Tailoring the Spacer Arm for Covalent Immobilization of Candida antarctica Lipase B—Thermal Stabilization by Bisepoxide-Activated Aminoalkyl Resins in Continuous-Flow Reactors
Abstract
:1. Introduction
2. Results and Discussion
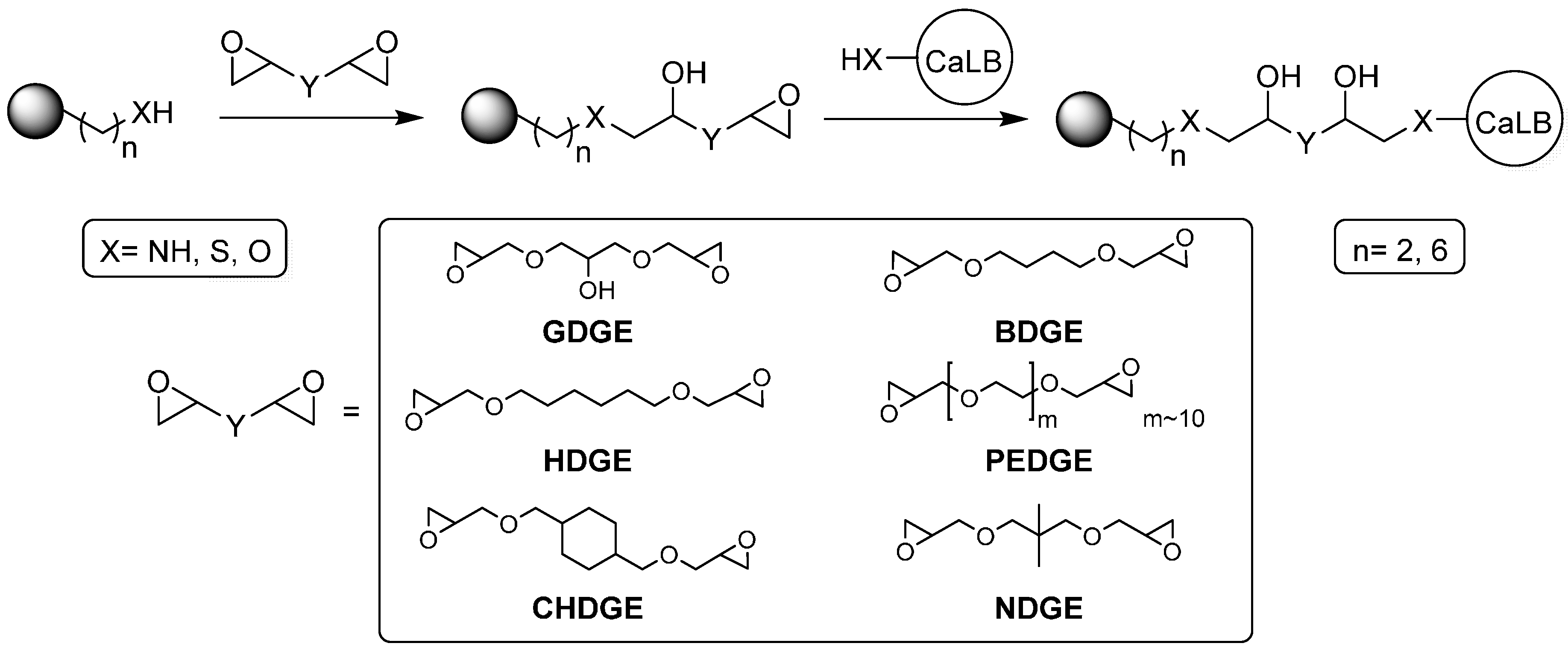
2.1. Selection and Bisepoxide Activation of Carriers Used for Covalent Immobilization of CaLB
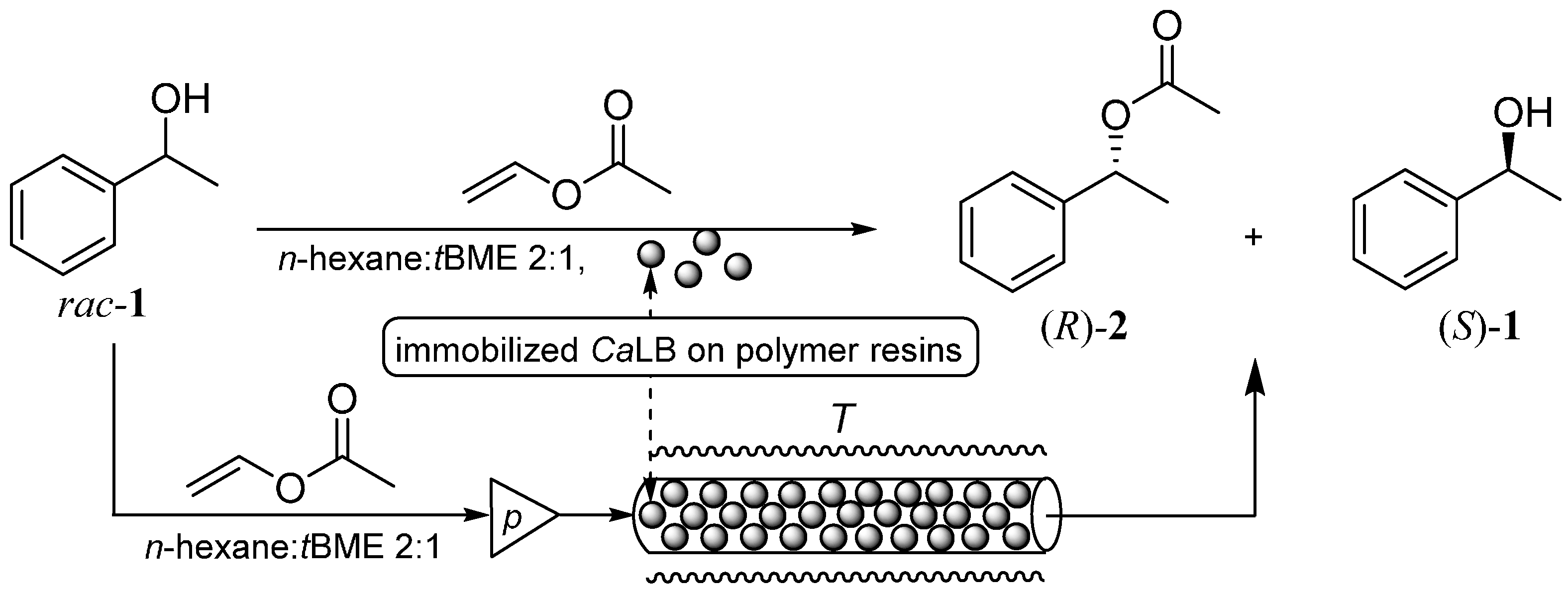
2.2. Covalent Immobilization of CaLB on Bisepoxide-Activated Supports and Its Properties in Kinetic Resolution of 1-Phenylethanol rac-1
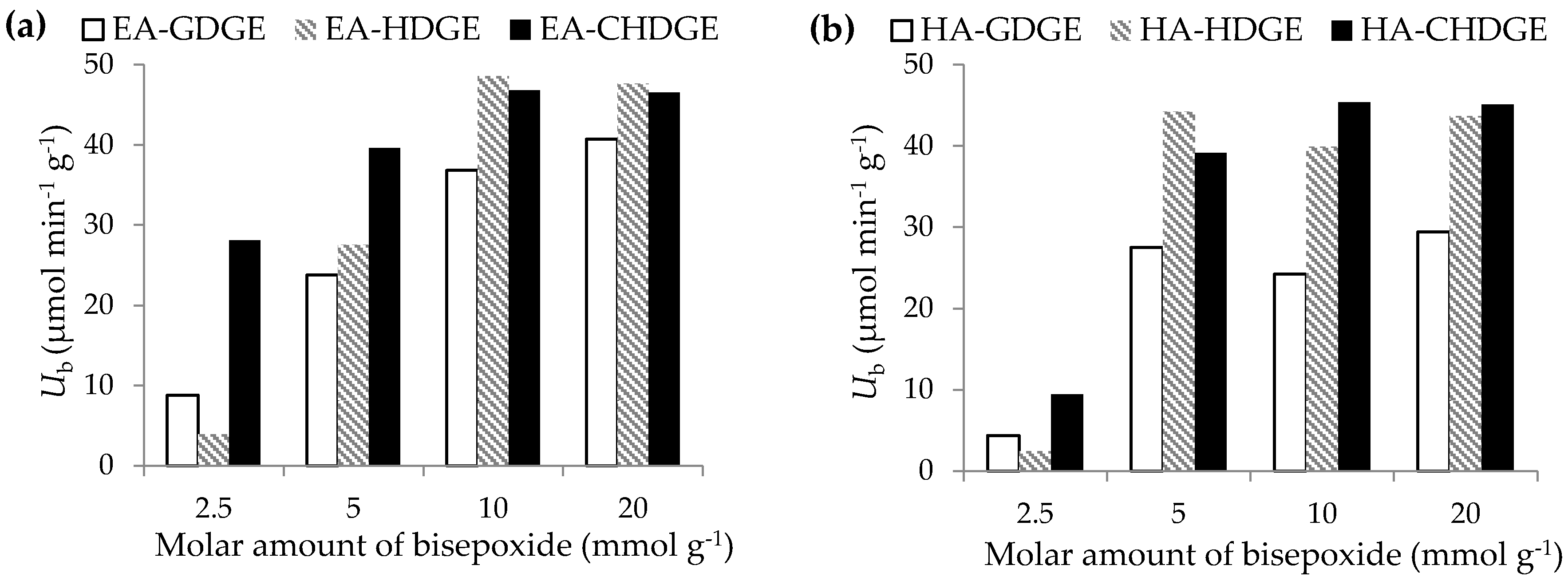
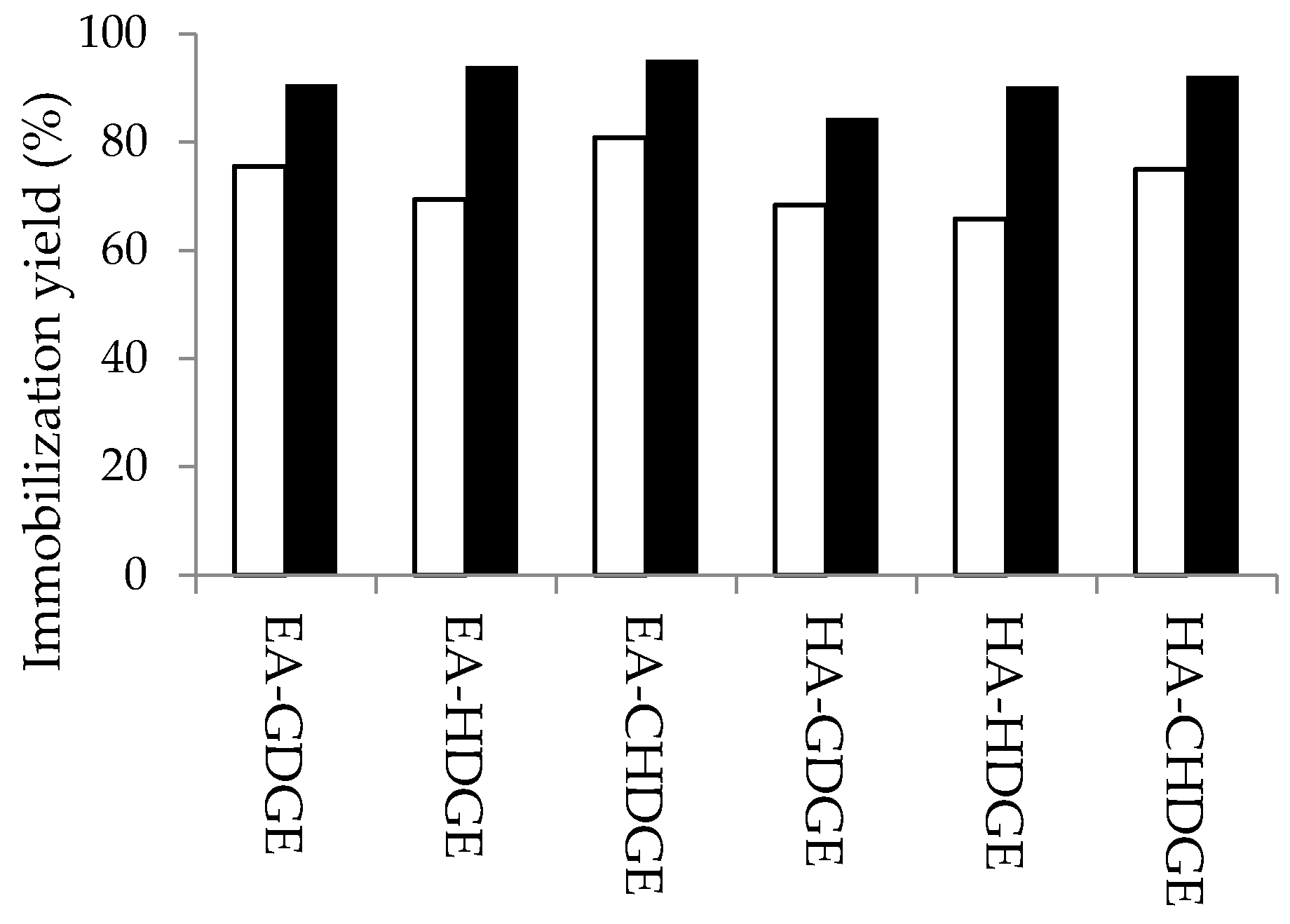
2.3. Optimization of the Bisepoxide Activation for Covalent Immobilization of CaLB
2.4. Operational Stability of the CaLB Preparations
2.5. Long-Term Storage Stability of the CaLB Preparations
2.6. Continuous-Flow Kinetic Resolution of Racemic 1-Phenylethanol (rac-1) Catalyzed by CaLB Preparations on Bisepoxide-Activated Resins—Substrate Concentration and Temperature Effects
2.7. Characterization of Support Morphology after Recycling and Application in Continuous-Flow Reactors
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Drying of the Aminoalkyl Polymer Resins
3.4. Surface Activation of Aminoalkyl Polymer Resins with Bisepoxides or Glutaraldehyde
3.5. Immobilization of CaLB on Bisepoxide-Activated Polymer Resins
3.6. Lipase Desorption Tests
3.7. Kinetic Resolution of 1-Phenylethanol (rac-1) Catalyzed by the CaLB Preparations in Batch Mode
3.8. Optimization of the Bisepoxide Activation of Alkylamino Polymer Resins
3.9. Recycling of the Immobilized CaLB Biocatalysts
3.10. Long-Term Stability of CaLB Derivatives (Storage at 4 °C)
3.11. Packed-Bed Columns Filled with Immobilized CaLB
3.12. Kinetic Resolution of 1-Phenylethanol (rac-1) Catalyzed by the CaLB Preparations in Continuous-Flow Mode
3.13. Effect of Temperature on Kinetic Resolution of 1-Phenylethanol (rac-1) Catalyzed by the CaLB Preparations in Continuous-Flow Mode
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CaLB | Candida antarctica lipase B |
| EA | ethylamine functionalized macroporous acrylate resin |
| HA | hexamethylamine functionalized macroporous acrylate resin |
| GDGE | glycerol diglycidyl ether |
| HDGE | 1,6-hexanediol diglycidyl ether |
| CHDGE | cyclohexanedimethanol diglycidyl ether |
| BDGE | 1,4-butanediol diglycidyl ether |
| NDGE | neopentylglycol diglycidyl ether |
| PEDGE | polyethyleneglycol diglycidyl ether |
| GA | glutaraldehyde |
References
- Sandoval, G. Lipases and Phospholipases, Methods and Protocols; Humana Press: New York, NY, USA, 2012. [Google Scholar]
- Forde, J.; Vakurov, A.; Gibson, T.D.; Millner, P.; Whelehan, M.; Marison, I.W.; Ó’Fágáin, C. Chemical modification and immobilisation of lipase B from Candida antarctica onto mesoporous silicates. J. Mol. Catal. B Enzym. 2010, 66, 203–209. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Mendes, A.A.; Adriano, W.S.; Goncalves, L.R.B.; Giordano, R.L.C. Multipoint covalent immobilization of microbial lipase on chitosan and agarose activated by different methods. J. Mol. Catal. B Enzym. 2008, 51, 100–109. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl-Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Poppe, J.K.; Costa, A.P.O.; Brasil, M.C.; Rodrigues, R.C.; Ayub, M.A.Z. Multipoint covalent immobilization of lipases on aldehyde-activated support: Characterization and application in transesterification reaction. J. Mol. Catal. B Enzym. 2013, 94, 57–62. [Google Scholar] [CrossRef]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enz. Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Verger, R. Interfacial activation of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T.; Kazlauskas, R.J. Hydrolases in Organic Synthesis. In Regio- and Stereoselective Biotransformations, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2006; Chapter 5; pp. 61–183. [Google Scholar]
- Palomo, J.M.; Fernández-Lorente, G.; Mateo, C.; Fuentes, M.; Fernández-Lafuente, R.; Guisan, J.M. Modulation of the enantioselectivity of Candida antarctica B lipase via conformational engineering: Kinetic resolution of (±)-α-hydroxy-phenylacetic acid derivatives. Tetrahedron 2002, 13, 1337–1345. [Google Scholar] [CrossRef]
- Brady, L.; Brzozowski, A.M.; Derewenda, Z.S.; Dodson, E.; Dodson, G.; Tolley, S.; Turkenburg, J.P.; Christiansen, L.; Huge-Jensen, B.; Norskov, L.; et al. A serine protease triad forms the catalytic center of a triacylglycerol lipase. Nature 1990, 343, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.G.; Besteti, M.D.; Manoel, E.A.; da Saliva, A.A.T.; Almeida, R.V.; Simas, A.B.C.; Fernandez-Lafuente, R.; Pinto, J.C.; Freire, D.M.G. Preparation of core-shell polymer supports to immobilize lipase B from Candida antarctica. Effect of the support nature on catalytic properties. J. Mol. Catal. B Enzym. 2014, 100, 59–67. [Google Scholar] [CrossRef]
- Kramer, M.; Cruz, J.C.; Pfromm, P.H.; Rezac, M.E.; Czermak, P. Enantioselective transesterification by Candida antarctica Lipase B immobilized on fumed silica. J. Biotechnol. 2010, 150, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Víllora, G.; Gómez, D.; Gayo, A.B.; Sánchez-Conesa, J.A.; Rubio, M.; Iborra, J.L. Membrane reactor with immobilized Candida antarctica lipase B for ester synthesis in supercritical carbon dioxide. J. Supercrit. Fluids 2004, 29, 121–128. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Effect of immobilization protocol on the properties of lipase B from Candida antarctica in organic media: Enantiospecific production of atenolol acetate. J. Mol. Catal. B Enzym. 2011, 71, 124–132. [Google Scholar] [CrossRef]
- Uppenberg, J.; Hansen, M.T.; Patkar, S.; Jones, T.A. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure (Oxford, UK) 1994, 2, 293–308. [Google Scholar] [CrossRef]
- Uppenberg, J.; Ohrner, N.; Norin, M.; Hult, K.; Kleywegt, G.J.; Patkar, S.; Waagen, V.; Anthonsen, T.; Jones, T.A. Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry 1995, 34, 16838–16851. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Svensson, I.; Monduzzi, M.; Solinas, V.; Adlercreutz, P. The atypical lipase B from Candida antarctica is better adapted for organic media than the typical lipase from Thermomyces lanuginosa. Biochim. Biophys. Acta 2003, 1646, 145–151. [Google Scholar] [CrossRef]
- Martinelle, M.; Holmquist, M.; Hult, K. On the interfacial activation of Candida antarctica lipase A and B as compared with Humicola lanuginosa lipase. Biochim. Biophys. Acta 1995, 1258, 272–276. [Google Scholar] [CrossRef]
- Stauch, B.; Fisher, S.J.; Cianci, M. Open and closed states of Candida antarctica lipase B: Protonation and the mechanism of interfacial activation. J. Lipid Res. 2015, 56, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, M.; Stojanovic, M.; Banjanac, K.; Carevic, M.; Prlainovic, N.; Milosavic, N.; Bezbradica, D. Immobilization of lipase on epoxy-activated Purolite A109 and its post-immobilization stabilization. Process Biochem. 2014, 49, 637–646. [Google Scholar] [CrossRef]
- Kumar, D.; Nagar, S.; Bhushan, I.; Kumar, L.; Parshad, R.; Gupta, V.K. Covalent immobilization of organic solvent tolerant lipase on aluminium oxide pellets and its potential application in esterification reaction. J. Mol. Catal. B Enzym. 2013, 87, 51–61. [Google Scholar] [CrossRef]
- Barbosa, O.; Ariza, C.; Ortiz, C.; Torres, R. Kinetic resolution of (R/S)-propranolol (1-isopropylamino-3-(1-naphtoxy)-2-propranol) catalyzed by immobilized preparations of Candida antarctica lipase B (CAL-B). New Biotechnol. 2010, 27, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.; Idris, A.; Atta, M.; Loong, T.C. Covalent immobilization of Candida antarctica lipase B on nanopolystyrene and its application to microwave-assisted esterification. Chin. J. Catal. 2014, 35, 1555–1564. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012, 47, 1220–1227. [Google Scholar] [CrossRef]
- Poojari, Y.; Clarson, S.J. Thermal stability of Candida antarctica lipase B immobilized on macroporous acrylic resin particles in organic media. Biocatal. Agric. Biotechnol. 2013, 2, 7–11. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Avnir, D.; Braun, S.; Lev, O.; Ottolenghi, M. Enzymes and Other Proteins Entrapped in Sol-Gel Materials. Chem. Mater. 1994, 6, 1605–1614. [Google Scholar] [CrossRef]
- Torres, R.; Ortiz, C.; Pessela, B.C.C.; Palomo, J.M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Improvement of the enantioselectivity of lipase (fraction B) from Candida antarctica via adsorption on polyethyleneimine-agarose under different experimental conditions. Enzyme. Microb. Technol. 2006, 39, 167–171. [Google Scholar] [CrossRef]
- Lee, C.H.; Lin, T.S.; Mou, C.Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Boros, Z.; Abaháziová, E.; Oláh, M.; Sátorhelyi, P.; Erdélyi, B.; Poppe, L. Novel hydrophobic silica gels as carriers for lipases—Separation of lipase A and lipase B from Candida antarctica. Chim. Oggi/Chem. Today 2012, 30, 28–31. [Google Scholar]
- Blanco, R.M.; Terreros, P.; Munoz, N.; Serra, E. Ethanol improves lipase immobilization on a hydrophobic support. J. Mol. Catal. B Enzym. 2007, 47, 13–20. [Google Scholar] [CrossRef]
- Mendes, A.A.; de Castro, H.F.; Andrade, G.S.S.; Tardioli, P.W.; Giordano, R.L.C. Preparation and application of epoxy-chitosan/alginate support in the immobilization of microbial lipases by covalent attachment. React. Funct. Polym. 2013, 73, 160–167. [Google Scholar] [CrossRef]
- Bernal, C.; Urrutia, P.; Illanes, A.; Wilson, L. Hierarchical meso-macroporous silica grafted with glyoxyl groups: Opportunities for covalent immobilization of enzymes. New Biotechnol. 2013, 30, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Grazú, V.; Abian, O.; Mateo, C.; Batista-Viera, F.; Fernández-Lafuente, R.; Guisán, J.M. Stabilization of Enzymes by Multipoint Immobilization of Thiolated Proteins on New Epoxy-Thiol Supports. Biotechnol. Bioeng. 2005, 90, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Grazu, V.; Palomo, J.M.; López-Gallego, F.; Fernández-Lafuente, R.; Guisán, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional Supports in Enzyme Immobilization: From Traditional Immobilization Protocols to Opportunities in Tuning Enzyme Properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synt. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Mateo, C.; Torres, R.; Fernández-Lorente, G.; Ortiz, C.; Fuentes, M.; Hidalgo, A.; López-Gallego, F.; Abian, O.; Palomo, J.M.; Betancor, L.; et al. Epoxy-amino groups: A new tool for improved immobilization of proteins by the epoxy method. Biomacromolecules 2003, 4, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Vasylieva, N.; Barnych, B.; Meiller, A.; Maucler, C.; Pollegioni, L.; Lin, J.S.; Barbier, D.; Marinesco, S. Covalent enzyme immobilization by poly(ethylene glycol) diglycidyl ether (PEGDE) for microelectrode biosensor preparation. Biosens. Bioelectron. 2011, 26, 3993–4000. [Google Scholar] [CrossRef] [PubMed]
- Weiser, D.; Varga, A.; Kovács, K.; Nagy, F.; Szilágyi, A.; Vértessy, B.G.; Paizs, C.; Poppe, L. Bisepoxide Cross-Linked Enzyme Aggregates—Novel Immobilized Biocatalysts for Selective Biotransformations. ChemCatChem 2014, 6, 1463–1469. [Google Scholar]
- Bartha-Vári, J.H.; Toşa, M.I.; Irimie, F.D.; Weiser, D.; Boros, Z.; Vértessy, B.G.; Paizs, C.; Poppe, L. Immobilization of phenylalanine ammonia-lyase on single-walled carbon nanotubes for stereoselective biotransformations in batch and in continuous-flow modes. ChemCatChem 2015, 7, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Bencze, L.C.; Bartha-Vári, J.H.; Katona, G.; Toşa, M.I.; Paizs, C.; Irimie, F.D. Nanobioconjugates of Candida antarctica lipase B and single-walled carbon nanotubes in biodiesel production. Bioresour. Technol. 2016, 200, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Boros, Z.; Hornyánszky, G.; Nagy, J.; Poppe, L. Stereoselective Hydrolase-Catalyzed Processes in Continuous-Flow Mode. In Cascade Biocatalysis: Integrating Stereoselective and Environmentally Friendly Reactions; Wiley-VCH: Weinheim, Germany, 2014; pp. 199–230. [Google Scholar]
- Mak, X.Y.; Laurino, P.; Seeberger, P.H. Asymmetric reactions in continuous flow. Beilstein. J. Org. Chem. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Yuryev, R.; Strompen, S.; Liese, A. Coupled chemo(enzymatic) reactions in continuous flow. Beilstein. J. Org. Chem. 2011, 7, 1149–1467. [Google Scholar] [CrossRef] [PubMed]
- Csajági, C.; Szatzker, G.; Tőke, E.R.; Ürge, L.; Darvas, F.; Poppe, L. Enantiomer selective acylation of racemic alcohols by lipases in continuous-flow bioreactors. Tetrahedron Asymmetry 2008, 19, 237–246. [Google Scholar] [CrossRef]
- Boros, Z.; Falus, P.; Márkus, M.; Weiser, D.; Oláh, M.; Hornyánszky, G.; Nagy, J.; Poppe, L. How the mode of Candida antarctica lipase B immobilization affects the continuous-flow kinetic resolution of racemic amines at various temperatures. J. Mol. Catal. B Enzym. 2013, 85–86, 119–125. [Google Scholar]
- Falus, P.; Boros, Z.; Kovács, P.; Poppe, L.; Nagy, J. Lipase-Catalyzed Kinetic Resolution of 1-(2-Hydroxycyclohexyl)Indoles in Batch and Continuous-Flow Systems. J. Flow Chem. 2014, 4, 125–134. [Google Scholar] [CrossRef]
- Silva, M.V.M.; Bassut, J.F.; Junior, I.I.; de Souza, S.P.; Estrada, M.L.G.; Miranda, L.S.M.; de Souza, R.O.M.A. Lipase immobilization towards improved productivity on kinetic resolutions by a continuous-flow process. RSC Adv. 2015, 5, 102409–102415. [Google Scholar] [CrossRef]
- García-Verdugo, E.; Altava, B.; Burguete, M.I.; Lozano, P.; Luis, S.V. Ionic liquids and continuous flow processes: A good marriage to design sustainable processes. Green Chem. 2015, 17, 2693–2713. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Hellner, G.; Boros, Z.; Tomin, A.; Poppe, L. Novel sol-gel lipases by designed bioimprinting for continuous-flow kinetic resolutions. Adv. Synth. Catal. 2011, 353, 2481–2491. [Google Scholar] [CrossRef]
- Poppe, J.K.; Garcia-Galan, C.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized on hydrophobic supports. J. Mol. Catal. B Enzym. 2013, 94, 51–56. [Google Scholar] [CrossRef]
- Abaháziová, E.; Boros, Z.; Poppe, L. Additives enhancing the catalytic properties of lipase from Burkholderia cepacia immobilized on mixed-function-grafted mesoporous silica gel. Molecules 2014, 19, 9818–9837. [Google Scholar] [CrossRef] [PubMed]
- Boros, Z.; Weiser, D.; Márkus, M.; Abaháziová, E.; Magyar, Á.; Tomin, A.; Koczka, B.; Kovács, P.; Poppe, L. Hydrophobic adsorption and covalent immobilization of Candida antarctica lipase B on mixed-function-grafted silica gel supports for continuous-flow biotransformations. Process Biochem. 2013, 48, 1039–1047. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sample Availability: Samples of CaLB on bisepoxide-activated EA- or HA-resins are available from the authors.
 ) or hexylamine (
) or hexylamine (  ) resin activated by CHDGE (●), GDGE (▲) or HDGE (■). Relative activity (%) = activity in given cycle/activity in first cycle × 100 was determined in kinetic resolution of rac-1. Experiments were performed as described in Section 3.9.
) resin activated by CHDGE (●), GDGE (▲) or HDGE (■). Relative activity (%) = activity in given cycle/activity in first cycle × 100 was determined in kinetic resolution of rac-1. Experiments were performed as described in Section 3.9.
 ) or hexylamine (
) or hexylamine (  ) resin activated by CHDGE (●), GDGE (▲) or HDGE (■). Relative activity (%) = activity in given cycle/activity in first cycle × 100 was determined in kinetic resolution of rac-1. Experiments were performed as described in Section 3.9.
) resin activated by CHDGE (●), GDGE (▲) or HDGE (■). Relative activity (%) = activity in given cycle/activity in first cycle × 100 was determined in kinetic resolution of rac-1. Experiments were performed as described in Section 3.9.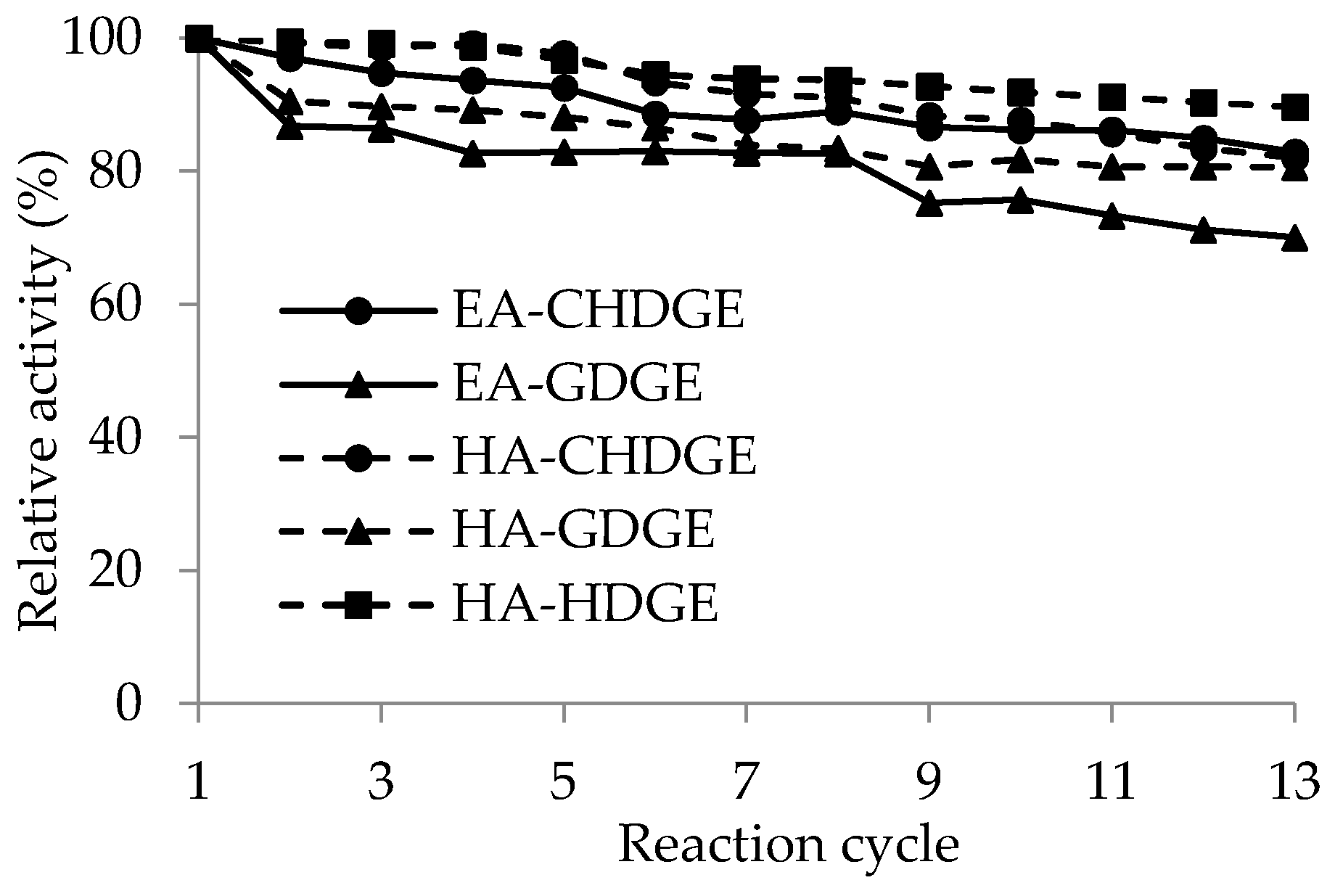
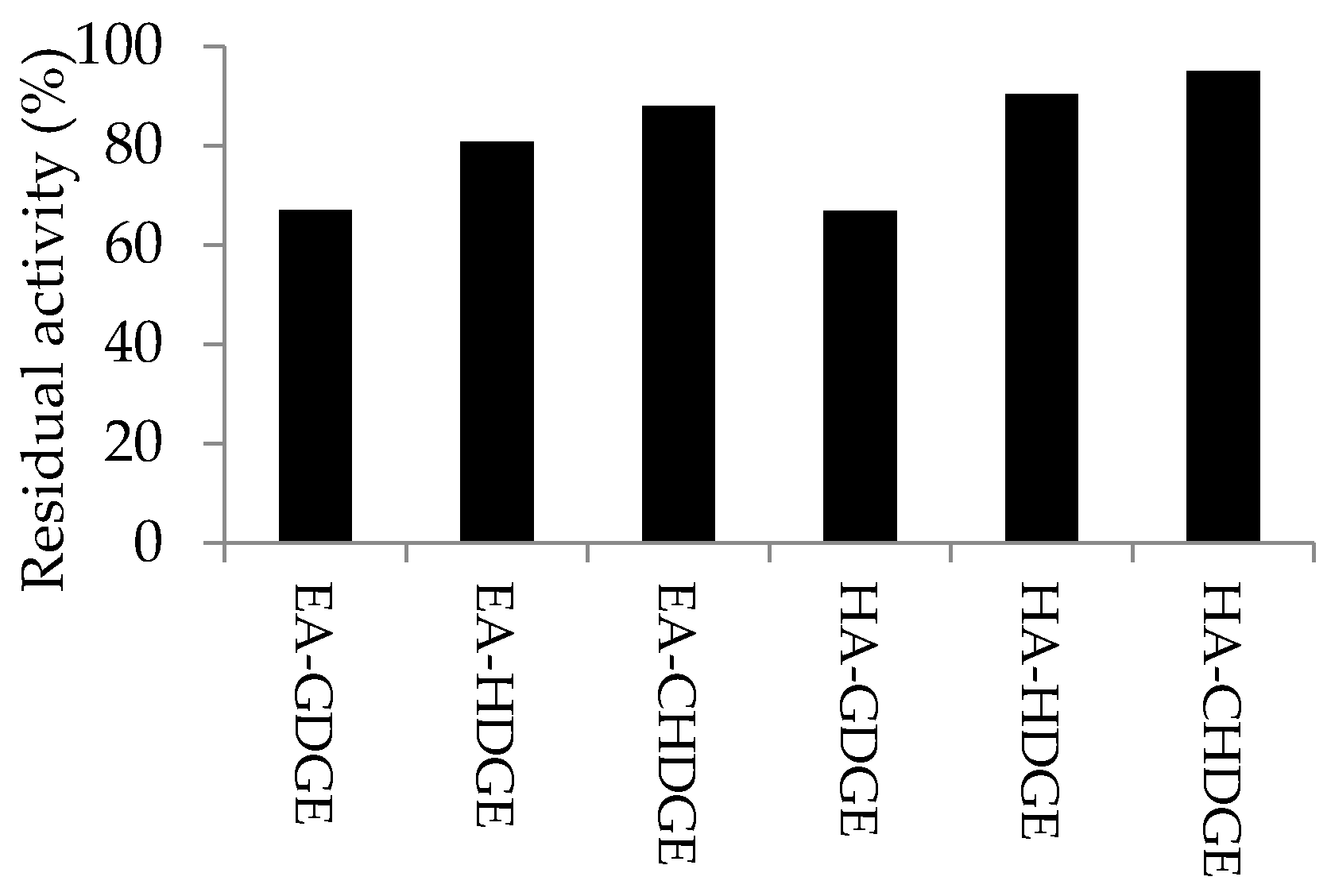
 ) or hexylamine (
) or hexylamine (  ) resin activated by CHDGE (●), GDGE (▲) or HDGE (■), compared to EP CaLB (
) resin activated by CHDGE (●), GDGE (▲) or HDGE (■), compared to EP CaLB (  in
in  ). (a) Effect of the substrate concentration on specific reaction rate (rflow); (b) Effect of temperature on specific reaction rate (rflow); (c) Effect of temperature on enantiomeric excess (ee); (d) Effect of temperature on enantiomeric ratio (E).
). (a) Effect of the substrate concentration on specific reaction rate (rflow); (b) Effect of temperature on specific reaction rate (rflow); (c) Effect of temperature on enantiomeric excess (ee); (d) Effect of temperature on enantiomeric ratio (E).
 ) or hexylamine (
) or hexylamine (  ) resin activated by CHDGE (●), GDGE (▲) or HDGE (■), compared to EP CaLB (
) resin activated by CHDGE (●), GDGE (▲) or HDGE (■), compared to EP CaLB (  in
in  ). (a) Effect of the substrate concentration on specific reaction rate (rflow); (b) Effect of temperature on specific reaction rate (rflow); (c) Effect of temperature on enantiomeric excess (ee); (d) Effect of temperature on enantiomeric ratio (E).
). (a) Effect of the substrate concentration on specific reaction rate (rflow); (b) Effect of temperature on specific reaction rate (rflow); (c) Effect of temperature on enantiomeric excess (ee); (d) Effect of temperature on enantiomeric ratio (E).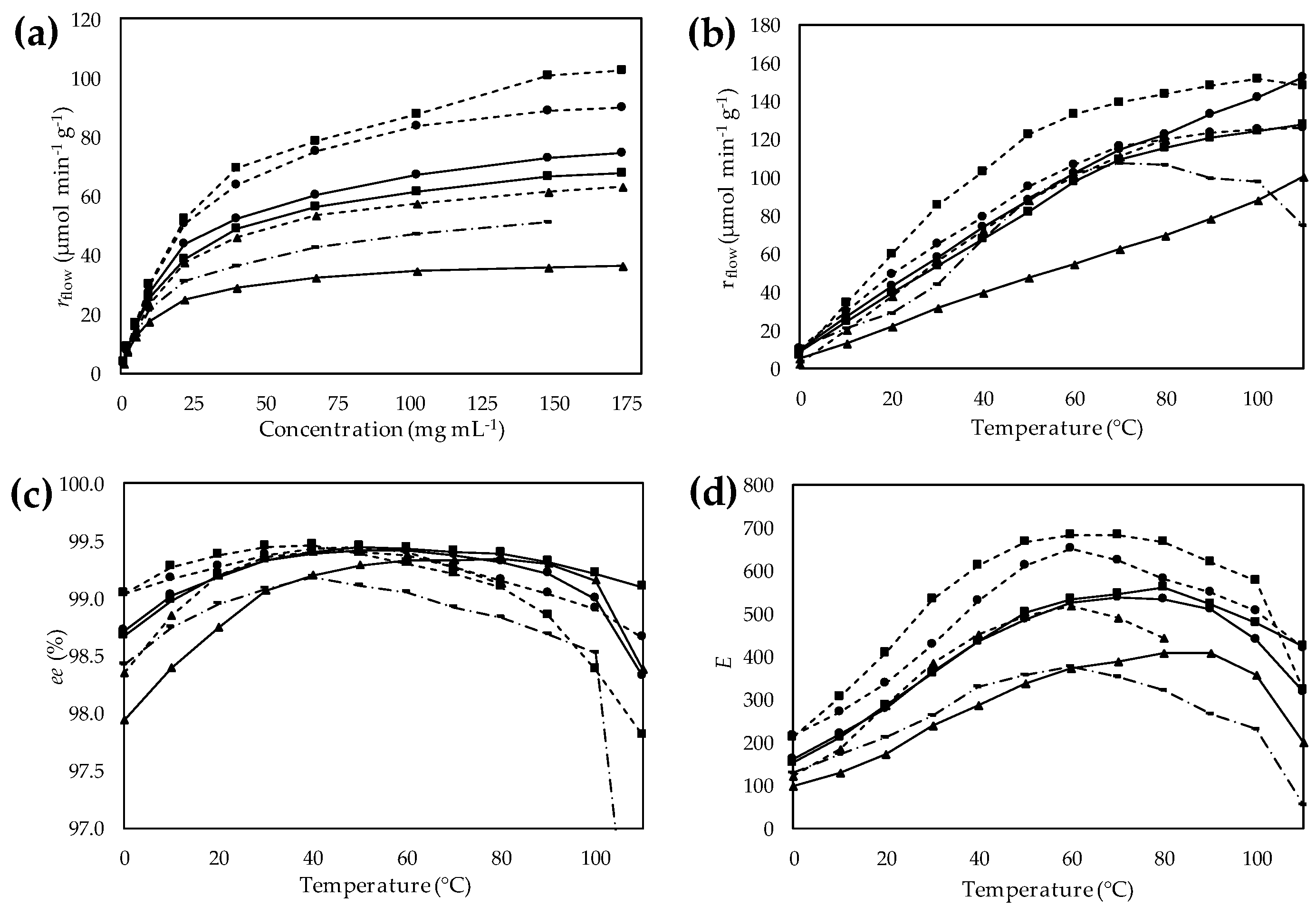
| Polymer Resin | Resin Name (Abbreviation) | Pore Size (nm) | Particle Size (µm) | Ion Exchange Capacity, Wet (µmol·g−1) | Water Retention (%) |
|---|---|---|---|---|---|
 | ReliZyme™ EA 403 (EA) | 40–60 | 100–300 | 500 | 60–70 |
 | ReliZyme™ HA 403 (HA) | 40–60 | 100–300 | 600 | 60–70 |
| Binding Function | Wash Resistance a (%) | Ub (µmol·min−1·g−1) | c (%) | ee(R)-2 (%) | E b |
|---|---|---|---|---|---|
| EP c | d | 39.6 | 28.9 | 99.1 | >200 |
| EA e | 7 | 0.4 | 0.3 | f | f |
| EA-GA | 39 | 10.2 | 7.4 | 98.9 | >200 |
| EA-GDGE | 53 | 23.7 | 17.4 | 99.4 | »200 |
| EA-BDGE | 22 | 8.5 | 6.2 | 98.2 | >200 |
| EA-HDGE | 58 | 27.5 | 20.2 | 99.4 | >200 |
| EA-CHDGE | 81 | 39.6 | 28.9 | 99.5 | »200 |
| EA-PEDGE | 7 | 2.2 | 1.6 | f | f |
| EA-NDGE | 30 | 12.0 | 8.8 | 98.6 | >200 |
| HA g | 11 | 0.2 | 0.2 | f | f |
| HA-GA | 51 | 11.9 | 8.7 | 98.9 | >200 |
| HA-GDGE | 64 | 23.8 | 17.4 | 99.6 | »200 |
| HA-BDGE | 51 | 24.5 | 17.9 | 99.2 | »200 |
| HA-HDGE | 82 | 44.2 | 32.4 | 99.5 | »200 |
| HA-CHDGE | 92 | 47.6 | 34.8 | 99.4 | »200 |
| HA-PEDGE | 17 | 6.3 | 4.6 | f | f |
| HA-NDGE | 49 | 20.2 | 14.7 | 99.1 | >200 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abaházi, E.; Lestál, D.; Boros, Z.; Poppe, L. Tailoring the Spacer Arm for Covalent Immobilization of Candida antarctica Lipase B—Thermal Stabilization by Bisepoxide-Activated Aminoalkyl Resins in Continuous-Flow Reactors. Molecules 2016, 21, 767. https://doi.org/10.3390/molecules21060767
Abaházi E, Lestál D, Boros Z, Poppe L. Tailoring the Spacer Arm for Covalent Immobilization of Candida antarctica Lipase B—Thermal Stabilization by Bisepoxide-Activated Aminoalkyl Resins in Continuous-Flow Reactors. Molecules. 2016; 21(6):767. https://doi.org/10.3390/molecules21060767
Chicago/Turabian StyleAbaházi, Emese, Dávid Lestál, Zoltán Boros, and László Poppe. 2016. "Tailoring the Spacer Arm for Covalent Immobilization of Candida antarctica Lipase B—Thermal Stabilization by Bisepoxide-Activated Aminoalkyl Resins in Continuous-Flow Reactors" Molecules 21, no. 6: 767. https://doi.org/10.3390/molecules21060767
APA StyleAbaházi, E., Lestál, D., Boros, Z., & Poppe, L. (2016). Tailoring the Spacer Arm for Covalent Immobilization of Candida antarctica Lipase B—Thermal Stabilization by Bisepoxide-Activated Aminoalkyl Resins in Continuous-Flow Reactors. Molecules, 21(6), 767. https://doi.org/10.3390/molecules21060767







