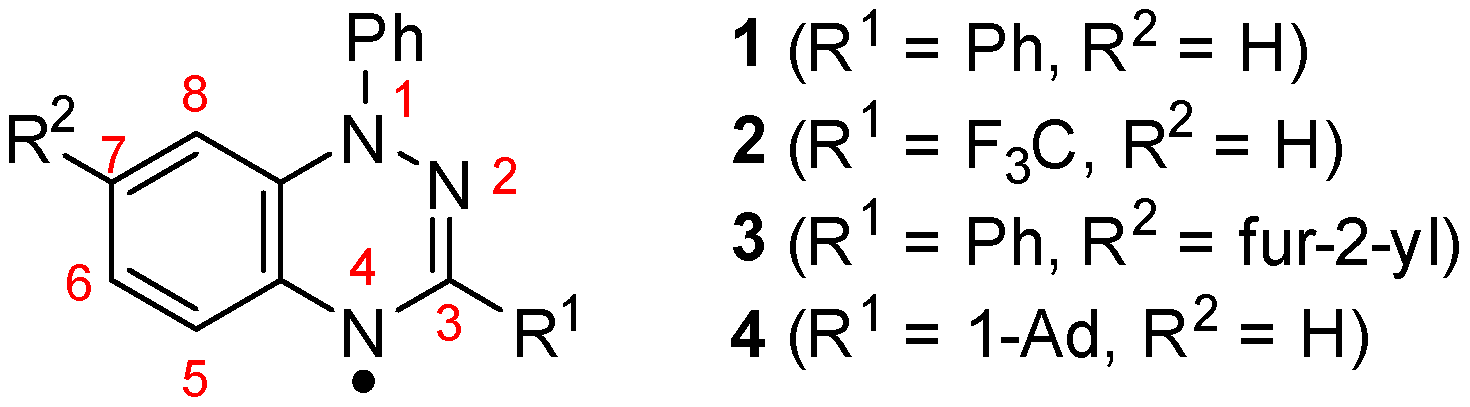Abstract
3-Adamantyl-1-phenyl-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl (4) crystallizes as chains of radicals where the spin bearing benzotriazinyl moieties are isolated from each other. Magnetic susceptibility studies in the 5–300 K temperature region indicate that radical 4 demonstrates typical paramagnetic behavior stemming from non-interacting S = ½ spins.
Keywords:
1,2,4-benzotriazinyls; hydrazyls; adamantyl; paramagnet; organic radicals; crystal packing 1. Introduction
1,4-Dihydrobenzo[e][1,2,4]triazin-4-yls a sub-class of the hydrazyl family of radicals has attracted considerable attention owing to their robust thermal, moisture and air stability. The first 1,2,4-benzotriazinyl radical was reported by Blatter in 1968 (Blatter radical 1, Chart 1) [1], and, aside from electrochemical studies by Neugebauer [2,3,4,5], this stable radical remained relatively obscure. Nevertheless, the recent development of improved syntheses of 1,2,4-benzotriazinyls [6,7,8,9,10] have led to numerous new derivatives, some of which display a range of low dimensional magnetic properties [11,12,13,14,15,16,17,18,19,20,21,22], while others have been used as radical initiators in polymer chemistry [23,24,25], and as sensors of picric acid [18].
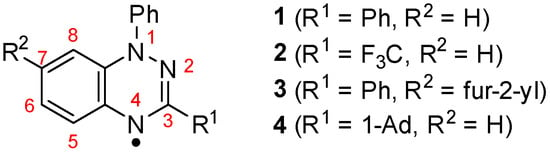
Chart 1.
Structures of 1,2,4-benzotriazin-4-yls 1–4 and IUPAC numbering of the ring system.
Solid-state σ or π dimerization is suppressed in the majority of these radicals, nevertheless, for two derivatives radicals within the π-stacked columns associate leading to dimers with singlet ground states: (a) 1-phenyl-3-trifluoromethyl-1,4-dihydrobenzo[e]-[1,2,4]triazin-4-yl (2) demonstrates an abrupt fully reversible spin transition at 58(2) K between a diamagnetic low temperature phase and a paramagnetic high temperature phase [16] and (b) 7-(fur-2-yl)-1,3-diphenyl-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl (3) has a singlet ground state below 100 K and a thermally accessible triplet state [13].
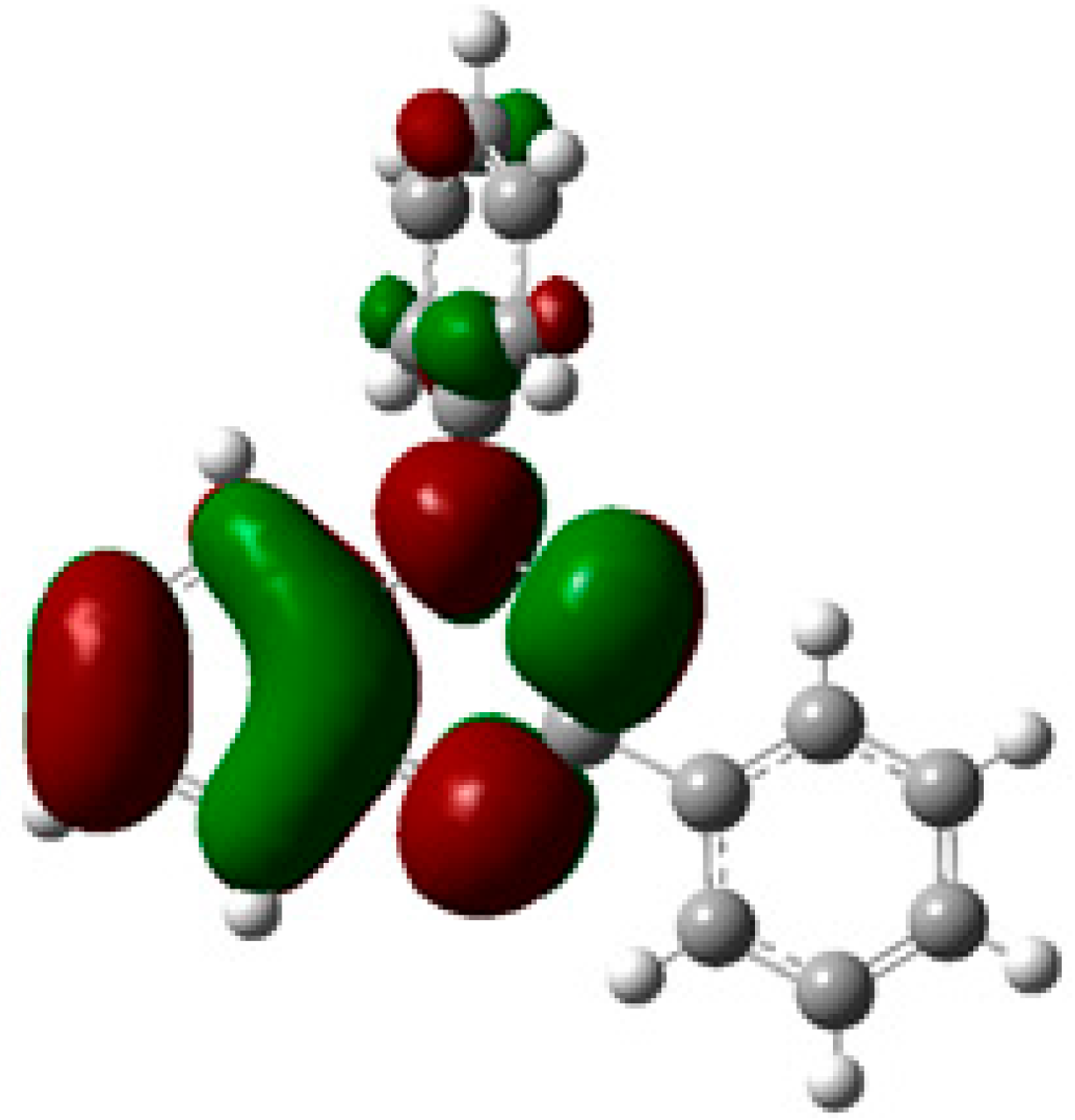
The extensive delocalization of the singly occupied molecular orbital (SOMO) orbital density over the benzo-fused hydrazonyl and the N1-Ph (Figure 1), indicates that positive orbital overlap and π-stacking, can be achieved in a variety of different association modes, and a number of different solid-state packing motifs have been reported [11,12,13,14,15,16,17,18,19,20,21,22]. In the majority of crystals structures reported to date, 1,2,4-benzotriazinyls π stack to form 1-D columns. The propensity of 1,2,4-benzotriazinyls to form columns could potentially be overcome by strategic introduction of bulky substituents in the periphery. To test this hypothesis we prepared a 1,2,4-benzotriazinyl substituted with an adamantyl group at C-3. Herein, we report the crystal structure and magnetic properties of the 3-adamantyl-1-phenyl-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl (4).
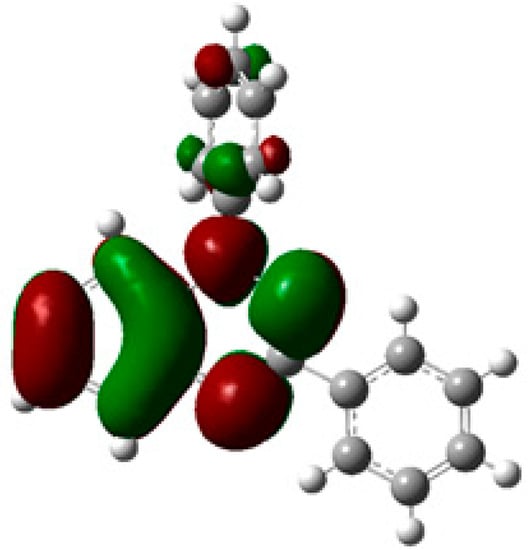
Figure 1.
SOMO of Blatter radical 1 calculated at the UB3LYP/6-311+G(d,p).
2. Results and Discussion
2.1. Synthesis, Electrochemical and EPR Data
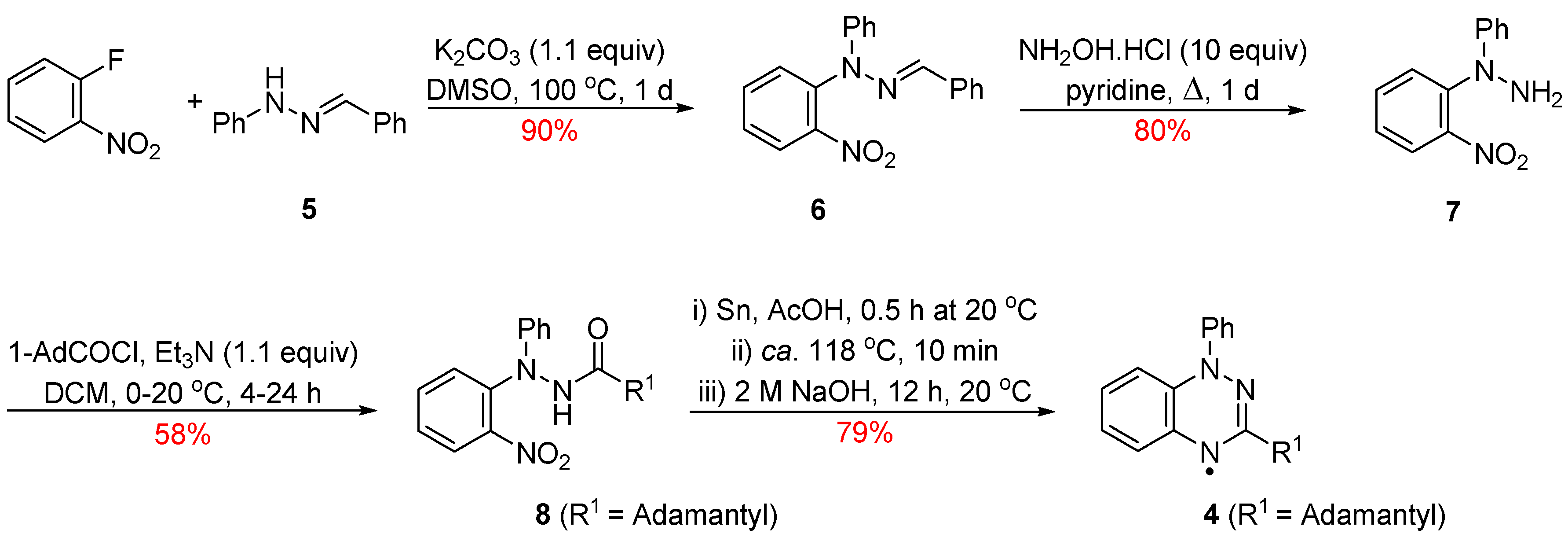
Radical 4 was previously prepared via a multistep route (Scheme 1) starting from the readily available 1-benzylidene-2-phenylhydrazine (5) [10]. Reaction of the benzylidene 5 with 1-fluoro-2-nitrobenzene and K2CO3 in DMSO at ca. 100 °C for 1 day gave 2-benzylidene-1-(2-nitrophenyl)-1-phenylhydrazine (6) which upon treatment with excess NH2OH·HCl in pyridine and heating to ca. 80 °C for 1 day released 1-(2-nitrophenyl)-1-phenylhydrazine (7) in 80% yield. Acetylation and subsequent mild reduction of the nitro group followed by an acid-mediated cyclodehydration gave the fused triazine which upon alkali treatment afforded the desired adamantly-substituted radical 4.
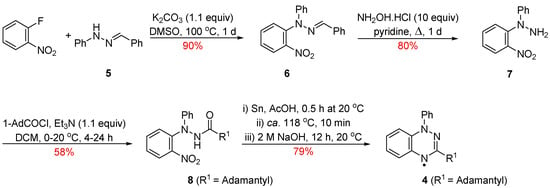
Scheme 1.
Synthesis of the 3-adamantyl-1,2,4-benzotriazinyl 4.
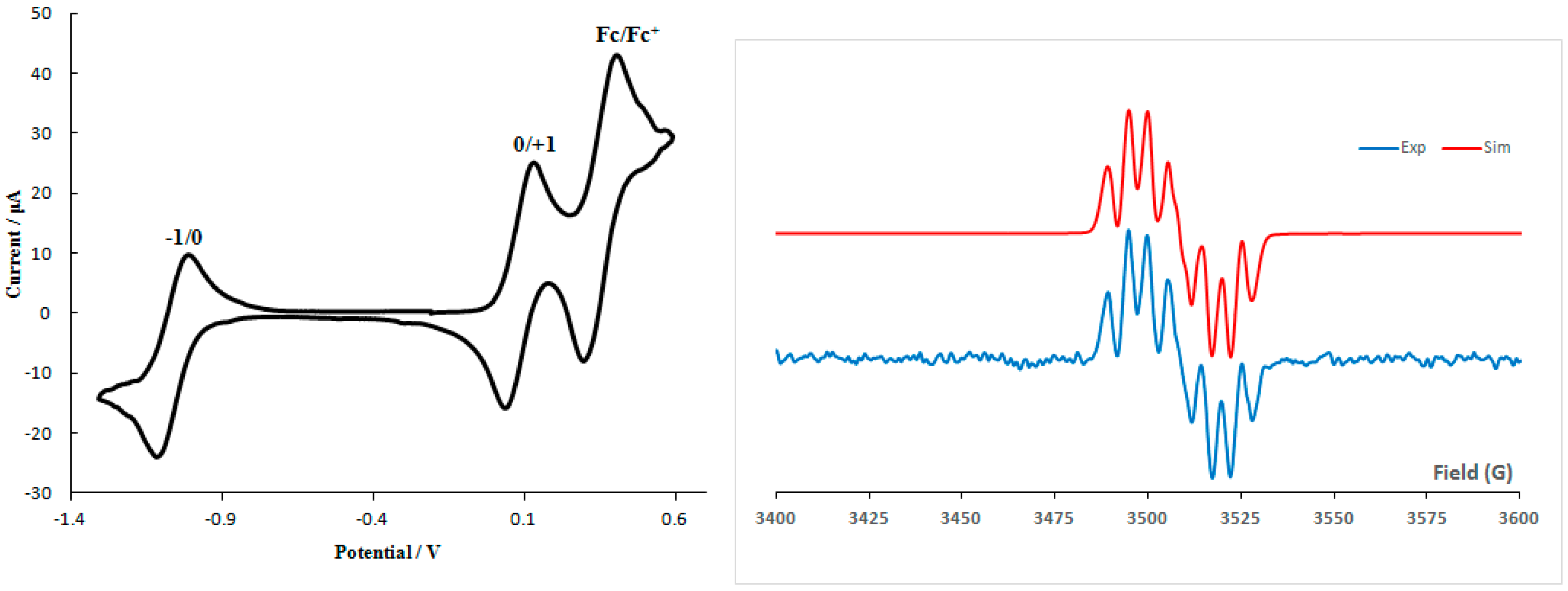
The redox behavior of radical 4 is typical of 1,2,4-benzotriazinyls exhibiting two fully reversible waves corresponding to the −1/0 and 0/+1 processes (Figure 2, left) [11,12,13,14,15,16,17,18,19,20,21,22]. Worthy of note is that the oxidation potential of E1/20/+1 = 0.08 V vs Fc/Fc+ couple makes radical 4 the best 1,2,4-benzotriazinyl donor reported to date. The solution EPR spectrum of radical 4 (Figure 2, right) exhibits the typical 1,2,4-benzotriazinyl seven-line multiplets consistent with coupling of the unpaired electron with the three similar but slightly nonequivalent 14N nuclei. The hyperfine coupling constants (hfcc), determined by the simulation of the EPR spectrum, are aN(1) = 7.51, aN(2) = 4.94 and aN(4) = 5.13 G with g = 2.0040.
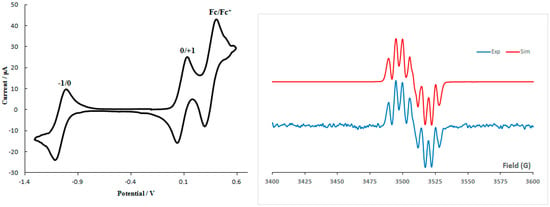
Figure 2.
Cyclic voltammetry (left) and solution EPR spectrum (right) of radical 4.
2.2. X-ray Studies
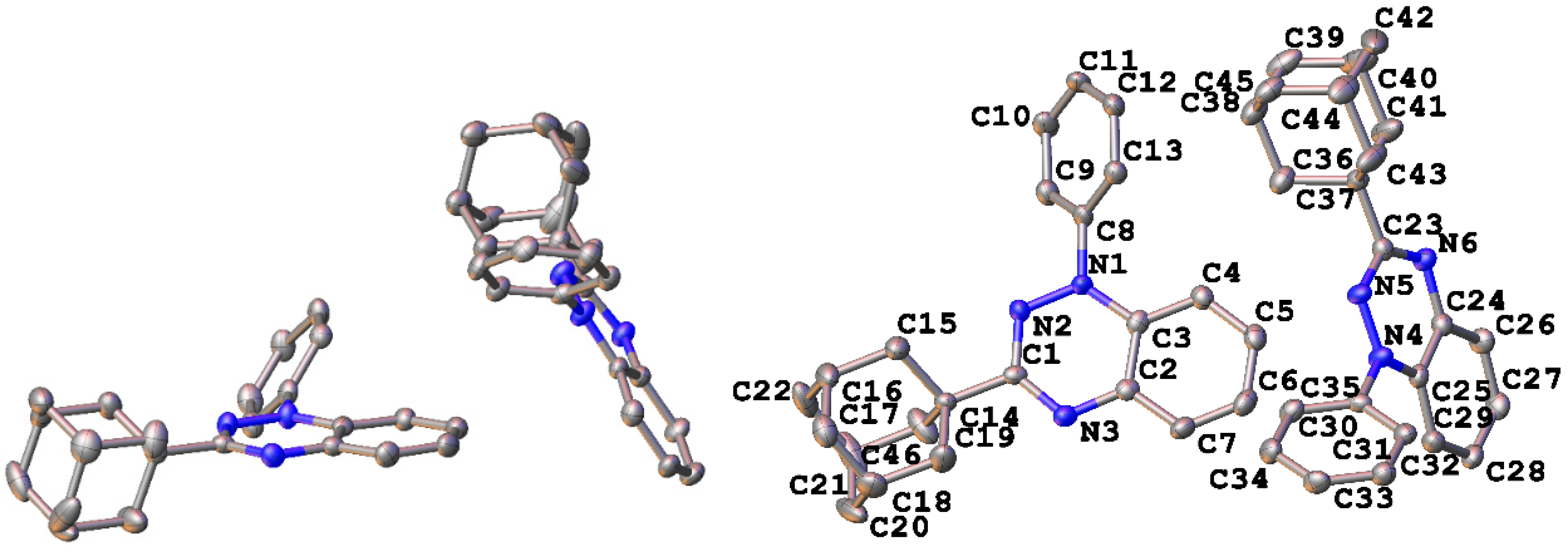
Suitable single crystals of radical 4 for X-ray diffraction studies were obtained by slow cooling of a concentrated c-hexane solution. Radical 4 adopted the orthorhombic space group Pna21 with two molecules in the asymmetric unit. These two molecules are oriented in a dihedral angle of 56.9° with respect to each other (Figure 3). The intramolecular geometry of radical 4 is typical of other 1,2,4-benzotriazinyls [11,12,13,14,15,16,17,18,19,20,21,22].
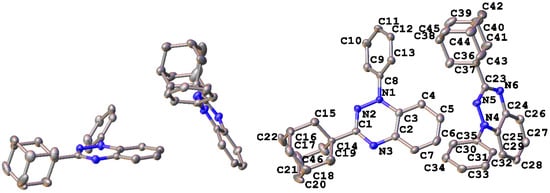
Figure 3.
Asymmetric unit (left) and crystallographic atom numbering for 3-adamantyl-1,2,4-benzotriazinyl 4 (right). Hydrogen atoms omitted for clarity.
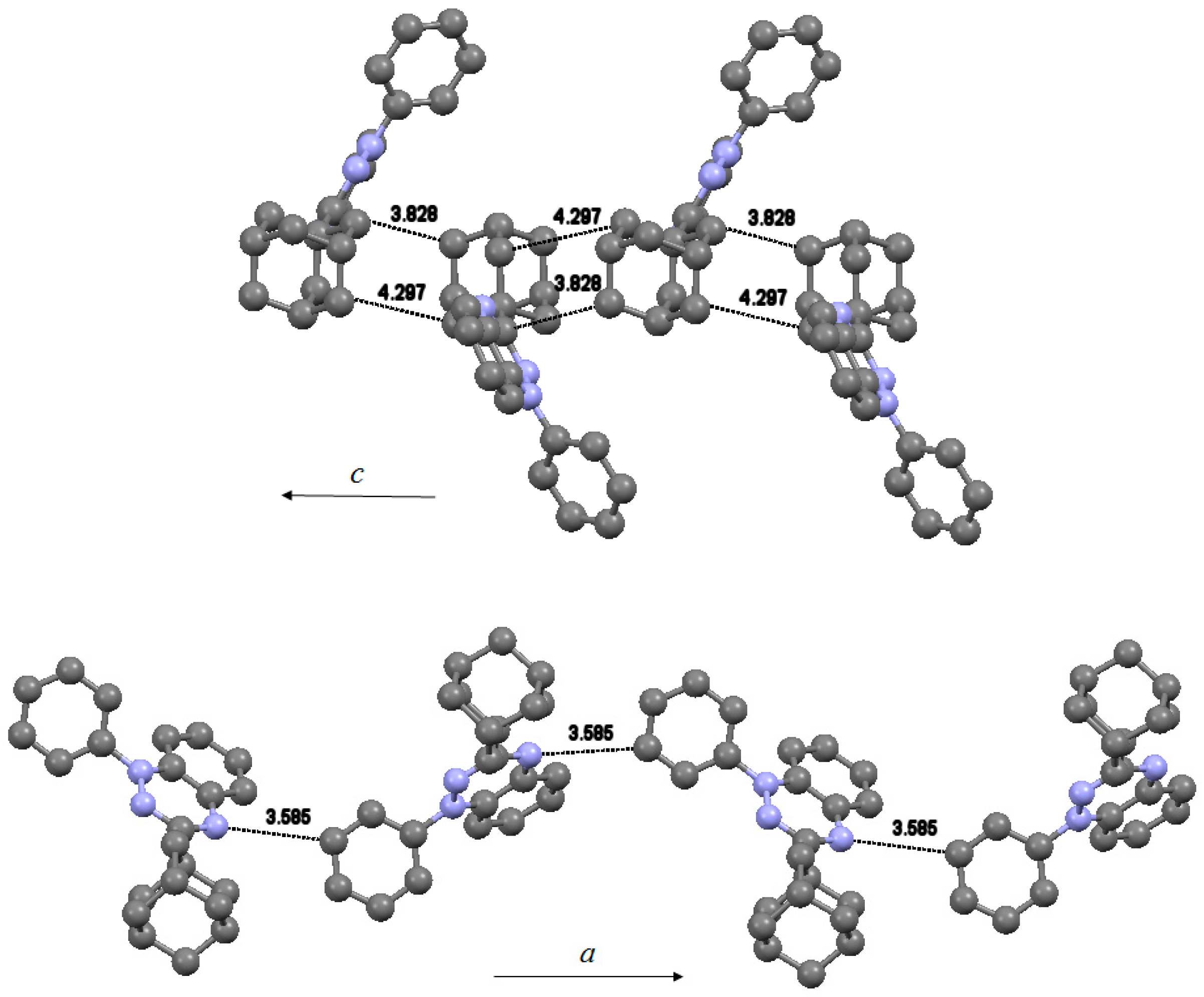
Each molecule in the asymmetric unit forms a linear chain of radicals at regular distances. Radicals in the chain running parallel to c-axis (Figure 4, top) have two close contacts, one between neighboring adamantyls [dC46…C19 = 4.297(5)°] and one between adamantyl and benzotriazinyl [dC20…C1 = 3.828(5)°]. Radicals within this chain are related by a 2-fold screw axis with direction (0, 0, 1) at 0,0,z and screw component (0, 0, ½). Radicals in the second chain running parallel to a-axis (Figure 4, bottom) are connected via a weak hydrogen contact between neighboring benzotriazinyls [dN6…C34 = 3.585(4)°]. This contact may propagate an antiferromagnetic interaction between the positive spin density on N6 and the negative spin density on C34. Carbon C34 has minimal orbital density in the SOMO, however, through spin polarization, it possesses significant negative spin density. The radical chains are related by two glide planes, one perpendicular to (0, 1, 0) with glide component (½, 0, 0) and one perpendicular to (1, 0, 0) with glide component (0, ½, ½). No formation of columns by π-stacked radicals was observed. The spin bearing benzotriazinyl moieties do not interact with each other. The related 3-(tert-butyl)-1-phenyl-1,4-dihydro-1,2,4-benzotriazin-4-yl was shown to crystallize in 1-D columns without π-π interactions between the benzotriazinyl moieties [5,20]. This radical demonstrated typical paramagnetic behavior as it followed the Curie-Weiss law with θ = −0.3 K [5].
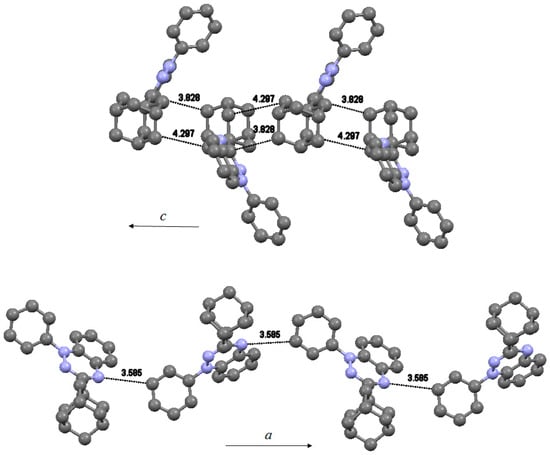
Figure 4.
Solid-state packing of radical 4 demonstrating the formation of chains along c-axis (top) and along a-axis (bottom).
2.3. Magnetic Susceptibility Studies
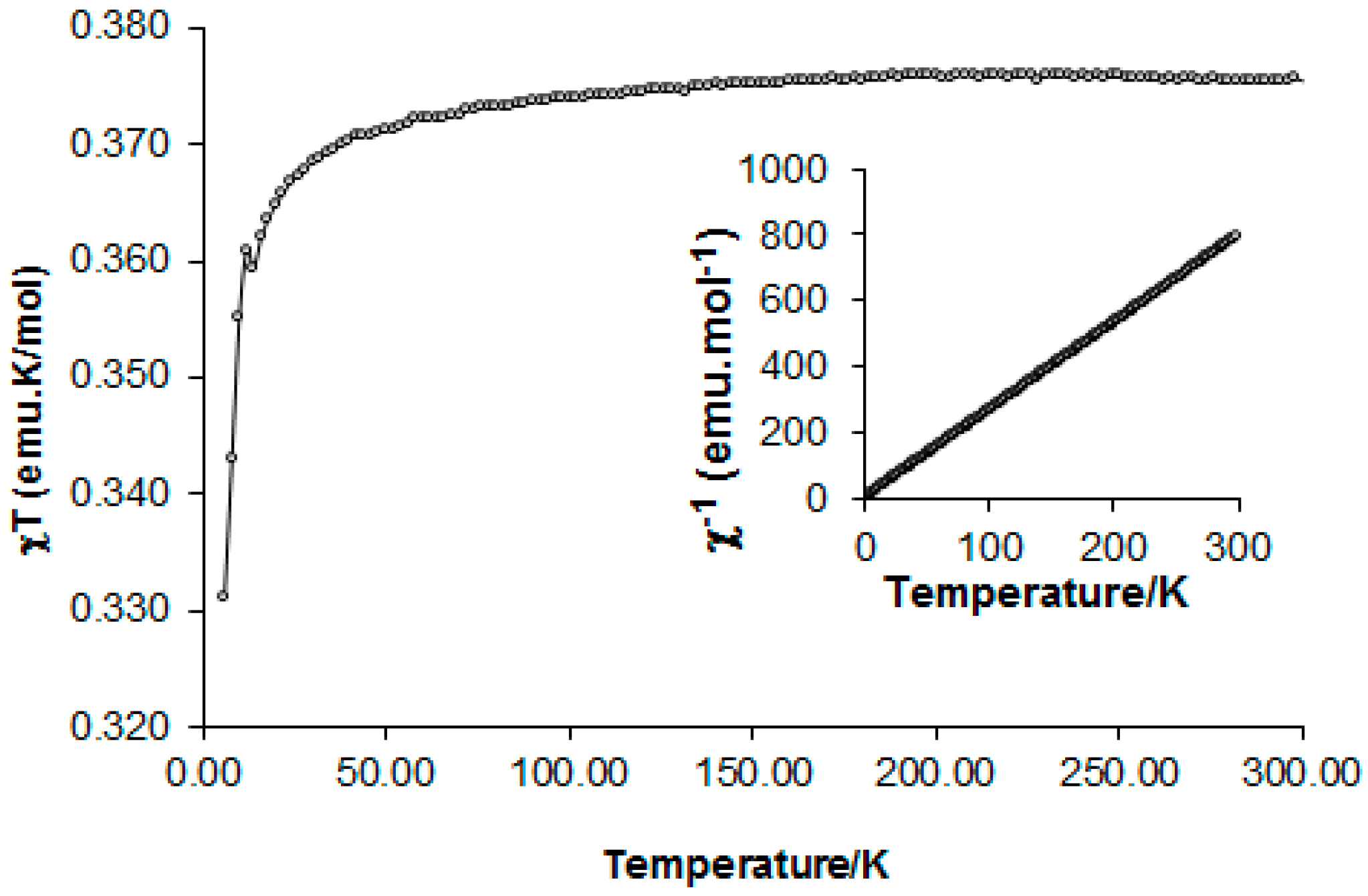
Variable-temperature magnetic-susceptibility measurements on radical 4 were obtained using a SQUID magnetometer in the temperature region 5–300 K in an applied field of 0.4 T. Data were collected in both warming and cooling modes with no significant differences in sample susceptibility. The temperature dependence of χT is shown in Figure 5.
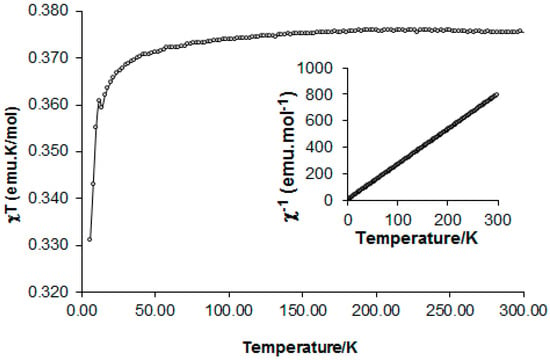
Figure 5.
Temperature dependence of χT upon heating from 5 to 300 K. Inset: Curie-Weiss behavior, C = 0.377 emu K∙mol−1 and θ = −0.61 K.
The inverse of molar susceptibility (1/χ) followed the Curie-Weiss law (Figure 5, inset) with C = 0.377 emu K∙mol−1 and θ = −0.61 K. On cooling from 300 K down to ca. 50 K, χT remained stable with a value of 0.375 emu K∙mol−1 expected for an S = ½ paramagnet. Below 50 K, χT gradually decreased as antiferromagnetic interactions along the a-axis chain (weak hydrogen dN6…C34 contacts) became dominant.
3. Experimental Sections
Synthetic procedure: The synthesis of radical 4 was published previously [10].
Instrumental analyses: Cyclic voltammetry (CV) measurements were performed on a Princeton Applied Research Potentiostat/Galvanostat 263A apparatus (Oak Ridge, TN, USA). The concentration of the benzotriazinyl radical 4 used was 1 mM in CH2Cl2. A 0.1 M CH2Cl2 solution of tetra-butylammonium tetrafluoroborate (n-Bu4BF4) was used as electrolyte. The electrolyte was dried for four days in the vacuum oven at 100 °C prior to the use. The reference electrode was Ag/AgCl and the scan rate was 50 mV/s. Ferrocene was used as an internal reference; the E1/2(ox) of ferrocene in this system was 0.352 V [26]. EPR spectra were recorded on a Bruker EMXplus X-band EPR spectrometer (Bruker, Billerica, MA, USA) at room temperature in dilute solution of CH2Cl2. For the EPR spectrum, the microwave power was in the region 5–70 mW with modulation frequencies of 50 or 100 kHz and modulation amplitudes of 0.5–1.0 Gpp. Simulations of the solution spectrum was made using Winsim software [27]. X-ray data of 4 (CCDC 1474192) [28] were collected on an Oxford-Diffraction Supernova diffractometer, equipped with a CCD area detector utilizing Mo-Kα radiation (λ = 0.71073 Å). A suitable crystal was attached to glass fibers using paratone-N oil and transferred to a goniostat where they were cooled for data collection. Unit cell dimensions were determined and refined by using 4562 (3.85 ≤ θ ≤ 27.68°) reflections. Empirical absorption corrections (multi-scan based on symmetry-related measurements) were applied using CrysAlis RED software [29]. The structures were solved by direct method and refined on F2 using full-matrix least squares using SHELXL97 [30,31]. Software packages used: CrysAlis CCD [29] for data collection, CrysAlis RED [29] for cell refinement and data reduction, WINGX for geometric calculations [32] and Mercury 3.1 (CCDC, Cambridge, UK) [33]. The non-H atoms were treated anisotropically. The hydrogen atoms were placed in calculated, ideal positions and refined as riding on their respective carbon atoms. Magnetic properties were studied by using a Quantum Design SQUID MPMS2 field-shielded magnetometer (Quantum Design Inc., San Diego, CA, USA). The DC (direct current) magnetic moment was measured for 43 mg sample of radical 4, placed in gelatin capsules held by polyethylene straw. The magnetic susceptibilities were measured in the temperature range of 5–300 K in an applied field of 0.4 T. Data were collected in both warming and cooling modes with no significant differences in sample susceptibility.
Crystal refinement data (4): C23H24N3, M = 342.45, Orthorhombic, space group Pna21, a = 18.5886(10) Å, b = 19.3115(9) Å, c = 9.9148(6) Å, α = 90°, β = 90°, γ = 90°, V = 35.59(2) Å3, Z = 8, T = 100(2) K, ρcalcd = 1.278 g·cm−3, 2θmax = 25. Refinement of 470 parameters on 5357 independent reflections out of 12978 measured reflections (Rint = 0.0438) led to R1 = 0.04761 [I > 2σ(I)], wR2 = 0.1154 (all data), and S = 1.050 with the largest difference peak and hole of 0.227 and −0.176 × 10−3, respectively.
4. Conclusions
Introduction of the bulky substituent adamantyl to the C-3 position of the 1,2,4-benzotriazinyl framework successfully inhibited the formation of π-stacked radical columns, but instead of promoting higher dimensional frameworks, only isolated radicals were obtained. This led to non-interacting S = ½ spins and a typical paramagnetic behavior. Our results indicate that there is a fine balance to be reached between steric perturbation and π-stacking interactions if materials with interesting magnetic and transport properties are to be developed.
Acknowledgments
The authors thank the University of Cyprus (medium-size grants), the Cyprus Research Promotion Foundation (grants no. ΥΓΕΙΑ/ΒΙΟΣ/0308(ΒΙΕ)/13, ΝΕΚΥΠ/0308/02 and ΑΝΑΒΑΘΜΙΣΗ/0308/32) and the following organizations in Cyprus for generous donations of chemicals and glassware: the State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd. and Biotronics Ltd. Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility in the University of Cyprus.
Author Contributions
Christos P. Constantinides and Panayiotis A. Koutentis have contributed to the conception, drafting and critical revision of the submitted manuscript. Andrey A. Berezin and Georgia A. Zissimou have contributed to the synthesis of the radical and collection of data. Maria Manoli has acquired the X-ray diffraction data and solved the structure. Gregory M. Leitus has acquired the magnetometry data.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Blatter, H.M.; Lukaszewshki, H. A new stable free radical. Tetrahedron Lett. 1968, 9, 2701–2705. [Google Scholar] [CrossRef]
- Neugebauer, F.A.; Umminger, I. Über 1,4-Dihydro-1,2,4-benzotriazinyl-Radikale. Chem. Ber. 1980, 113, 1205–1225. [Google Scholar] [CrossRef]
- Neugebauer, F.A.; Umminger, I. 1,4-Dihydro-1,2,4-benzotriazin-Radikalkationen. Chem. Ber. 1981, 114, 2423–2430. [Google Scholar] [CrossRef]
- Neugebauer, F.A.; Rimmler, G. ENDOR and Triple Resonance Studies of 1,4-Dihydro-1,2,4-benzotriazinyl Radicals and 1,4-Dihydro-1,2,4-benzotriazine Radical Cations. Magn. Reson. Chem. 1988, 26, 595–600. [Google Scholar] [CrossRef]
- Mukai, K.; Inoue, K.; Achiwa, N.; Jamali, J.B.; Krieger, C.; Neugebauer, F.A. Magnetic properties of 1,4-dihydro-1,2,4-benzotriazin-4-yl radicals. Chem. Phys. Lett. 1994, 224, 569–575. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Lo Re, D. Catalytic Oxidation of N-Phenylamidrazones to 1,3-Diphenyl-1,4-dihydro-1,2,4-benzotriazin-4-yls: An Improved Synthesis of Blatter’s Radical. Synthesis 2010, 2075–2079. [Google Scholar] [CrossRef]
- Constantinides, C.P.; Koutentis, P.A.; Loizou, G. Synthesis of 7-aryl/heteraryl-1,3-diphenyl-1,2,4-benzotriazinyls via palladium catalyzed Stille and Suzuki-Miyaura reactions. Org. Biomol. Chem. 2011, 9, 3122–3125. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Constantinides, C.P.; Drouza, C.; Manoli, M.; Koutentis, P.A. From Blatter Radical to 7-Substituted 1,3-Diphenyl-1,4-dihydrothiazolo[5’,4’:4,5]benzo[1,2-e][1,2,4]triazin-4-yls: Toward Multifunctional Materials. Org. Lett. 2012, 14, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Constantinides, C.P.; Mirallai, S.I.; Manoli, M.; Cao, L.L.; Rawson, J.M.; Koutentis, P.A. Synthesis and properties of imidazolo-fused benzotriazinyl radicals. Org. Biomol. Chem. 2013, 11, 6780–6795. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Zissimou, G.A.; Constantinides, C.P.; Beldjoudi, Y.; Rawson, J.M.; Koutentis, P.A. Route to Benzo- and Pyrido-Fused 1,2,4-Triazinyl Radicals via N’-(Het)aryl-N’-[2-nitro(het)aryl]hydrazides. J. Org. Chem. 2014, 79, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Koutentis, P.A.; Krassos, H.; Rawson, J.M.; Tasiopoulos, A.J. Characterization and Magnetic Properties of a “Super Stable” Radical 1,3-Diphenyl-7-trifluoromethyl-1,4-dihydro-1,2,4-benzotriazin-4-yl. J. Org. Chem. 2011, 76, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Koutentis, P.A.; Rawson, J.M. Antiferromagnetic Interactions in 1D Heisenberg Linear Chains of 7-(4-Fluorophenyl) and 7-Phenyl-Substituted 1,3-Diphenyl-1,4-dihydro-1,2,4-benzotriazin-4-yl Radicals. Chem. Eur. J. 2012, 18, 15433–15438. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Carter, E.; Murphy, D.M.; Manoli, M.; Leitus, G.M.; Bendikov, M.; Rawson, J.M.; Koutentis, P.A. Spin-triplet excitons in 1,3-diphenyl-7-(fur-2-yl)-1,4-dihydro-1,2,4-benzotriazin-4-yl. Chem. Commun. 2013, 49, 8662–8664. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Berezin, A.A.; Manoli, M.; Leitus, G.M.; Bendikov, M.; Rawson, J.M.; Koutentis, P.A. Effective exchange coupling in alternating-chains of a π-extended 1,2,4-benzotriazin-4-yl. New J. Chem. 2014, 38, 949–954. [Google Scholar] [CrossRef]
- Constantinides, C.P.; Berezin, A.A.; Manoli, M.; Leitus, G.M.; Zissimou, G.Z.; Bendikov, M.; Rawson, J.M.; Koutentis, P.A. Structural, Magnetic, and Computational Correlations of Some Imidazolo-Fused 1,2,4-Benzotriazinyl Radicals. Chem. Eur. J. 2014, 20, 5388–5396. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.P.; Berezin, A.A.; Zissimou, G.Z.; Manoli, M.; Leitus, G.M.; Bendikov, M.; Probert, M.R.; Rawson, J.M.; Koutentis, P.A. A Magnetostructural Investigation of an Abrupt Spin Transition for 1-Phenyl-3-trifluoromethyl-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl. J. Am. Chem. Soc. 2014, 136, 11906–11909. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Cramen, J.; McDonald, R.; Frank, N.L. Ferromagnetic spin-delocalized electron donors for multifunctional materials: π-Conjugated benzotriazinyl radicals. Chem. Comm. 2011, 47, 3201–3203. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Miao, M.-S.; Kemei, M.C.; Seshadri, R.; Wudl, F. The Pyreno-Triazinyl Radical–Magnetic and Sensor Properties. Isr. J. Chem. 2014, 54, 774–778. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miura, Y.; Yoshioka, N. Introduction of Three Aryl Groups to Benzotriazinyl Radical by Suzuki-Miyaura Cross-coupling Reaction. Chem. Lett. 2014, 43, 1236–1238. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miura, Y.; Yoshioka, N. Synthesis and properties of the 3-tert-butyl-7-trifluoromethyl-1,4-dihydro-1-phenyl-1,2,4-benzotriazin-4-yl radical. New J. Chem. 2015, 39, 4783–4789. [Google Scholar] [CrossRef]
- Miura, Y.; Yoshioka, N. π-Stacked structure of thiadiazolo-fused benzotriazinyl radical: Crystal structure and magnetic properties. Chem. Phys. Lett. 2015, 626, 11–14. [Google Scholar] [CrossRef]
- Fumanal, M.; Vela, S.; Novoa, J.J.; Ribas-Arino, J. Towards the tailored design of benzotriazinyl-based organic radicals displaying a spin transition. Chem. Commun. 2015, 51, 15776–15779. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, M.; Berezin, A.A.; Koutentis, P.A.; Krasia-Christoforou, T. Benzotriazinyl-mediated controlled radical polymerization of styrene. Polym. Int. 2014, 63, 674–679. [Google Scholar] [CrossRef]
- Areephong, J.; Treat, N.; Kramer, J.W.; Christianson, M.D.; Hawker, C.J.; Collins, H.A. Triazine Mediated Living Radical Controlled Polymerization. WO 2015/061189 A1, 30 April 2015. [Google Scholar]
- Areephong, J.; Mattson, K.M.; Treat, N.J.; Poelma, S.O.; Kramer, J.W.; Sprafke, H.A.; Latimer, A.A.; Read de Alaniz, J.; Hawker, C.J. Triazine-Mediated Controlled Radical Polymerization: New Unimolecular Initiators. Polym. Chem. 2016, 7, 370–374. [Google Scholar] [CrossRef]
- Dietrich, M.; Heinze, J. On the Determination of Redox Potentials of Highly Reactive Aromatic Mono- and Multications. J. Am. Chem. Soc. 1990, 112, 5142–5145. [Google Scholar] [CrossRef]
- Duling, D.R. Simulation of Multiple Isotropic Spin Trap EPR Spectra. J. Magn. Reson. Ser. B 1994, 104, 105–110. [Google Scholar] [CrossRef]
- CCDC 1474192 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk.
- CrysAlis CCD and CrysAlis RED, version 1.171.32.15; Oxford Diffraction Ltd: Abingdon, Oxford, UK, 2008.
- Sheldrick, G.M. SHELXL 97-A Program for the Refinement of Crystal Structure; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta. Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the radical is available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).