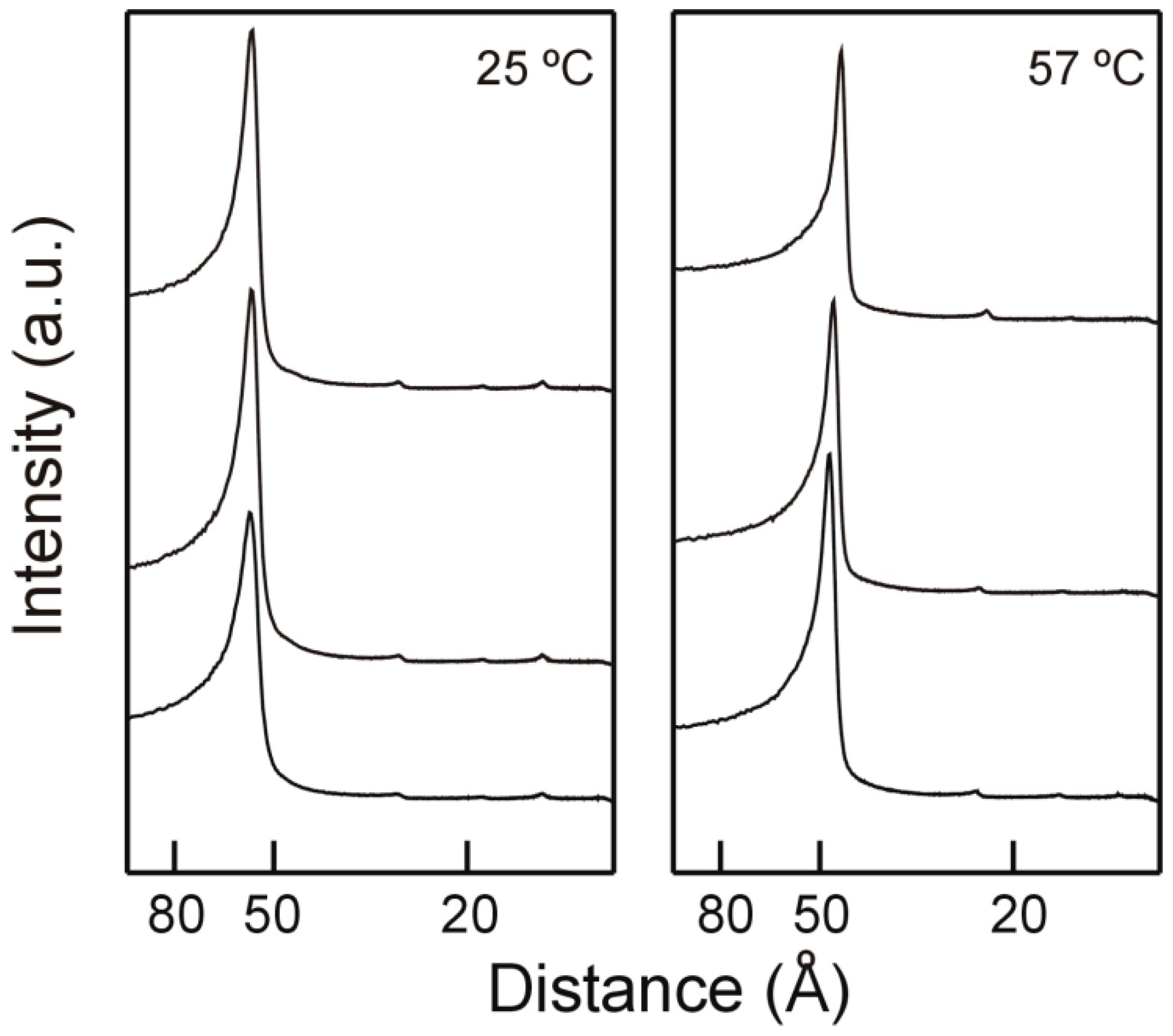
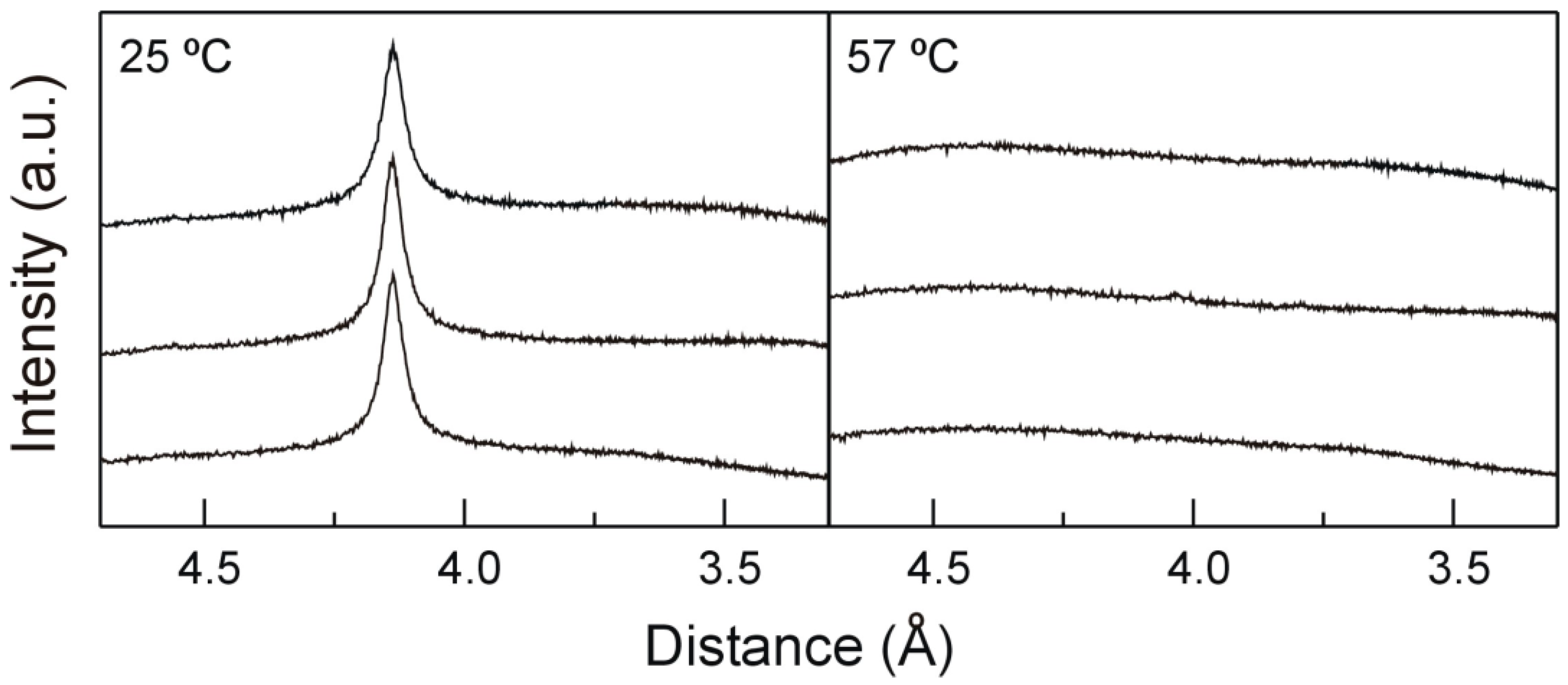
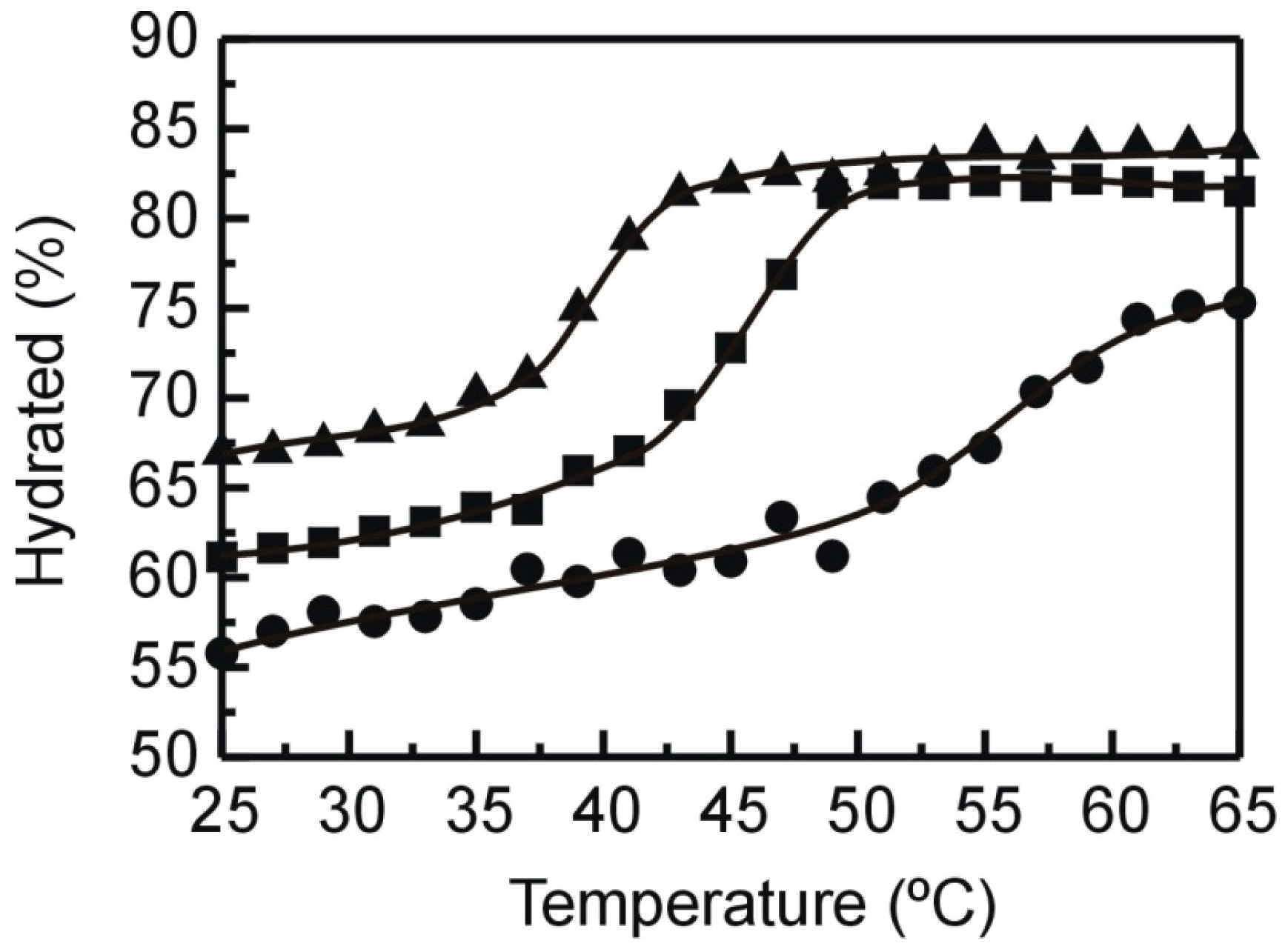
2.2. X-ray Diffraction
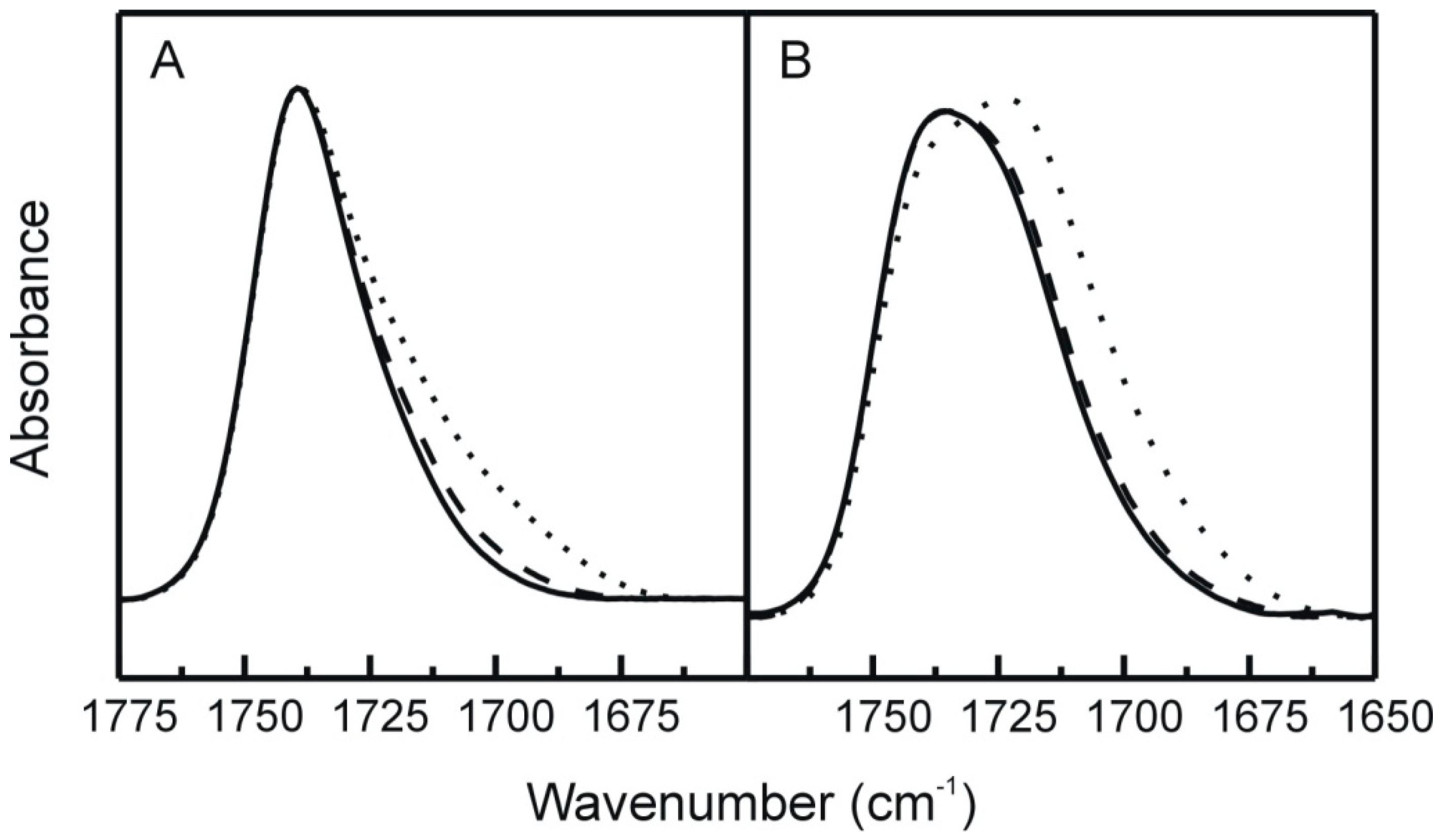
2.3. FT-IR
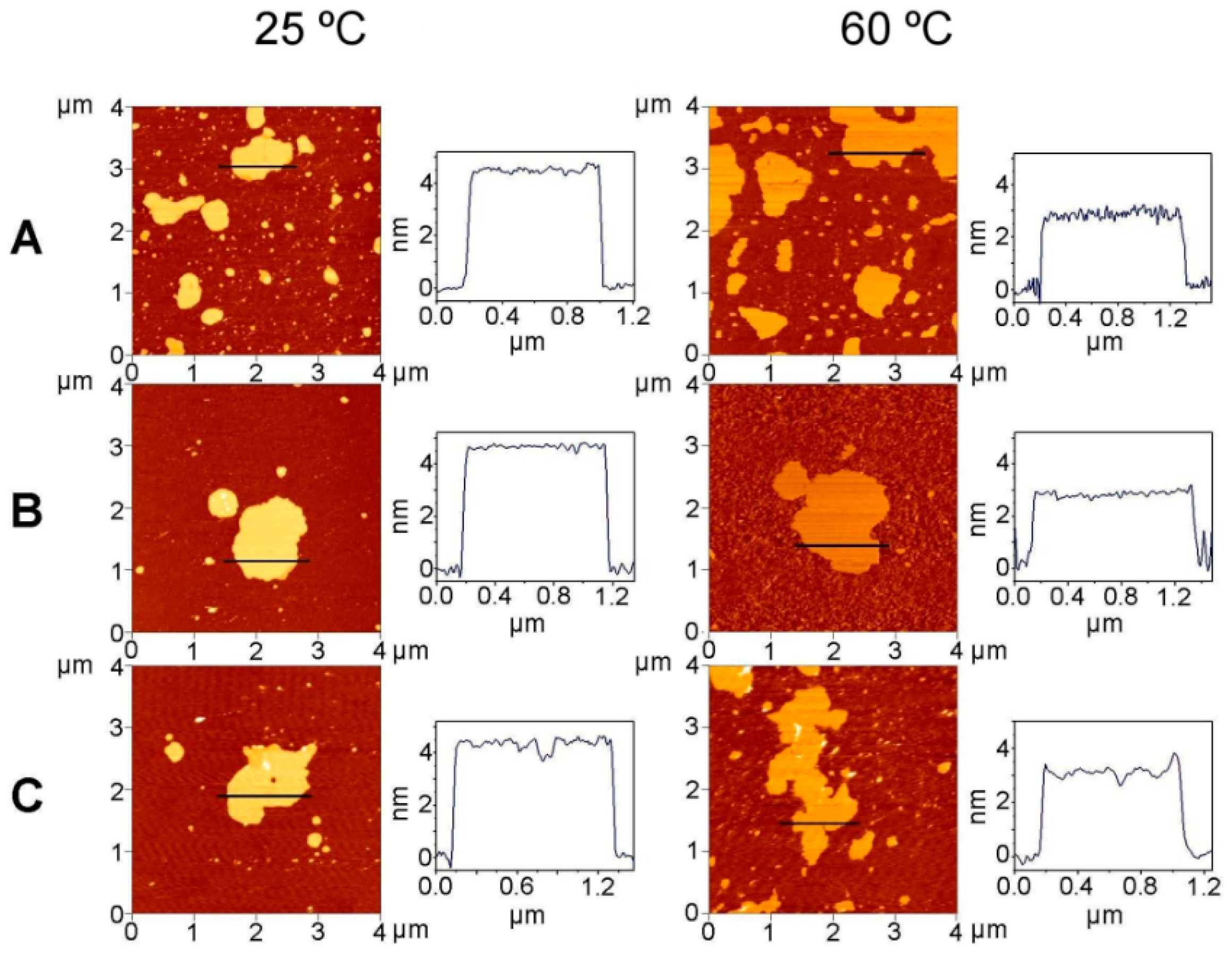
2.4. AFM
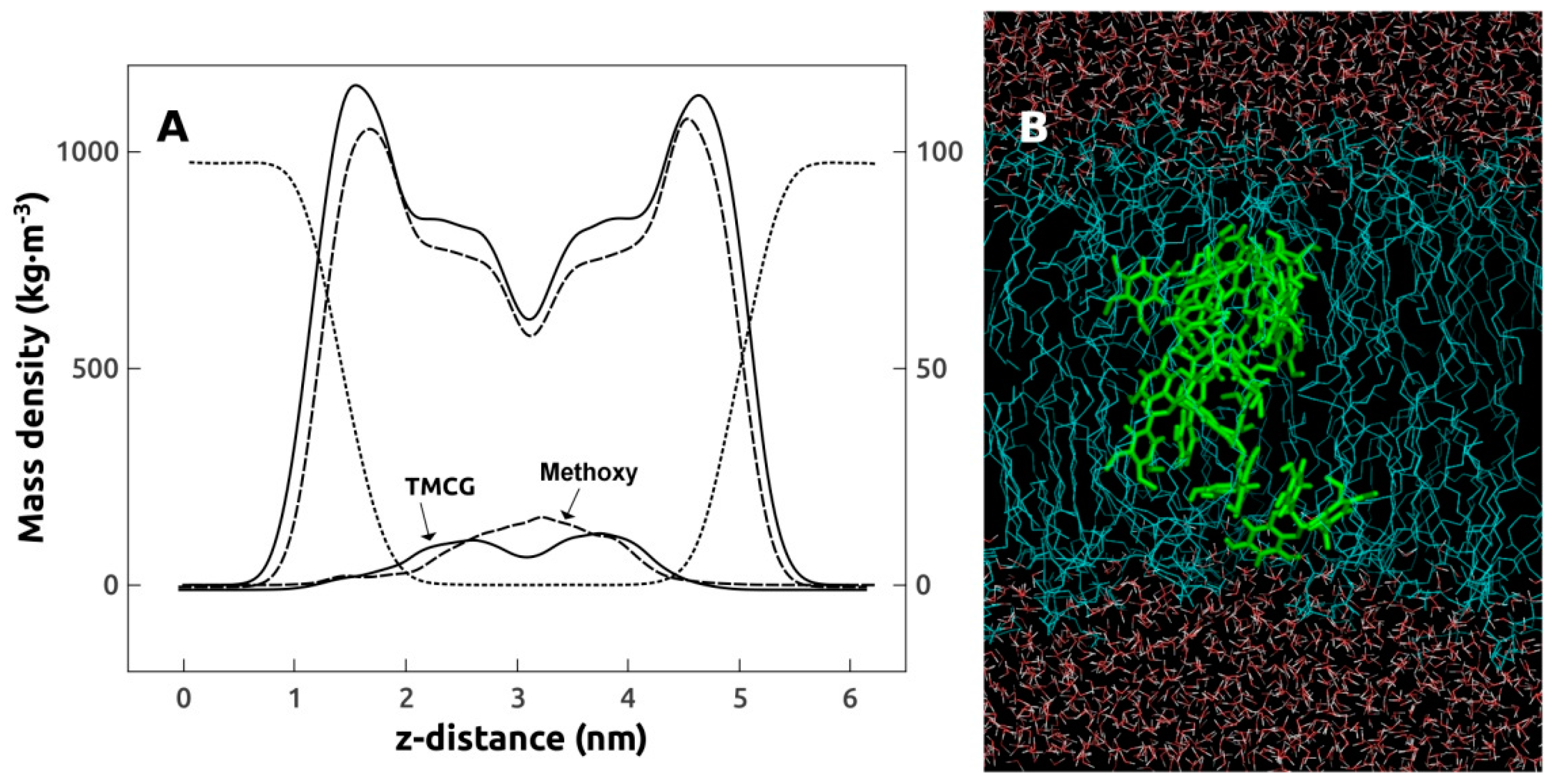
2.5. Molecular Dynamics Simulations
3. Materials and Methods
3.1. Materials
3.2. Differential Scanning Calorimetry (DSC)
3.3. X-Ray Diffraction
3.4. Infrared Spectroscopy (FT-IR)
3.5. Atomic Force Micorsocopy (AFM)
3.6. Molecular Dynamics Simulation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Hendrich, B.A. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Dróżdż, P.; Biesaga, M.; Pyrzynska, K. Screening of the antioxidant properties and polyphenol composition of aromatised green tea infusions. J. Sci. Food Agric. 2012, 92, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Park, J.C.; Taguchi, H.; Fukushima, K.; Hyon, S.H.; Takatori, K. Antifungal susceptibility of epigallocatechin 3-O-gallate (EGCg) on clinical isolates of pathogenic yeasts. Biochem. Biophys. Res. Commun. 2006, 347, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Oh, Y.J.; Lim, J.; Youn, M.; Lee, I.; Pak, H.K.; Park, W.; Jo, W.; Park, S. AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against gram-positive and gram-negative bacteria. Food Microbiol. 2012, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Muthu, M.; Paul, D.; Kim, D.-H.; Chun, S. Bactericidal activity of green tea extracts: The importance of catechin containing nano particles. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Hong, J. Prevention of chronic diseases by tea: Possible mechanisms and human relevance. Annu. Rev. Nutr. 2013, 33, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 2014, 141, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Lee, C. Reversal of Cisplatin resistance by epigallocatechin gallate is mediated by downregulation of axl and tyro 3 expression in human lung cancer cells. Korean J. Physiol. Pharmacol. 2014, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hagen, R.M.; Chedea, V.S.; Mintoff, C.P.; Bowler, E.; Morse, H.R.; Ladomery, M.R. Epigallocatechin-3-gallate promotes apoptosis and expression of the caspase 9a splice variant in PC3 prostate cancer cells. Int. J. Oncol. 2013, 43, 194–200. [Google Scholar] [PubMed]
- Hu, G.; Zhang, L.; Rong, Y.; Ni, X.; Sun, Y. Downstream carcinogenesis signaling pathways by green tea polyphenols: A translational perspective of chemoprevention and treatment for cancers. Curr. Drug Metab. 2014, 15, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Peran, E.; Cabezas-Herrera, J.; Garcia-Canovas, F.; Durrant, M.C.; Thorneley, R.N.; Rodríguez-López, J.N. The antifolate activity of tea catechins. Cancer Res. 2005, 65, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Peran, E.; Cabezas-Herrera, J.; Sánchez-del-Campo, L.; Rodríguez-López, J.N. Effects of folate cycle disruption by the green tea polyphenol epigallocatechin-3-gallate. Int. J. Biochem. Cell Biol. 2007, 39, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Ayala, M.; Sánchez-del-Campo, L.; Montenegro, M.F.; Chazarra, S.; Tárraga, A.; Cabezas-Herrera, J.; Rodríguez-López, J.N. Comparison of a pair of synthetic tea-catechin-derived epimers: Synthesis, antifolate activity, and tyrosinase-mediated activation in melanoma. Chem. Med. Chem. 2011, 6, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Weinstein, I.B. Modulation of signal transduction by tea catechins and related phytochemicals. Mutat. Res. 2005, 591, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsson, H.I.; Koeppe, R.E.; Andersen, O.S. Effects of green tea catechins on gramicidin channel function and inferred changes in bilayer properties. FEBS Lett. 2011, 585, 3101–3105. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Nagao, T.; Ingolfsson, H.I.; Maxfield, F.R.; Andersen, O.S.; Kopelovich, L.; Weinstein, I.B. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007, 67, 6493–6501. [Google Scholar] [CrossRef] [PubMed]
- Maeyama, R.; Kwon, I.K.; Mizunoe, Y.; Anderson, J.M.; Tanaka, M.; Matsuda, T. Novel bactericidal surface: Catechin-loaded surface-erodible polymer prevents biofilm formation. J. Biomed. Mater. Res. 2005, 75, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Tao, M.-H.; Hung, T.-M.; Chen, J.-C.; Lin, Z.-J.; Huang, C. (–)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus. Antivir. Res. 2014, 111, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Watanabe, T.; Mondal, A.; Suzuki, K.; Kurusu-Kanno, M.; Li, Z.; Yamazaki, T.; Fujiki, H.; Suganuma, M. Mechanism-based inhibition of cancer metastasis with (–)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2014, 443, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Stereospecificity in membrane effects of catechins. Chem. Biol. Interact. 2001, 134, 41–54. [Google Scholar] [CrossRef]
- Nakayama, T.; Hashimoto, T.; Kajiya, K.; Kumazawa, S. Affinity of polyphenols for lipid bilayers. Biofactors 2000, 13, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hung, W.-C.; Chen, F.-Y.; Lee, C.-C.; Huang, H.W. Interaction of tea catechin (−)-epigallocatechin gallate with lipid bilayers. Biophys. J. 2009, 96, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Caturla, N.; Vera-Samper, E.; Villalain, J.; Mateo, C.R.; Micol, V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef]
- How, C.W.; Teruel, J.A.; Ortiz, A.; Montenegro, M.F.; Rodríguez-López, J.N.; Aranda, F.J. Effects of a synthetic antitumoral catechin and its tyrosinase-processed product on the structural properties of phosphatidylcholine membranes. Biochim. Biophys. Acta 2014, 1838, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.A.H.; McElhaney, R.N. Calorimetric and spectroscopic studies of the polymorphic phase behaviour of a homologous series of n.saturated 1,2-diacylphosphatidylethanolamines. Biophys. J. 1993, 64, 1081–1096. [Google Scholar] [CrossRef]
- Ortiz, A.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Manresa, A.; Aranda, F.J. Interactions of a Rhodococcus sp. biosurfactant trehalose lipid with phosphatidylethanolamine membranes. Biochim. Biophys. Acta 2008, 1778, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, V. X-ray diffraction studies of lipid-water systems. In Biological Membranes; Chapman, D., Ed.; Academic Press: New York, NY, USA, 1968; pp. 71–123. [Google Scholar]
- Marsh, D. Handbook of Lipid Bilayers; CRC Press: Boca Raton, FL, USA, 1990; p. 173. [Google Scholar]
- Tamba, Y.; Ohba, S.; Kubota, M.; Yoshioka, H.; Yoshioka, H.; Yamazaki, M. Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys. J. 2007, 92, 3178–3194. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, A.; Luzzati, V.; Reman, F.C. Structure and polymorphism of the hydrocarbon chains of lipids: A study of lecithin–water phases. J. Mol. Biol. 1973, 25, 711–733. [Google Scholar] [CrossRef]
- Seddon, J.M.; Harlos, K.; Marsh, D. Metastability and polymorphism in the gel and fluid bilayer phases of dilauroylphosphatidylethanolamine. Two crystalline forms in excess water. J. Biol. Chem. 1983, 25, 3850–3854. [Google Scholar]
- Smith, E.A.; Dea, P.K. Differential Scanning Calorimetry Studies of Phospholipid Membranes: The Interdigitated Gel Phase. In Applications of Calorimetry in a Wide Context-Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; Elkordy, A.A., Ed.; InTech Open Access: Rijeka, Croatia, 2013; pp. 407–444. [Google Scholar]
- Veiro, J.A.; Nambi, P.; Rowe, E.S. Effect of Alcohols on the Phase Transitions of Dihexadecylphosphatidylcholine. Biochim. Biophys. Acta 1988, 943, 108–111. [Google Scholar] [CrossRef]
- Kusube, M.; Matsuki, H.; Kaneshina, S. Thermotropic and barotropic phase transitions of N-methylated dipalmitoylphosphatidylethanolamine bilayers. Biochim. Biophys. Acta 2005, 1668, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tamai, N.; Goto, M.; Matsuki, H.; Kaneshina, S. A Mechanism of Pressure-Induced Interdigitation of Lipid Bilayers. J. Phys. Conf. Ser. 2010, 215, 012161. [Google Scholar] [CrossRef]
- McIntosh, T.J. Hydration Properties of Lamellar and Non-lamellar Phases of Phosphatidylcholine and Phosphatidylethanolamine. Chem. Phys. Lipids 1996, 81, 117–131. [Google Scholar] [CrossRef]
- Mantsch, H.H.; McElhaney, R.N. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids 1991, 57, 213–226. [Google Scholar] [CrossRef]
- Blume, A.; Hübner, W.; Messner, G. Fourier transform infrared spectroscopy of 13C=O labelled phospholipids. Hydrogen bonding to carbonyl groups. Biochemistry 1988, 27, 8239–8249. [Google Scholar] [CrossRef] [PubMed]
- Nussio, M.R.; Voelcker, N.H. Lateral heterogeneities in supported bilayers from pure and mixed phosphatidylethanolamine demonstrating hydrogen bonding capacity samples. Biointerphases 2008, 3, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Yarrow, F.; Kuipers, B.W.M. AFM study of the thermotropic behaviour of supported DPPC bilayers with and without the model peptide WALP23. Chem. Phys. Lipids 2011, 164, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-del-Campo, L.; Tárraga, A.; Montenegro, M.F.; Cabezas-Herrera, J.; Rodríguez-López, J.N. Melanoma activation of 3-o-(3,4,5-trimethoxybenzoyl)-(−)-epicatechin to a potent irreversible inhibitor of dihydrofolate reductase. Mol. Pharm. 2009, 6, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.J.F.; van Gent, C.M.; Pries, C. A rapid and sensitive sub-micro phosphorus determination. Anal. Chim. Acta 1961, 24, 203–204. [Google Scholar] [CrossRef]
- Teruel, J.A.; Ortiz, A.; Aranda, F.J. Influence of organotin compounds on phosphatidylserine membranes. Appl. Organometal. Chem. 2004, 18, 111–116. [Google Scholar] [CrossRef]
- Piggot, T.J.; Holdbrook, D.A.; Khalid, S. Electroporation of the E. coli and S. Aureus Membranes: Molecular Dynamics Simulations of Complex Bacterial Membranes. J. Phys. Chem. B 2011, 115, 13381–13388. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.M.; Martinez, L. Packing optimization for automated generation of complex system′s initial configurations for molecular dynamics and docking. J. Comput. Chem. 2003, 24, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Intermolecular Forces; Pullman, B., Reidel, D., Eds.; Springer: Dordrecht, The Netherlands, 1981; pp. 331–342. [Google Scholar]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.W.; Pandit, S.A.; Scott, H.L.; Jakobsson, E. An improved united atom force field for simulation of mixed lipid bilayers. J. Phys. Chem. B 2009, 113, 2748–2763. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.L.C. The PyMOL Molecular Graphics System, Version 1.5.0.1. Available online: http://www.pymol.org (accessed on 1 May 2010).
- Leonov, A.; Arlia-Ciommo, A.; Piano, A.; Svistkova, V.; Lutchman, V.; Medkour, Y.; Titorenko, V.I. Longevity Extension by Phytochemicals. Molecules 2015, 20, 6544–6572. [Google Scholar] [CrossRef] [PubMed]
- Casali, C.; Weber, K.; Favale, N.O.; Fernández Tome, M.C. Environmental hyperosmolality regulates phospholipid biosynthesis in the renal epithelial cell line MDCK. J. Lipid Res. 2013, 54, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, R.E., II. Bilayer Thickness and Membrane Protein Function: An Energetic Perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado, F.; Teruel, J.A.; Casado, S.; Ortiz, A.; Rodríguez-López, J.N.; Aranda, F.J. Location and Effects of an Antitumoral Catechin on the Structural Properties of Phosphatidylethanolamine Membranes. Molecules 2016, 21, 829. https://doi.org/10.3390/molecules21070829
Casado F, Teruel JA, Casado S, Ortiz A, Rodríguez-López JN, Aranda FJ. Location and Effects of an Antitumoral Catechin on the Structural Properties of Phosphatidylethanolamine Membranes. Molecules. 2016; 21(7):829. https://doi.org/10.3390/molecules21070829
Chicago/Turabian StyleCasado, Francisco, José A. Teruel, Santiago Casado, Antonio Ortiz, José N. Rodríguez-López, and Francisco J. Aranda. 2016. "Location and Effects of an Antitumoral Catechin on the Structural Properties of Phosphatidylethanolamine Membranes" Molecules 21, no. 7: 829. https://doi.org/10.3390/molecules21070829
APA StyleCasado, F., Teruel, J. A., Casado, S., Ortiz, A., Rodríguez-López, J. N., & Aranda, F. J. (2016). Location and Effects of an Antitumoral Catechin on the Structural Properties of Phosphatidylethanolamine Membranes. Molecules, 21(7), 829. https://doi.org/10.3390/molecules21070829






