Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management
Abstract
:1. Mycotoxigenic Fusarium and Fusarium-Related Diseases
2. Fusarium Mycotoxins
2.1. Trichothecenes
2.2. Zearalenone
2.3. Fumonisins
2.4. Emerging Fusarium Toxins
3. Legislation on Fusarium Toxins in Cereal
4. Factors affecting Fusarium Toxins Production
4.1. Effect of Climate Events on FHB, Maize ear Rots and Mycotoxin
4.2. Fungal Interactions in Cereals: Consequences for Fusarium Development and Mycotoxin
4.3. Stress Factors
5. Fusarium Disease and Toxins Management
5.1. Tillage and Crop Rotation
5.2. Cultivar Selection
5.3. Planting and Weed Management
5.4. Irrigation and Fertilization Regimes
5.5. Insect Management
5.6. Chemical and Biological Control
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize Ear Rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. A 2015, 32, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Placinta, C.; D’Mello, J.P.; Macdonald, A.M. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Regional economic impacts of Fusarium Head Blight in wheat and barley. Appl. Econ. Perspect. Policy 2004, 26, 332–347. [Google Scholar] [CrossRef]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Perrone, G.; Mulè, G. Biodiversity of complexes of mycotoxigenic fungal species associated with Fusarium ear rot of maize and Aspergillus rot of grape. Int. J. Food Microbiol. 2007, 119, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; De Haas, B.H.; Kastelein, P.; Burgers, S.; Waalwijk, C. Population dynamics of Fusarium spp. and Microdochium nivale in crops and crop residues of winter wheat. Phytopathology 2007, 97, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Backhouse, D. Global distribution of Fusarium graminearum, F. asiaticum and F. boothii from wheat in relation to climate. Eur. J. Plant Pathol. 2014, 139, 161–173. [Google Scholar] [CrossRef]
- Yli-Mattila, T. Ecology and evolution of toxigenic Fusarium species in cereals in Northern Europe and Asia. J. Plant Pathol. 2010, 92, 7–18. [Google Scholar]
- Aoki, T.; Ward, T.J.; Kistler, H.C.; O’donnell, K. Systematics, phylogeny and trichothecene mycotoxin potential of Fusarium head blight cereal pathogens. JSM Mycotoxins 2012, 62, 91–102. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Osborne, L.E.; Stein, J.M. Epidemiology of Fusarium head blight on small-grain cereals. Int. J. Food Microbiol. 2007, 119, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Ward, T.J.; Ballois, N.; Iancu, G.; Ioos, R. Diversity of the Fusarium graminearum species complex on French cereals. Eur. J. Plant Pathol. 2014, 138, 133–148. [Google Scholar] [CrossRef]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Puri, K.D.; Saucedo, E.S.; Zhong, S. Molecular characterization of Fusarium head blight pathogens sampled from a naturally infected disease nursery used for wheat breeding programs in China. Plant Dis. 2012, 96, 1280–1285. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Jestoi, M.; Parikka, P.; Hietaniemi, V.; Gagkaeva, T.; Sarlin, T.; Haikara, A.; Laaksonen, S.; Rizzo, A. Real-time PCR detection and quantification of Fusarium poae, F. graminearum, F. sporotrichioides and F. langsethiae in cereal grains in Finland and Russia. Arch. Phytopathol. Plant Prot. 2008, 41, 243–260. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and other selected Fusarium toxins in Swedish oats—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, S.A. Fusarium poae: A pathogen that needs more attention. J. Plant Pathol. 2009, 91, 25–36. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins ssociated with Head Blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Covarelli, L.; Stifano, S.; Beccari, G.; Raggi, L.; Lattanzio, V.M.T.; Albertini, E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonisin production, pathogenicity and genetic variability. Food Microbiol. 2012, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gatch, E.W.; Munkvold, G.P. Fungal species composition in maize stalks in relation to European corn borer injury and transgenic insect protection. Plant Dis. 2002, 86, 1156–1162. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Associations of planting date, drought stress, and insects with Fusarium ear rot and fumonisin B1 contamination in California maize. Food Addit. Contam. A 2010, 27, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.E.; Howard, R.J. Biology of maize kernel infection by Fusarium verticillioides. Mol. Plant. Microbe Interact. 2010, 23, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, E.; Ellner, F. Distribution of disease symptoms and mycotoxins in maize ears infected by Fusarium culmorum and Fusarium graminearum. Mycotoxin Res. 2015, 31, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. Factors that affect the occurrence of fumonisin. Environ. Health Perspect. 2001, 109, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Kebebe, A.Z.; Reid, L.M.; Zhu, X.; Wu, J.; Woldemariam, T.; Voloaca, C.; Xiang, K. Relationship between kernel drydown rate and resistance to gibberella ear rot in maize. Euphytica 2015, 201, 79–88. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Desjardins, A.E. Fumonisins in maize: Can we reduce their occurrence? Plant Dis. 1997, 81, 556–565. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Effects of planting date and environmental factors on Fusarium ear rot symptoms and fumonisin B1 accumulation in maize grown in six North American locations. Plant Pathol. 2012, 61, 1130–1142. [Google Scholar] [CrossRef]
- Balconi, C.; Berardo, N.; Locatelli, S.; Lanzanova, C.; Torri, A.; Redaelli, R. Evaluation of ear rot (Fusarium verticillioides) resistance and fumonisin accumulation in Italian maize inbred lines. Phytopathol. Mediterr. 2014, 53, 14–26. [Google Scholar]
- Cao, A.; Santiago, R.; Ramos, A.J.; Souto, X.C.; Aguín, O.; Malvar, R.A.; Butrón, A. Critical environmental and genotypic factors for Fusarium verticillioides infection, fungal growth and fumonisin contamination in maize grown in northwestern Spain. Int. J. Food Microbiol. 2014, 177, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fandohan, P.; Hell, K.; Marasas, W.F.O.; Wingfield, M.J. Infection of maize by Fusarium species and contamination with fumonisin in Africa. Afr. J. Biotechnol. 2003, 2, 570–579. [Google Scholar]
- Mohammadi, A.; Shams-Ghahfarokhi, M.; Nazarian-Firouzabadi, F.; Kachuei, R.; Gholami-Shabani, M.; Razzaghi-Abyaneh, M. Gibberella fujikuroi species complex isolated from maize and wheat in Iran: Distribution, molecular identification and fumonisin B1 in vitro biosynthesis. J. Sci. Food Agric. 2016, 96, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Jurjevic, Z.; Wilson, D.M.; Wilson, J.P.; Geiser, D.M.; Juba, J.H.; Mubatanhema, W.; Widstrom, N.W.; Rains, G.C. Fusarium species of the Gibberella fujikuroi complex and fumonisin contamination of pearl millet and corn in Georgia, USA. Mycopathologia 2005, 159, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, R.; dos Santos, J.; Gomes, L.B.; Silva, C.N.; Tessmann, D.J.; Ferreira, F.D.; Machinski Junior, M.; Del Ponte, E.M. Fusarium species and fumonisins associated with maize kernels produced in Rio Grande do Sul State for the 2008/09 and 2009/10 growing seasons. Braz. J. Microbiol. 2013, 44, 89–95. [Google Scholar]
- Bottalico, A. Fusarium diseases of cereals: Species complex and related mycotoxin profiles, in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar]
- Vigier, B.; Reid, L.M.; Seifert, K.A.; Stewart, D.W.; Hamilton, R.I. Distribution and prediction of Fusarium species associated with maize ear rot in Ontario. Can. J. Plant Pathol. 1997, 19, 60–65. [Google Scholar] [CrossRef]
- Iglesias, J.; Presello, D.A.; Botta, G.; Lori, G.A.; Fauguel, C.M. Aggressiveness of Fusarium section Liseola isolates causing maize ear rot in Argentina. J. Plant Pathol. 2010, 92, 205–211. [Google Scholar]
- Wit, M.; Warzecha, R.; Mirzwa-Mroz, E.; Jabionska, E.; Ochodzki, P.; Waskiewicz, A.; Wakulinski, W. Susceptibility of flint and dent maize ears to Fusarium species. Phytopathologia 2011, 60, 35–45. [Google Scholar]
- Thrane, U.; Adler, A.; Clasen, P.-E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Sewram, V.; Mshicileli, N.; Shephard, G.S.; Vismer, H.F.; Rheeder, J.P.; Lee, Y.-W.; Leslie, J.F.; Marasas, W.F.O. Production of fumonisin B and C analogues by several Fusarium species. J. Agric. Food Chem. 2005, 53, 4861–4866. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R.; et al. New tricks of an old enemy: Isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ. Microbiol. 2015, 17, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M. Emerging Fusarium mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.E.; Hernández Pezzani, C.; Pacin, A. Mycotoxins occurrence in Argentina’s maize (Zea mays L.), from 1999 to 2010. Food Control 2012, 25, 660–665. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Bemvenuti, R.H.; Zorzete, P.; de Souza Garcia, F.; Corrêa, B. Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem. 2016, 196, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Tittlemier, S.A.; Roscoe, M.; Trelka, R.; Gaba, D.; Chan, J.M.; Patrick, S.K.; Sulyok, M.; Krska, R.; McKendry, T.; Gräfenhan, T. Fusarium damage in small cereal grains from Western Canada. 2. Occurrence of Fusarium toxins and their source organisms in durum wheat harvested in 2010. J. Agric. Food Chem. 2013, 61, 5438–5448. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Nie, D.; Ediage, E.N.; Yang, X.; Wang, J.; Chen, B.; Li, S.; On, S.L.W.; De Saeger, S.; Wu, A. Cumulative health risk assessment of co-occurring mycotoxins of deoxynivalenol and its acetyl derivatives in wheat and maize: Case study, Shanghai, China. Food Chem. Toxicol. 2014, 74, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.; Wang, L.; Chang, F.; Yang, L. Occurrence of deoxynivalenol in wheat, Hebei Province, China. Food Chem. B 2016, 197, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Vahčić, N.; Perši, N.; Ševelj, D.; Markov, K.; Frece, J. Fusarium mycotoxins’ occurrence in cereals harvested from Croatian fields. Food Control 2013, 32, 49–54. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Aureli, G.; Amoriello, T.; Belocchi, A.; D’Egidio, M.G.; Fornara, M.; Melloni, S.; Quaranta, F. Preliminary survey on the co-occurrence of DON and T2+HT2 toxins in durum wheat in Italy. Cereal Res. Commun. 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Leggieri, M.C.; Bertuzzi, T.; Pietri, A.; Battilani, P. Mycotoxin occurrence in maize produced in Northern Italy over the years 2009–2011: Focus on the role of crop related factors. Phytopathol. Mediterr. 2015, 54, 212–221. [Google Scholar]
- Blesa, J.; Moltó, J.-C.; El Akhdari, S.; Mañes, J.; Zinedine, A. Simultaneous determination of Fusarium mycotoxins in wheat grain from Morocco by liquid chromatography coupled to triple quadrupole mass spectrometry. Food Control 2014, 46, 1–5. [Google Scholar] [CrossRef]
- Czembor, E.; Stępień, Ł.; Waśkiewicz, A. Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS ONE 2015, 10, e0133644. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, M.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Fredlund, E. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; De Meulenaer, B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Xu, X.; Madden, L.V.; Edwards, S.G. Modeling the effects of environmental conditions on HT2 and T2 toxin accumulation in field oat grains. Phytopathology 2014, 104, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Xu, J.; Liu, X.; Yin, X.; Shi, J. Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province, China. Food Chem. 2014, 157, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Nooh, A.; Amra, H.; Youssef, M.M.; El-Banna, A.A. Mycotoxins and toxigenic fungi occurrence in Egyptian maize. Int. J. Adv. Res. 2014, 2, 521–532. [Google Scholar]
- Zaied, C.; Zouaoui, N.; Bacha, H.; Abid, S. Natural occurrence of zearalenone in Tunisian wheat grains. Food Control 2012, 25, 773–777. [Google Scholar] [CrossRef]
- Cendoya, E.; Monge, M.P.; Palacios, S.A.; Chiacchiera, S.M.; Torres, A.M.; Farnochi, M.C.; Ramirez, M.L. Fumonisin occurrence in naturally contaminated wheat grain harvested in Argentina. Food Control 2014, 37, 56–61. [Google Scholar] [CrossRef]
- Peluque, E.; Neres, N.B.; Michelin, E.C.; Reis, T.A.; Rosim, R.E.; Oliveira, C.A.F.; Sousa, R.L.M.; Corrêa, B.; Fernandes, A.M. Fumonisin B1 in cereal mixtures marketed in Brazil. Food Addit. Contam. B 2014, 7, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Scussel, V.M.; Savi, G.D.; Costas, L.L.F.; Xavier, J.J.M.; Manfio, D.; Bittencourt, K.O.; Aguiar, K.; Stein, S.M. Fumonisins in corn (Zea mays L.) from Southern Brazil. Food Addit. Contam. B 2014, 7, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Tao, B.; Pang, M.; Liu, Y.; Dong, J. Natural occurrence of fumonisins B1 and B2 in maize from three main maize-producing provinces in China. Food Control 2015, 50, 838–842. [Google Scholar] [CrossRef]
- Li, F.; Jiang, D.; Zheng, F.; Chen, J.; Li, W. Fumonisins B1, B2 and B3 in corn products, wheat flour and corn oil marketed in Shandong province of China. Food Addit. Contam. B 2015, 8, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.; Matute, J.; Gelineau-van Waes, J.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Voss, K.A.; Riley, R.T. Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in Latin America: Guatemala as a case study. World Mycotoxin J. 2014, 8, 143–159. [Google Scholar] [CrossRef]
- Van Rensburg, B.J.; McLaren, N.W.; Flett, B.C.; Schoeman, A. Fumonisin producing Fusarium spp. and fumonisin contamination in commercial South African maize. Eur. J. Plant Pathol. 2015, 141, 491–504. [Google Scholar] [CrossRef]
- Shank, R.A.; Foroud, N.A.; Hazendonk, P.; Eudes, F.; Blackwell, B.A. Current and future experimental strategies for structural analysis of trichothecene mycotoxins—A prospectus. Toxins 2011, 3, 1518–1553. [Google Scholar] [CrossRef] [PubMed]
- Koch, P. State of the art of trichothecenes analysis. Toxicol. Lett. 2004, 153, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Yoshizawa, T.; Shier, W.T. Cytotoxicity and phytotoxicity of trichothecene mycotoxins produced by Fusarium spp. Toxicon 2013, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.C.; Clear, R.M.; O’Donnell, K.; McCormick, S.; Turkington, T.K.; Tekauz, A.; Gilbert, J.; Kistler, H.C.; Busman, M.; Ward, T.J. Diversity of Fusarium head blight populations and trichothecene toxin types reveals regional differences in pathogen composition and temporal dynamics. Fungal Genet. Biol. 2015, 82, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Clear, R.M.; Rooney, A.P.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.E.; Gilbert, J.; Geiser, D.M.; Nowicki, T.W. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol. 2008, 45, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Gale, L.R.; Harrison, S.A.; Ward, T.J.; O’Donnell, K.; Milus, E.A.; Gale, S.W.; Kistler, H.C. Nivalenol-type populations of Fusarium graminearum and F. asiaticum are prevalent on wheat in Southern Louisiana. Phytopathology 2011, 101, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to Deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J. 2004, 73, 1–42. [Google Scholar]
- Cortinovis, C.; Caloni, F.; Schreiber, N.B.; Spicer, L.J. Effects of fumonisin B1 alone and combined with deoxynivalenol or zearalenone on porcine granulosa cell proliferation and steroid production. Theriogenology 2014, 81, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, F.; Caloni, F.; Schutz, L.F.; Totty, M.L.; Spicer, L.J. Individual and combined effects of deoxynivalenol and α-zearalenol on cell proliferation and steroidogenesis of granulosa cells in cattle. Environ. Toxicol. Pharmacol. 2015, 40, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, M.; Müller, H.M.; Ernst, K.; Sondermann, S.; Liebscher, M.; Schlecker, C.; Wischer, G.; Drochner, W.; Hartung, K.; Piepho, H.-P. Occurrence and distribution of 13 trichothecene toxins in naturally contaminated maize plants in Germany. Toxins 2012, 4, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Flannery, B.M.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.; Pestka, J.J. Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem. Toxicol. 2012, 50, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA J. 2013, 11. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Puel, O.; Oswald, I.P. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2014, 89, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Haratian, M.; Sharifnabi, B.; Alizadeh, A.; Safaie, N. PCR analysis of the Tri13 gene to determine the genetic potential of Fusarium graminearum isolates from Iran to produce nivalenol and deoxynivalenol. Mycopathologia 2008, 166, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Pogoda, F.; Pallez, M.; Lazic, J.; Hoffmann, L.; Pasquali, M. Evidence for a reversible drought induced shift in the species composition of mycotoxin producing Fusarium head blight pathogens isolated from symptomatic wheat heads. Int. J. Food Microbiol. 2014, 182–183, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Fels-Klerx, H.J.V.D.; Klemsdal, S.; Hietaniemi, V.; Lindblad, M.; Ioannou-Kakouri, E.; Asselt, E.D.V. Mycotoxin contamination of cereal grain commodities in relation to climate in North West Europe. Food Addit. Contam. A 2012, 29, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No. 1126/2007 on maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, 255, 14–17. [Google Scholar]
- Fredlund, E.; Gidlund, A.; Pettersson, H.; Olsen, M.; Börjesson, T. Real-time PCR detection of Fusarium species in Swedish oats and correlation to T-2 and HT-2 toxin content. World Mycotoxin J. 2010, 3, 77–88. [Google Scholar] [CrossRef]
- Edwards, S.G.; Imathiu, S.M.; Ray, R.V.; Back, M.; Hare, M.C. Molecular studies to identify the Fusarium species responsible for HT-2 and T-2 mycotoxins in UK oats. Int. J. Food Microbiol. 2012, 156, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher-Wolz, U.; Heine, K.; Schneider, K. Report on Toxicity Data on Trichothecene Mycotoxins HT-2 and T-2 Toxins. CT/EFSA/CONTAM/2010/03. Available online: http://www.efsa.europa.eu/en/scdocs/scdoc/65e.htm (accessed on 9 April 2016).
- European Food Safety Authority. Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 1–187. [Google Scholar]
- Yang, L.; Yu, Z.; Hou, J.; Deng, Y.; Zhou, Z.; Zhao, Z.; Cui, J. Toxicity and oxidative stress induced by T-2 toxin and HT-2 toxin in broilers and broiler hepatocytes. Food Chem. Toxicol. 2016, 87, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.T.; Ciasca, B.; Haidukowski, M.; Infantino, A.; Visconti, A.; Pascale, M. Mycotoxin profile of Fusarium langsethiae isolated from wheat in Italy: Production of type-A trichothecenes and relevant glucosyl derivatives. J. Mass Spectrom. 2013, 48, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9. [Google Scholar] [CrossRef]
- Cortinovis, C.; Pizzo, F.; Spicer, L.J.; Caloni, F. Fusarium mycotoxins: Effects on reproductive function in domestic animals—A review. Theriogenology 2013, 80, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Glenn, A.E. Mycotoxigenic Fusarium species in animal feed. Anim. Feed Sci. Technol. 2007, 137, 213–240. [Google Scholar] [CrossRef]
- Edwards, S. Zearalenone risk in European wheat. World Mycotoxin J. 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Zheng, H.; He, C.; Zhang, H. T-2 toxin, zearalenone and fumonisin B1 in feedstuffs from China. Food Addit. Contam. B 2013, 6, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Yazar, S.; Omurtag, G.Z. Fumonisins, trichothecenes and zearalenone in cereals. Int. J. Mol. Sci. 2008, 9, 2062–2090. [Google Scholar] [CrossRef] [PubMed]
- Marasas, W.F.O. Fumonisins: History, world-wide occurrence and impact. In Fumonisins in Food; Jackson, L.S., DeVries, J.W., Bullerman, L.B., Eds.; Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 1996; pp. 1–17. [Google Scholar]
- Marín, S.; Magan, N.; Ramos, A.J.; Sanchis, V. Fumonisin-producing strains of Fusarium: A review of their ecophysiology. J. Food Prot. 2004, 67, 1792–1805. [Google Scholar] [PubMed]
- Caloni, F.; Cortinovis, C. Effects of fusariotoxins in the equine species. Vet. J. 2010, 186, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Garbetta, A.; Debellis, L.; de Girolamo, A.; Schena, R.; Visconti, A.; Minervini, F. Dose-dependent lipid peroxidation induction on ex vivo intestine tracts exposed to chyme samples from fumonisins contaminated corn samples. Toxicol. In Vitro 2015, 29, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to fumonisins as undesirable substances in animal feed. EFSA J. 2005, 235, 1–32. [Google Scholar]
- Bartók, T.; Szécsi, Á.; Szekeres, A.; Mesterházy, Á.; Bartók, M. Detection of new fumonisin mycotoxins and fumonisin-like compounds by reversed-phase high-performance liquid chromatography/electrospray ionization ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2447–2462. [Google Scholar] [CrossRef] [PubMed]
- Bartók, T.; Tölgyesi, L.; Szekeres, A.; Varga, M.; Bartha, R.; Szécsi, Á.; Bartók, M.; Mesterházy, Á. Detection and characterization of twenty-eight isomers of fumonisin B1 (FB1) mycotoxin in a solid rice culture infected with Fusarium verticillioides by reversed-phase high-performance liquid chromatography/electrospray ionization time-of-flight and ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 35–42. [Google Scholar] [PubMed]
- Szécsi, Á.; Szekeres, A.; Bartók, T.; Oros, G.; Bartók, M.; Mesterházy, Á. Fumonisin B1-4-producing capacity of Hungarian Fusarium verticillioides isolates. World Mycotoxin J. 2010, 3, 67–76. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Mateo, E.M.; González-Jaén, M.T.; Jiménez, M.; Vázquez, C.; Patiño, B. Contamination of barley seeds with Fusarium species and their toxins in Spain: An integrated approach. Food Addit. Contam. A 2013, 30, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Fotso, J.; Leslie, J.F.; Smith, J.S. Production of beauvericin, moniliformin, fusaproliferin, and fumonisins B1, B2, and B3 by fifteen ex-type strains of Fusarium species. Appl. Environ. Microbiol. 2002, 68, 5195–5197. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Meca, G.; Uhlig, S.; Ritieni, A. Fusaproliferin, beauvericin and enniatins: Occurrence in food—A review. World Mycotoxin J. 2012, 5, 71–81. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12. [Google Scholar] [CrossRef]
- Mallebrera, B.; Juan-Garcia, A.; Font, G.; Ruiz, M.-J. Mechanisms of beauvericin toxicity and antioxidant cellular defense. Toxicol. Lett. 2016, 246, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Vongvilai, P.; Isaka, M.; Kittakoop, P.; Srikitikulchai, P.; Kongsaeree, P.; Prabpai, S.; Thebtaranonth, Y. Isolation and structure elucidation of enniatins L, M1, M2, and N: Novel hydroxy analogs. Helv. Chim. Acta 2004, 87, 2066–2073. [Google Scholar] [CrossRef]
- Feifel, S.C.; Schmiederer, T.; Hornbogen, T.; Berg, H.; Süssmuth, R.D.; Zocher, R. In vitro synthesis of new enniatins: Probing the α-d-hydroxy carboxylic acid binding pocket of the multienzyme enniatin synthetase. ChemBioChem 2007, 8, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Bolechová, M.; Benešová, K.; Běláková, S.; Čáslavský, J.; Pospíchalová, M.; Mikulíková, R. Determination of seventeen mycotoxins in barley and malt in the Czech Republic. Food Control 2015, 47, 108–113. [Google Scholar] [CrossRef]
- Meca, G.; Zinedine, A.; Blesa, J.; Font, G.; Mañes, J. Further data on the presence of Fusarium emerging mycotoxins enniatins, fusaproliferin and beauvericin in cereals available on the Spanish markets. Food Chem. Toxicol. 2010, 48, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Meca, G.; Mañes, J.; Font, G. Further data on the occurrence of Fusarium emerging mycotoxins enniatins (A, A1, B, B1), fusaproliferin and beauvericin in raw cereals commercialized in Morocco. Food Control 2011, 22, 1–5. [Google Scholar] [CrossRef]
- Oueslati, S.; Meca, G.; Mliki, A.; Ghorbel, A.; Mañes, J. Determination of Fusarium mycotoxins enniatins, beauvericin and fusaproliferin in cereals and derived products from Tunisia. Food Control 2011, 22, 1373–1377. [Google Scholar] [CrossRef]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of the Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. A 2016. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.B.; Font, G.; Ruiz, M.J.; Ferrer, E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chem. 2012, 135, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-E.; Kim, B.H.; Lee, C. Occurrence of Fusarium mycotoxin beauvericin in animal feeds in Korea. Anim. Feed Sci. Technol. 2010, 157, 190–194. [Google Scholar] [CrossRef]
- Logrieco, A.; Rizzo, A.; Ferracane, R.; Ritieni, A. Occurrence of beauvericin and enniatins in wheat affected by Fusarium avenaceum head blight. Appl. Environ. Microbiol. 2002, 68, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Fumero, M.V.; Reynoso, M.M.; Chulze, S. Fusarium temperatum and Fusarium subglutinans isolated from maize in Argentina. Int. J. Food Microbiol. 2015, 199, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Marín, R.; Lino, C.M.; Mañes, J. Evaluation of enniatins A, A1, B, B1 and beauvericin in Portuguese cereal-based foods. Food Addit. Contam. A 2012, 29, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Velázquez, W.P.; Figueroa-Gómez, R.M.; Barberis, M.; Reynoso, M.M.; Rojo, F.G.A.; Chulze, S.N.; Torres, A.M. Fusarium species (section Liseola) occurrence and natural incidence of beauvericin, fusaproliferin and fumonisins in maize hybrids harvested in Mexico. Mycotoxin Res. 2011, 27, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Basílico, M.L.Z.; Pose, G.; Ludemann, V.; Pinto, V.E.F.; Aríngoli, E.E.; Ritieni, A.; Basílico, J.C. Fungal diversity and natural occurrence of fusaproliferin, beauvericin, deoxynivalenol and nivalenol in wheat cultivated in Santa Fe Province, Argentina. Mycotoxin Res. 2010, 26, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Mulè, G.; Ritieni, A.; Logrieco, A. Further data on the production of beauvericin, enniatins and fusaproliferin and toxicity to Artemia salina by Fusarium species of Gibberella fujikuroi species complex. Int. J. Food Microbiol. 2007, 118, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Logrieco, A.; Ritieni, A.; Randazzo, G.; Bottalico, A.; Macchia, L. Beauvericin and Fusaproliferin: Emerging Fusarium Toxins; Bulletin of the Institute for Comprehensive Agricultural Sciences, Kinki University; Kinki University: Higashiosaka, Japan, 1998; Volume 6, pp. 13–21. [Google Scholar]
- Ritieni, A.; Monti, S.M.; Randazzo, G.; Logrieco, A.; Moretti, A.; Peluso, G.; Ferracane, R.; Fogliano, V. Teratogenic effects of fusaproliferin on chicken embryos. J. Agric. Food Chem. 1997, 45, 3039–3043. [Google Scholar] [CrossRef]
- Prosperini, A.; Meca, G.; Font, G.; Ruiz, M.J. Study of the cytotoxic activity of beauvericin and fusaproliferin and bioavailability in vitro on Caco-2 cells. Food Chem. Toxicol. 2012, 50, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.; Stahr, H.M.; Logrieco, A.; Moretti, A.; Ritieni, A. Occurrence of fusaproliferin and beauvericin in Fusarium-contaminated livestock feed in Iowa. Appl. Environ. Microbiol. 1998, 64, 3923–3926. [Google Scholar] [PubMed]
- Shephard, G.S.; Burger, H.-M.; Gambacorta, L.; Krska, R.; Powers, S.P.; Rheeder, J.P.; Solfrizzo, M.; Sulyok, M.; Visconti, A.; Warth, B.; et al. Mycological analysis and multimycotoxins in maize from rural subsistence farmers in the former Transkei, South Africa. J. Agric. Food Chem. 2013, 61, 8232–8240. [Google Scholar] [CrossRef] [PubMed]
- Steyn, M.; Thiel, P.G.; Van Schalkwyk, G.C. Isolation and purification of moniliformin. J.-Assoc. Off. Anal. Chem. 1978, 61, 578–580. [Google Scholar] [PubMed]
- Chelkowski, J.; Zawadzki, M.; Zajkowski, P.; Logrieco, A.; Bottalico, A. Moniliformin production by Fusarium species. Mycotoxin Res. 1990, 6, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Thiel, P.G. A molecular mechanism for the toxic action of moniliformin, a mycotoxin produced by Fusarium moniliforme. Biochem. Pharmacol. 1978, 27, 483–486. [Google Scholar] [CrossRef]
- Peltonen, K.; Jestoi, M.; Eriksen, G. Health effects of moniliformin: A poorly understood Fusarium mycotoxin. World Mycotoxin J. 2010, 3, 403–414. [Google Scholar] [CrossRef]
- Scarpino, V.; Blandino, M.; Negre, M.; Reyneri, A.; Vanara, F. Moniliformin analysis in maize samples from North-West Italy using multifunctional clean-up columns and the LC-MS/MS detection method. Food Addit. Contam. A 2013, 30, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Torp, M.; Jarp, J.; Parich, A.; Gutleb, A.C.G.; Krska, R. Moniliformin in Norwegian grain. Food Addit. Contam. 2004, 21, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.L.; Nielsen, K.F.; Thrane, U. Analysis of moniliformin in maize plants using hydrophilic interaction chromatography. J. Agric. Food Chem. 2007, 55, 9764–9768. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Codex General Standard for Contaminants and Toxins in Food and Feed; CODEX STAN 193-1995 (Rev.1–1997); in Joint FAO/WHO Food Standards Programme; FAO, WHO: Rome, Italy, 2000. [Google Scholar]
- Codex Alimentarius Commission. Code of practice for the prevention and reduction of mycotoxin contamination in cereals, including annexes on ochratoxin A, zearalenone, fumonisins and tricothecenes (CAC/RCP 51-2003). In Prevention and Reduction of Food and Feed Contamination; FAO, WHO: Rome, Italy, 2003; pp. 1–13. [Google Scholar]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Report of the ninth session of the codex committee on contaminants in foods. In Joint FAO/WHO Food Standards Programme; FAO, WHO: New Delhi, India, 2015. [Google Scholar]
- Leslie, J.F.; Bandyopadhyay, R.; Visconti, A. Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; CABI: Wallingford, UK, 2008. [Google Scholar]
- Lee, T.; Lee, S.-H.; Lee, S.-H.; Shin, J.Y.; Yun, J.-C.; Lee, Y.-W.; Ryu, J.-G. Occurrence of Fusarium mycotoxins in rice and its milling by-products in Korea. J. Food Prot. 2011, 74, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilância Sanitária (ANVISA). Resolução RDC n° 7, de 18 de Fevereiro de 2011. Diário Oficial da União—Seção 1, n° 37, 22 de Fevereiro de 2011; ANVISA: Brasília, Brazil, 2011. [Google Scholar]
- Health Canada. Canadian Standards (Maximum Limits) for Various Chemical Contaminants in Foods. 2016. Available online: http://www.hc-sc.gc.ca/fn-an/securit/chem-chim/contaminants-guidelines-directives-eng.php (accessed on 9 April 2016).
- National Health and Family Planning of People’s Republic of China (NFHPC). China GB 2761-2011 Maximum Levels of Mycotoxins in Foods. 2011. Available online: http://www.cirs-group.com/food/news/GB_2761-2011_maximum_levels_mycotoxins.html (accessed on 9 April 2016).
- Ministry of Health of the Russian Federation. Amendments and Additions No. 18 to Hygienic Requirements for Safety and Nutrition Value of Food Products; 2.3.2.1078-01; Ministry of Health of the Russian Federation: Moscow, Russia, 2010.
- US Food and Drug Administration (US FDA). Guidance for Industry: Fumonisin Levels in Human Foods and Animal Feeds; Final Guidance; US FDA: Silver Spring, MD, USA, 2001.
- US Food and Drug Administration (US FDA). Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain by-Products Used for Animal Feed; US FDA: Silver Spring, MD, USA, 2010.
- European Commission. Commission Recommendation (2006/576/CE) on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- European Commission. Commission Recommendation (2013/165/EU) on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, 91, 12–15. [Google Scholar]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals. In Epidemiology of Mycotoxin Producing Fungi; Springer: Berlin, Germany, 2003; pp. 755–768. [Google Scholar]
- Isebaert, S.; de Saeger, S.; Devreese, R.; Verhoeven, R.; Maene, P.; Heremans, B.; Haesaert, G. Mycotoxin-producing Fusarium species occurring in winter wheat in Belgium (Flanders) during 2002–2005. J. Phytopathol. 2009, 157, 108–116. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Meier, A.; Dehne, H.-W.; Sulyok, M.; Krska, R.; Steiner, U. Spatial variability of Fusarium head blight pathogens and associated mycotoxins in wheat crops. Plant Pathol. 2010, 59, 671–682. [Google Scholar] [CrossRef]
- Paterson, R.R. M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium species. J. R. Soc. Interface 2011, 8, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Schmidt-Heydt, M.; Cárdenas-Chávez, D.L.; Parra, R.; Geisen, R.; Magan, N. Integrating toxin gene expression, growth and fumonisin B1 and B2 production by a strain of Fusarium verticillioides under different environmental factors. J. R. Soc. Interface 2013, 10, 20130320. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.L.; Martins, H.M. Influence of water activity, temperature and incubation time on the simultaneous production of deoxynivalenol and zearalenone in corn (Zea mays) by Fusarium graminearum. Food Chem. 2002, 79, 315–318. [Google Scholar] [CrossRef]
- Marín, P.; Magan, N.; Vázquez, C.; González-Jaén, M.T. Differential effect of environmental conditions on the growth and regulation of the fumonisin biosynthetic gene FUM1 in the maize pathogens and fumonisin producers Fusarium verticillioides and Fusarium proliferatum. FEMS Microbiol. Ecol. 2010, 73, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Savard, M.E.; Schaafsma, A.W. Mycotoxin production by Fusarium moniliforme and Fusarium proliferatum from Ontario and occurrence of fumonisin in the 1993 corn crop. Can. J. Plant Pathol. 1995, 17, 233–239. [Google Scholar]
- Picot, A.; Hourcade-Marcolla, D.; Barreau, C.; Pinson-Gadais, L.; Caron, D.; Richard-Forget, F.; Lannou, C. Interactions between Fusarium verticillioides and Fusarium graminearum in maize ears and consequences for fungal development and mycotoxin accumulation. Plant Pathol. 2012, 61, 140–151. [Google Scholar] [CrossRef]
- Fitt, B.D.; Huang, Y.-J.; van den Bosch, F.; West, J.S. Coexistence of related pathogen species on arable crops in space and time. Phytopathology 2006, 44, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, J.J. Mutualism, facilitation, and the structure of ecological communities positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend. Bioscience 2001, 51, 235–246. [Google Scholar]
- Mehl, H.L.; Cotty, P.J. Influence of plant host species on intraspecific competition during infection by Aspergillus flavus. Plant Pathol. 2013, 62, 1310–1318. [Google Scholar] [CrossRef]
- Cooney, J.M.; Lauren, D.R.; di Menna, M.E. Impact of competitive fungi on trichothecene production by Fusarium graminearum. J. Agric. Food Chem. 2001, 49, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Steier, I.; Köppen, R.; Siegel, D.; Proske, M.; Korn, U.; Koch, M. Cocultivation of phytopathogenic Fusarium and Alternaria strains affects fungal growth and mycotoxin production. J. Appl. Microbiol. 2012, 113, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Monger, W.; Ritieni, A.; Nicholson, P. Effect of temperature and duration of wetness during initial infection periods on disease development, fungal biomass and mycotoxin concentrations on wheat inoculated with single, or combinations of, Fusarium species. Plant Pathol. 2007, 56, 943–956. [Google Scholar] [CrossRef]
- Pan, J.J.; May, G. Fungal-fungal associations affect the assembly of endophyte communities in maize (Zea mays). Microb. Ecol. 2009, 58, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Siou, D.; Gélisse, S.; Laval, V.; Suffert, F.; Lannou, C. Mutual exclusion between fungal species of the FHB complex in a wheat spike. Appl. Environ. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blightin wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Audenaert, K.; Van Broeck, R.; Bekaert, B.; De Witte, F.; Heremans, B.; Messens, K.; Höfte, M.; Haesaert, G. Fusarium head blight (FHB) in Flanders: Population diversity, inter-species associations and DON contamination in commercial winter wheat varieties. Eur. J. Plant Pathol. 2009, 125, 445–458. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Oerke, E.-C.; Dehne, H.-W.; Steiner, U. Interactions of Fusarium species during prepenetration development. Fungal Biol. 2012, 116, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, S.; Bonner, C.T.; Wang, L.; Blackwell, B.; Rowland, O.; Subramaniam, R. Intraspecies interaction of Fusarium graminearum contributes to reduced toxin production and virulence. Mol. Plant Microbe Interact. 2015, 28, 1256–1267. [Google Scholar] [PubMed]
- Siou, D.; Gélisse, S.; Laval, V.; Elbelt, S.; Repinçay, C.; Bourdat-Deschamps, M.; Suffert, F.; Lannou, C. Interactions between head blight pathogens: Consequences for disease development and toxin production in wheat spikes. Appl. Environ. Microbiol. 2015, 81, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Marín, S.; Sanchis, V.; Ramos, A.J.; Vinas, I.; Magan, N. Environmental factors, in vitro interactions, and niche overlap between Fusarium moniliforme, F. proliferatum, and F. graminearum, Aspergillus and Penicillium species from maize grain. Mycol. Res. 1998, 102, 831–837. [Google Scholar] [CrossRef]
- Zorzete, P.; Castro, R.S.; Pozzi, C.R.; Israel, A.L.M.; Fonseca, H.; Yanaguibashi, G.; Corrêa, B. Relative populations and toxin production by Aspergillus flavus and Fusarium verticillioides in artificially inoculated corn at various stages of development under field conditions. J. Sci. Food Agric. 2008, 88, 48–55. [Google Scholar] [CrossRef]
- Velluti, A.; Marín, S.; Gonzalez, R.; J Ramos, A.; Sanchis, V. Fumonisin B1, zearalenone and deoxynivalenol production by Fusarium moniliforme, F. proliferatum and F. graminearum in mixed cultures on irradiated maize kernels. J. Sci. Food Agric. 2001, 81, 88–94. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Plant stress and mycotoxin accumulation in maize. Agrochimica 2014, 58, 116–127. [Google Scholar]
- Etcheverry, M. Aflatoxin B1, zearalenone and deoxynivalenol production by Aspergillus parasiticus and Fusarium graminearum in interactive cultures on irradiated corn kernels. Mycopathologia 1998, 142, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Bruns, H.A. Controlling aflatoxin and fumonisin in maize by crop management. Toxin Rev. 2003, 22, 153–173. [Google Scholar] [CrossRef]
- Folcher, L.; Jarry, M.; Weissenberger, A.; Gérault, F.; Eychenne, N.; Delos, M.; Regnault-Roger, C. Comparative activity of agrochemical treatments on mycotoxin levels with regard to corn borers and Fusarium mycoflora in maize (Zea mays L.) fields. Crop Prot. 2009, 28, 302–308. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Shier, W.T.; Johnson, B.J.; Phillips, N.A.; Weaver, M.A.; Abel, C.A.; Bruns, H.A. Aflatoxin and fumonisin in corn (Zea mays) infected by common smut Ustilago maydis. Plant Dis. 2015, 99, 1236–1240. [Google Scholar] [CrossRef]
- Afifi, M.; Swanton, C. Maize seed and stem roots differ in response to neighbouring weeds. Weed Res. 2011, 51, 442–450. [Google Scholar] [CrossRef]
- Robertson, A.E.; Munkvold, G.P.; Hurburgh, C.R.; Ensley, S. Effects of natural hail damage on ear rots, mycotoxins, and grain quality characteristics of corn. Agron. J. 2011, 103, 193–199. [Google Scholar] [CrossRef]
- Lobell, D.B.; Asner, G.P. Climate and management contributions to recent trends in U.S. agricultural yields. Science 2003, 299. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Santiago, R.; Ramos, A.J.; Marín, S.; Reid, L.M.; Butrón, A. Environmental factors related to fungal infection and fumonisin accumulation during the development and drying of white maize kernels. Int. J. Food Microbiol. 2013, 164, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, G.; Zhang, C. Interaction between reactive oxygen species and nitric oxide in drought-induced abscisic acid synthesis in root tips of wheat seedlings. Funct. Plant Biol. 2001, 28, 1055–1061. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D. G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Ponts, N.; Pinson-Gadais, L.; Verdal-Bonnin, M.-N.; Barreau, C.; Richard-Forget, F. Accumulation of deoxynivalenol and its 15-acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum. FEMS Microbiol. Lett. 2006, 258, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Bogialli, S.; Bortolini, C.; Tapparo, A.; Causin, R. In vitro production of fumonisins by Fusarium verticillioides under oxidative stress induced by H2O2. J. Agric. Food Chem. 2015, 63, 4879–4885. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, G.; Quaranta, F.; Desiderio, E.; Visconti, A. Fumonisin contamination of maize hybrids visibly damaged by Sesamia. J. Sci. Food Agric. 2003, 83, 13–18. [Google Scholar] [CrossRef]
- Kerchev, P.I.; Fenton, B.; Foyer, C.H.; Hancock, R.D. Plant responses to insect herbivory: Interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant Cell Environ. 2012, 35, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Walker, F.; Hoeglinger, B.; Buchenauer, H. Solvolysis procedures for the determination of bound residues of the mycotoxin deoxynivalenol in Fusarium species infected grain of two winter wheat cultivars preinfected with barley yellow dwarf virus. J. Agric. Food Chem. 2005, 53, 6864–6869. [Google Scholar] [CrossRef] [PubMed]
- De Zutter, N.; Audenaert, K.; Ameye, M.; De Boevre, M.; De Saeger, S.; Haesaert, G.; Smagghe, G. The plant response induced in wheat ears by a combined attack of Sitobion avenae aphids and Fusarium graminearum boosts the fungal infection and its deoxynivalenol production. Mol. Plant Pathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Couedelo, L.; Pinson-Gadais, L.; Verdal-Bonnin, M.-N.; Barreau, C.; Richard-Forget, F. Fusarium response to oxidative stress by H2O2 is trichothecene chemotype-dependent. FEMS Microbiol. Lett. 2009, 293, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; Maclean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, G.; Jiang, C.; Xu, J.-R.; Wang, C. Fgk3 glycogen synthase kinase is important for development, pathogenesis, and stress responses in Fusarium graminearum. Sci. Rep. 2015, 5, 8504. [Google Scholar] [CrossRef] [PubMed]
- Ameye, M.; Audenaert, K.; de Zutter, N.; Steppe, K.; van Meulebroek, L.; Vanhaecke, L.; de Vleesschauwer, D.; Haesaert, G.; Smagghe, G. Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against Fusarium graminearum but boosts deoxynivalenol production. Plant Physiol. 2015, 167, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Scarpino, V.; Vanara, F.; Sulyok, M.; Krska, R.; Reyneri, A. Role of the European corn borer (Ostrinia nubilalis) on contamination of maize with 13 Fusarium mycotoxins. Food Addit. Contam. A 2015, 32, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Cao, A.; Malvar, R.A.; Butrón, A. Is it possible to control fumonisin contamination in maize kernels by using genotypes resistant to the Mediterranean corn borer? J. Econ. Entomol. 2013, 106, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Scully, B.T.; Krakowsky, M.D.; Ni, X.; Wilson, J.P.; Lee, R.D.; Guo, B.Z. Preharvest aflatoxin contamination of corn and other grain crops grown on the US Southeastern Coastal Plain. Toxin Rev. 2009, 28, 169–179. [Google Scholar] [CrossRef]
- Barry, D.; Zuber, M.S.; Lillehoj, E.B.; McMillian, W.W.; Adams, N.J.; Kwolek, W.F.; Widstrom, N.W. Evaluation of two arthropod vectors as inoculators of developing maize ears with Aspergillus flavus. Environ. Entomol. 1985, 14, 634–636. [Google Scholar] [CrossRef]
- Bricchi, I.; Leitner, M.; Foti, M.; Mithöfer, A.; Boland, W.; Maffei, M.E. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: Early signaling and volatile emission in Lima bean (Phaseolus lunatus L.). Planta 2010, 232, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Jih, P.-J.; Lin, H.-H.; Lin, J.-S.; Chang, L.-L.; Shen, Y.-H.; Jeng, S.-T. Nitric oxide activates superoxide dismutase and ascorbate peroxidase to repress the cell death induced by wounding. Plant Mol. Biol. 2011, 77, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Pinson-Gadais, L.; Barreau, C.; Richard-Forget, F.; Ouellet, T. Exogenous H2O2 and catalase treatments interfere with Tri genes expression in liquid cultures of Fusarium graminearum. FEBS Lett. 2007, 581, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.W.; Miller, J.D.; Savard, M.E.; Ewing, R.J. Ear rot development and mycotoxin production in corn in relation to inoculation method, corn hybrid, and species of Fusarium. Can. J. Plant Pathol. 1993, 15, 185–192. [Google Scholar] [CrossRef]
- Page, E.R.; Liu, W.; Cerrudo, D.; Lee, E.A.; Swanton, C.J. Shade avoidance influences stress tolerance in maize. Weed Sci. 2011, 59, 326–334. [Google Scholar] [CrossRef]
- Hol, W.H.G.; de Boer, W.; ten Hooven, F.; van der Putten, W.H. Competition increases sensitivity of wheat (Triticum aestivum) to biotic plant-soil feedback. PLoS ONE 2013, 8, e66085. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.L.; Cassin, A.; Bacic, A. Quantitative proteomic analysis of wheat cultivars with differing drought stress tolerance. Front. Plant Sci. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Williams, W.P.; Windham, G.L.; Pringle, H.C.; Xie, W.; Shier, W.T. Aflatoxin and fumonisin contamination of commercial corn (Zea mays) hybrids in Mississippi. J. Agric. Food Chem. 2002, 50, 5246–5254. [Google Scholar] [CrossRef] [PubMed]
- Pascale, M.; Visconti, A.; Chelkowski, J. Ear rot susceptibility and mycotoxin contamination of maize hybrids inoculated with Fusarium species under field conditions. In Mycotoxins in Plant Disease; Springer: Berlin, Germany, 2002; pp. 645–651. [Google Scholar]
- Abbas, H.K.; Mascagni, H.J., Jr.; Bruns, H.A.; Shier, W.T. Effect of planting density, irrigation regimes, and maize hybrids with varying ear size on yield, and aflatoxin and fumonisin contamination levels. Am. J. Plant Sci. 2012, 3, 1341–1354. [Google Scholar] [CrossRef]
- Teller, R.S.; Schmidt, R.J.; Whitlow, L.W.; Kung, L., Jr. Effect of physical damage to ears of corn before harvest and treatment with various additives on the concentration of mycotoxins, silage fermentation, and aerobic stability of corn silage. J. Dairy Sci. 2012, 95, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Arimura, G.-I.; Uchtenhagen, H.; Bossi, S.; Bertea, C.M.; Cucuzza, L.S.; Novero, M.; Volpe, V.; Quadro, S.; et al. Effects of feeding Spodoptera littoralis on Lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006, 140, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Reyneri, A.; Bruno, G.; D’Egidio, M.G.; Balconi, C. Linee Guida per il Controllo Delle Micotossine Nella Granella di Mais e di Frumento, 2016. Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/9703 (accessed on 9 April 2016). (In Italian)
- Rossi, V.; Scandolara, A.; Battilani, P. Effect of environmental conditions on spore production by Fusarium verticillioides, the causal agent of maize ear rot. Eur. J. Plant Pathol. 2009, 123, 159–169. [Google Scholar] [CrossRef]
- Keller, M.D.; Bergstrom, G.C.; Shields, E.J. The aerobiology of Fusarium graminearum. Aerobiologia 2014, 30, 123–136. [Google Scholar] [CrossRef]
- Nicolardot, B.; Bouziri, L.; Bastian, F.; Ranjard, L. A microcosm experiment to evaluate the influence of location and quality of plant residues on residue decomposition and genetic structure of soil microbial communities. Soil Biol. Biochem. 2007, 39, 1631–1644. [Google Scholar] [CrossRef]
- Khonga, E.B.; Sutton, J.C. Inoculum production and survival of Gibberella zeae in maize and wheat residues. Can. J. Plant Pathol. 1988, 10, 232–239. [Google Scholar] [CrossRef]
- Cotten, T.K.; Munkvold, G.P. Survival of Fusarium moniliforme, F. proliferatum, and F. subglutinans in maize stalk residue. Phytopathology 1998, 88, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Dick, W.A. Bacterial community diversity in soil under two tillage practices as determined by pyrosequencing. Microb. Ecol. 2015, 70, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Degrune, F.; Theodorakopoulos, N.; Dufrêne, M.; Colinet, G.; Bodson, B.; Hiel, M.-P.; Taminiau, B.; Nezer, C.; Daube, G.; Vandenbol, M. No favorable effect of reduced tillage on microbial community diversity in a silty loam soil (Belgium). Agric. Ecosyst. Environ. 2016, 224, 12–21. [Google Scholar] [CrossRef]
- Sipilä, T.P.; Yrjälä, K.; Alakukku, L.; Palojärvi, A. Cross-site soil microbial communities under tillage regimes: Fungistasis and microbial biomarkers. Appl. Environ. Microbiol. 2012, 78, 8191–8201. [Google Scholar] [CrossRef] [PubMed]
- Peigné, J.; Messmer, M.; Aveline, A.; Berner, A.; Mäder, P.; Carcea, M.; Narducci, V.; Samson, M.-F.; Thomsen, I.K.; Celette, F.; et al. Wheat yield and quality as influenced by reduced tillage in organic farming. Org. Agric. 2013, 4, 1–13. [Google Scholar] [CrossRef]
- Marocco, A.; Gavazzi, C.; Pietri, A.; Tabaglio, V. On fumonisin incidence in monoculture maize under no-till, conventional tillage and two nitrogen fertilisation levels. J. Sci. Food Agric. 2008, 88, 1217–1221. [Google Scholar] [CrossRef]
- Blandino, M.; Haidukowski, M.; Pascale, M.; Plizzari, L.; Scudellari, D.; Reyneri, A. Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crops Res. 2012, 133, 139–149. [Google Scholar] [CrossRef]
- Ono, E.Y.S.; Moreno, E.C.; Ono, M.A.; Rossi, C.N.; Saito, G.H.; Vizoni, É.; Sugiura, Y.; Hirooka, E.Y. Effect of cropping systems and crop successions on fumonisin levels in corn from Northern Paraná State, Brazil. Eur. J. Plant Pathol. 2011, 131, 653–660. [Google Scholar] [CrossRef]
- Dill-Macky, R.; Jones, R.K. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Shah, D.A.; Bergstrom, G.C. Spatial patterns of Fusarium head blight in New York wheat fields suggest role of airborne inoculum. Plant Health Prog. 2003, 10. [Google Scholar] [CrossRef]
- Maldonado-Ramirez, S.L.; Schmale, D.G.; Shields, E.J.; Bergstrom, G.C. The relative abundance of viable spores of Gibberella zeae in the planetary boundary layer suggests the role of long-distance transport in regional epidemics of Fusarium head blight. Agric. For. Meteorol. 2005, 132, 20–27. [Google Scholar] [CrossRef]
- Bateman, G.L.; Gutteridge, R.J.; Gherbawy, Y.; Thomsett, M.A.; Nicholson, P. Infection of stem bases and grains of winter wheat by Fusarium culmorum and F. graminearum and effects of tillage method and maize-stalk residues. Plant Pathol. 2007, 56, 604–615. [Google Scholar] [CrossRef]
- Landschoot, S.; Audenaert, K.; Waegeman, W.; Pycke, B.; Bekaert, B.; de Baets, B.; Haesaert, G. Connection between primary Fusarium inoculum on gramineous weeds, crop residues and soil samples and the final population on wheat ears in Flanders, Belgium. Crop Prot. 2011, 30, 1297–1305. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Tamburic-Ilincic, L.; Hooker, D.C. Effect of previous crop, tillage, field size, adjacent crop, and sampling direction on airborne propagules of Gibberella zeae/Fusarium graminearum, Fusarium head blight severity, and deoxynivalenol accumulation in winter wheat. Can. J. Plant Pathol. 2005, 27, 217–224. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Steiner, B.; Lemmens, M.; Ruckenbauer, P. Resistance to Fusarium head blight in winter wheat: Heritability and trait associations. Crop Sci. 2000, 40, 1012–1018. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Buerstmayr, H. Comparative mapping of quantitative trait loci for Fusarium head blight resistance and anther retention in the winter wheat population Capo × Arina. Theor. Appl. Genet. 2015, 128, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Nakajima, T.; Arai, M.; Suzuki, F.; Tomimura, K. Effect of the timing of fungicide application on Fusarium head blight and mycotoxin accumulation in closed-flowering barley. Plant Dis. 2008, 92, 1164–1170. [Google Scholar] [CrossRef]
- Nakajima, T.; Yoshida, M.; Tomimura, K. Effect of lodging on the level of mycotoxins in wheat, barley, and rice infected with the Fusarium graminearum species complex. J. Gen. Plant Pathol. 2008, 74, 289–295. [Google Scholar] [CrossRef]
- Chetouhi, C.; Bonhomme, L.; Lecomte, P.; Cambon, F.; Merlino, M.; Biron, D.G.; Langin, T. A proteomics survey on wheat susceptibility to Fusarium head blight during grain development. Eur. J. Plant Pathol. 2015, 141, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Chetouhi, C.; Bonhomme, L.; Lasserre-Zuber, P.; Cambon, F.; Pelletier, S.; Renou, J.-P.; Langin, T. Transcriptome dynamics of a susceptible wheat upon Fusarium head blight reveals that molecular responses to Fusarium graminearum infection fit over the grain development processes. Funct. Integr. Genomics 2016, 16, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Shaner, G. Scab of wheat: Prospects for control. Plant Dis. 1994, 78, 760–766. [Google Scholar]
- Burt, C.; Steed, A.; Gosman, N.; Lemmens, M.; Bird, N.; Ramirez-Gonzalez, R.; Holdgate, S.; Nicholson, P. Mapping a Type 1 FHB resistance on chromosome 4AS of Triticum macha and deployment in combination with two Type 2 resistances. Theor. Appl. Genet. 2015, 128, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Lu, Q.; Lillemo, M.; Skinnes, H.; He, X.; Shi, J.; Ji, F.; Dong, Y.; Bjornstad, A. Anther extrusion and plant height are associated with Type I resistance to Fusarium head blight in bread wheat line ‘Shanghai-3/Catbird’. Theor. Appl. Genet. 2013, 126, 317–334. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Singh, P.K.; Schlang, N.; Duveiller, E.; Dreisigacker, S.; Payne, T.; He, Z. Characterization of Chinese wheat germplasm for resistance to Fusarium head blight at CIMMYT, Mexico. Euphytica 2014, 195, 383–395. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Richard-Forget, F.; Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Ji, F.; Wu, J.; Zhao, H.; Xu, J.; Shi, J. Relationship of deoxynivalenol content in grain, chaff, and straw with Fusarium head blight severity in wheat varieties with various levels of resistance. Toxins 2015, 7, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Xiang, K.; Zhang, Z.M.; Reid, L.M.; Zhu, X.Y.; Yuan, G.S.; Pan, G.T. A meta-analysis of QTL associated with ear rot resistance in maize. Maydica 2010, 55, 281–290. [Google Scholar]
- Martin, M.; Miedaner, T.; Dhillon, B.S.; Ufermann, U.; Kessel, B.; Ouzunova, M.; Schipprack, W.; Melchinger, A.E. Colocalization of QTL for Gibberella ear rot resistance and low mycotoxin contamination in early European maize. Crop Sci. 2011, 51, 1935–1945. [Google Scholar] [CrossRef]
- Horne, D.W.; Eller, M.S.; Holland, J.B. Responses to recurrent index selection for reduced Fusarium ear rot and lodging and for increased yield in maize. Crop Sci. 2016, 56, 85–94. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A. Effect of maize hybrid maturity and grain hardness on fumonisin and zearalenone contamination. Ital. J. Agron. 2008, 3, 107–117. [Google Scholar] [CrossRef]
- Pietri, A.; Battilani, P.; Gualla, A.; Bertuzzi, T. Mycotoxin levels in maize produced in northern Italy in 2008 as influenced by growing location and FAO class of hybrid. World Mycotoxin J. 2012, 5, 409–418. [Google Scholar] [CrossRef]
- Battilani, P.; Formenti, S.; Ramponi, C.; Rossi, V. Dynamic of water activity in maize hybrids is crucial for fumonisin contamination in kernels. J. Cereal Sci. 2011, 54, 467–472. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Battilani, P. Role of maize hybrids and their chemical composition in Fusarium infection and fumonisin production. J. Agric. Food Chem. 2012, 60, 3800–3808. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Pons, S.; Pinson-Gadais, L.; Picot, A.; Marchegay, G.; Bonnin-Verdal, M.-N.; Ducos, C.; Barreau, C.; Roucolle, J.; Sehabiague, P. Chlorogenic acid and maize ear rot resistance: A dynamic study investigating Fusarium graminearum development, deoxynivalenol production, and phenolic acid accumulation. Mol. Plant. Microbe Interact. 2012, 25, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Bowers, E.; Hellmich, R.; Munkvold, G. Comparison of fumonisin contamination using HPLC and ELISA methods in Bt and near-isogenic maize hybrids infested with European corn borer or Western bean cutworm. J. Agric. Food Chem. 2014, 62, 6463–6472. [Google Scholar] [CrossRef] [PubMed]
- Choo, T.M.; Martin, R.A.; Savard, M.E.; Blackwell, B. Effects of planting date and earliness on deoxynivalenol contamination in barley under natural epidemic conditions. Can. J. Plant Sci. 2014, 94, 1363–1371. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Vanara, F. Effect of sowing time on toxigenic fungal infection and mycotoxin contamination of maize kernels. J. Phytopathol. 2009, 157, 7–14. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Has, V.; Melidis, V.; Has, I.; Mylonas, I.; Evgenidis, G.; Copandean, A.; Ninou, E.; Fasoula, V.A. Maize hybrids less dependent on high plant densities improve resource-use efficiency in rainfed and irrigated conditions. Field Crops Res. 2011, 120, 345–351. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Vanara, F. Effect of plant density on toxigenic fungal infection and mycotoxin contamination of maize kernels. Field Crops Res. 2008, 106, 234–241. [Google Scholar] [CrossRef]
- Postic, J.; Cosic, J.; Vrandecic, K.; Jurkovic, D.; Saleh, A.A.; Leslie, J.F. Diversity of Fusarium species isolated from weeds and plant debris in Croatia. J. Phytopathol. 2012, 160, 76–81. [Google Scholar] [CrossRef]
- Mourelos, C.A.; Malbrán, I.; Balatti, P.A.; Ghiringhelli, P.D.; Lori, G.A. Gramineous and non-gramineous weed species as alternative hosts of Fusarium graminearum, causal agent of Fusarium head blight of wheat, in Argentina. Crop Prot. 2014, 65, 100–104. [Google Scholar] [CrossRef]
- Teich, A.H.; Nelson, K. Survey of Fusarium head blight and possible effects of cultural practices in wheat fields in Lambton County in 1983. Can. Plant Dis. Surv. 1984, 64, 11–13. [Google Scholar]
- Kremer, R.J.; Means, N.E. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur. J. Agron. 2009, 31, 153–161. [Google Scholar] [CrossRef]
- Duke, S.O.; Wedge, D.E.; Cerdeira, A.L.; Matallo, M.B. Interactions of synthetic herbicides with plant disease and microbial herbicides. In Novel Biotechnologies for Biocontrol Agent Enhancement and Management; Springer: Berlin, Germany, 2007; pp. 277–296. [Google Scholar]
- Powell, J.R.; Swanton, C.J. A critique of studies evaluating glyphosate effects on diseases associated with Fusarium spp. Weed Res. 2008, 48, 307–318. [Google Scholar] [CrossRef]
- Johal, G.S.; Huber, D.M. Glyphosate effects on diseases of plants. Eur. J. Agron. 2009, 31, 144–152. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Selles, F.; Gehl, D.; DePauw, R.M.; Zentner, R.P. Crop production factors associated with Fusarium head blight in spring wheat in eastern Saskatchewan. Crop Sci. 2005, 45, 1908–1916. [Google Scholar] [CrossRef]
- Reddy, K.N.; Abbas, H.K.; Zablotowicz, R.M.; Abel, C.A.; Koger, C.H. Mycotoxin occurrence and Aspergillus flavus soil propagules in a corn and cotton glyphosate-resistant cropping systems. Food Addit. Contam. 2007, 24, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, M.-E.; Vanasse, A.; Rioux, S.; Bourget, N.; Dion, Y.; Tremblay, G. Effect of glyphosate on Fusarium head blight in wheat and barley under different soil tillages. Plant Dis. 2011, 96, 338–344. [Google Scholar] [CrossRef]
- Gautam, P.; Dill-Macky, R. Impact of moisture, host genetics and Fusarium graminearum isolates on Fusarium head blight development and trichothecene accumulation in spring wheat. Mycotoxin Res. 2012, 28, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Wegulo, S.N.; Baenziger, P.S.; Hernandez Nopsa, J.; Bockus, W.W.; Hallen-Adams, H. Management of Fusarium head blight of wheat and barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Kharbikar, L.L.; Dickin, E.T.; Edwards, S.G. Impact of post-anthesis rainfall, fungicide and harvesting time on the concentration of deoxynivalenol and zearalenone in wheat. Food Addit. Contam. A 2015, 32, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Torelli, E.; Firrao, G.; Bianchi, G.; Saccardo, F.; Locci, R. The influence of local factors on the prediction of fumonisin contamination in maize. J. Sci. Food Agric. 2012, 92, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Ariño, A.; Herrera, M.; Juan, T.; Estopañan, G.; Carramiñana, J.J.; Rota, C.; Herrera, A. Influence of agricultural practices on the contamination of maize by fumonisin mycotoxins. J. Food Prot. 2009, 72, 898–902. [Google Scholar] [PubMed]
- Heier, T.; Jain, S.K.; Kogel, K.-H.; Pons-Kühnemann, J. Influence of N-fertilization and fungicide strategies on Fusarium head blight severity and mycotoxin content in winter wheat. J. Phytopathol. 2005, 153, 551–557. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.; Løes, A.-K.; Kristoffersen, A.B. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. A 2012, 29, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Reyneri, A.; Vanara, F. Influence of nitrogen fertilization on mycotoxin contamination of maize kernels. Crop Prot. 2008, 27, 222–230. [Google Scholar] [CrossRef]
- Hajiboland, R. Effect of micronutrient deficiencies on plants stress responses. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, Ny, USA, 2012; pp. 283–329. [Google Scholar]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil 2012, 368, 73–85. [Google Scholar] [CrossRef]
- Miller, J.D.; Culley, J.; Fraser, K.; Hubbard, S.; Meloche, F.; Ouellet, T.; Lloyd Seaman, W.; Seifert, K.A.; Turkington, K.; Voldeng, H. Effect of tillage practice on Fusarium head blight of wheat. Can. J. Plant Pathol. 1998, 20, 95–103. [Google Scholar] [CrossRef]
- Chen, G.; Hinds, J.; Zobel, E.; Rosario-Lebron, A.; Hooks, C.R.R. Evaluation of prophylactic sprays on pest abundance, foliar damage and yield in winter wheat. Int. J. Pest Manag. 2015, 61, 161–170. [Google Scholar] [CrossRef]
- Bagga, P. Fusarium Head Blight (FHB) of wheat: Role of host resistance, wheat aphids, insecticide and strobilurin fungicide in disease control in Punjab, India. Cereal Res. Commun. 2008, 36, 667–670. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Vanara, F.; Scopel, C.; Causin, R.; Blandino, M. Relationship between European Corn Borer injury, Fusarium proliferatum and F. subglutinans infection and moniliformin contamination in maize. Field Crops Res. 2015, 183, 69–78. [Google Scholar] [CrossRef]
- Watrous, K.; Rice, K.; Fleischer, S.; Smiles, S. Evaluation of foliar insecticides for the control of Lepidopterans, 2014. Arthropod Manag. Tests 2015, 40, E73. [Google Scholar] [CrossRef]
- Saladini, M.A.; Blandino, M.; Reyneri, A.; Alma, A. Impact of insecticide treatments on Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae) and their influence on the mycotoxin contamination of maize kernels. Pest Manag. Sci. 2008, 64, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Papst, C.; Utz, H.F.; Melchinger, A.E.; Eder, J.; Magg, T.; Klein, D.; Bohn, M. Mycotoxins produced by Fusarium spp. in isogenic Bt vs. non-Bt maize hybrids under European corn borer pressure. Agron. J. 2005, 97, 219–224. [Google Scholar]
- D’Angelo, D.L.; Bradley, C.A.; Ames, K.A.; Willyerd, K.T.; Madden, L.V.; Paul, P.A. Efficacy of fungicide applications during and after anthesis against Fusarium head blight and deoxynivalenol in soft red winter wheat. Plant Dis. 2014, 98, 1387–1397. [Google Scholar] [CrossRef]
- Ruske, R.E.; Gooding, M.J.; Jones, S.A. The effects of adding picoxystrobin, azoxystrobin and nitrogen to a triazole programme on disease control, flag leaf senescence, yield and grain quality of winter wheat. Crop Prot. 2003, 22, 975–987. [Google Scholar] [CrossRef]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Ioos, R.; Belhadj, A.; Menez, M.; Faure, A. The effects of fungicides on Fusarium spp. and Microdochium nivale and their associated trichothecene mycotoxins in French naturally-infected cereal grains. Crop Prot. 2005, 24, 894–902. [Google Scholar] [CrossRef]
- De Curtis, F.; De Cicco, V.; Haidukowski, M.; Pascale, M.; Somma, S.; Moretti, A. Effects of agrochemical treatments on the occurrence of Fusarium ear rot and fumonisin contamination of maize in Southern Italy. Field Crops Res. 2011, 123, 161–169. [Google Scholar] [CrossRef]
- Willyerd, K.T.; Li, C.; Madden, L.V.; Bradley, C.A.; Bergstrom, G.C.; Sweets, L.E.; McMullen, M.; Ransom, J.K.; Grybauskas, A.; Osborne, L.; et al. Efficacy and stability of integrating fungicide and cultivar resistance to manage Fusarium Head Blight and deoxynivalenol in wheat. Plant Dis. 2012, 96, 957–967. [Google Scholar] [CrossRef]
- Haidukowski, M.; Visconti, A.; Perrone, G.; Vanadia, S.; Pancaldi, D.; Covarelli, L.; Balestrazzi, R.; Pascale, M. Effect of prothioconazole-based fungicides on Fusarium head blight, grain yield and deoxynivalenol accumulation in wheat under field conditions. Phytopathol. Mediterr. 2012, 51, 236–246. [Google Scholar]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Kokkonen, M.; Magan, N.; Medina, A. Comparative effects of fungicides and environmental factors on growth and T-2+ HT-2 toxin production by Fusarium sporotrichioides and Fusarium langsethiae strains on an oat-based matrix. World Mycotoxin J. 2014, 7, 177–186. [Google Scholar] [CrossRef]
- Edwards, S.; Anderson, E. Impact of agronomy on HT-2 and T-2 toxin content of oats. Plant Breed. Seed Sci. 2011, 63, 49–57. [Google Scholar] [CrossRef]
- Simpson, D.R.; Weston, G.E.; Turner, J.A.; Jennings, P.; Nicholson, P. Differential control of head blight pathogens of wheat by fungicides and consequences for mycotoxin contamination of grain. Eur. J. Plant Pathol. 2001, 107, 421–431. [Google Scholar] [CrossRef]
- Pirgozliev, S.R.; Edwards, S.G.; Hare, M.C.; Jenkinson, P. Effect of dose rate of azoxystrobin and metconazole on the development of Fusarium head blight and the accumulation of deoxynivalenol (DON) in wheat grain. Eur. J. Plant Pathol. 2002, 108, 469–478. [Google Scholar] [CrossRef]
- Audenaert, K.; Callewaert, E.; Höfte, M.; Saeger, S.D.; Haesaert, G. Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Microbiol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Marín, P.; de Ory, A.; Cruz, A.; Magan, N.; González-Jaén, M.T. Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling Fusarium verticillioides and Fusarium proliferatum growth rate and fumonisin biosynthesis. Int. J. Food Microbiol. 2013, 165, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Luongo, L.; Galli, M.; Corazza, L.; Meekes, E.; Haas, L.D.; Van Der Plas, C.L.; Köhl, J. Potential of fungal antagonists for biocontrol of Fusarium spp. in wheat and maize through competition in crop debris. Biocontrol Sci. Technol. 2005, 15, 229–242. [Google Scholar] [CrossRef]
- Chulze, S.N.; Palazzini, J.M.; Torres, A.M.; Barros, G.; Ponsone, M.L.; Geisen, R.; Schmidt-Heydt, M.; Köhl, J. Biological control as a strategy to reduce the impact of mycotoxins in peanuts, grapes and cereals in Argentina. Food Addit. Contam. A 2015, 32, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Mionetto, A.; Tiscornia, S.; Bettucci, L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015, 31, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Shearer, C.R.; Limay-Rios, V.; Zhou, T.; Raizada, M.N. Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Aiyaz, M.; Divakara, S.T.; Nayaka, S.C.; Hariprasad, P.; Niranjana, S.R. Application of beneficial rhizospheric microbes for the mitigation of seed-borne mycotoxigenic fungal infection and mycotoxins in maize. Biocontrol Sci. Technol. 2015, 25, 1105–1119. [Google Scholar] [CrossRef]
- Samsudin, N.I.p.; Magan, N. Efficacy of potential biocontrol agents for control of Fusarium verticillioides and fumonisin B1 under different environmental conditions. World Mycotoxin J. 2015, 9, 205–213. [Google Scholar] [CrossRef]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Palazzini, J.M.; Groenenboom-de Haas, B.H.; Torres, A.M.; Köhl, J.; Chulze, S.N. Biocontrol and population dynamics of Fusarium spp. on wheat stubble in Argentina. Plant Pathol. 2013, 62, 859–866. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Rasera, R.; Causin, R. Trichoderma harzianum seed treatment controls Fusarium verticillioides colonization and fumonisin contamination in maize under field conditions. Crop Prot. 2014, 65, 51–56. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Piccolo, E.; Scopel, C.; Causin, R. Trichoderma harzianum T22 induces in maize systemic resistance against Fusarium verticillioides. J. Plant Pathol. 2014, 96, 133–142. [Google Scholar]
- Moya-Elizondo, E.A.; Jacobsen, B.J. Integrated management of Fusarium crown rot of wheat using fungicide seed treatment, cultivar resistance, and induction of systemic acquired resistance (SAR). Biol. Control 2016, 92, 153–163. [Google Scholar] [CrossRef]
- Pani, G.; Scherm, B.; Azara, E.; Balmas, V.; Jahanshiri, Z.; Carta, P.; Fabbri, D.; Dettori, M.A.; Fadda, A.; Dessì, A.; et al. Natural and natural-like phenolic inhibitors of type B trichothecene in vitro production by the wheat (Triticum sp.) pathogen Fusarium culmorum. J. Agric. Food Chem. 2014, 62, 4969–4978. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ferrochio, L.; Cendoya, E.; Farnochi, M.C.; Massad, W.; Ramirez, M.L. Evaluation of ability of ferulic acid to control growth and fumonisin production of Fusarium verticillioides and Fusarium proliferatum on maize based media. Int. J. Food Microbiol. 2013, 167, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Roselló, J.; Sempere, F.; Sanz-Berzosa, I.; Chiralt, A.; Santamarina, M.P. Antifungal activity and potential use of essential oils against Fusarium culmorum and Fusarium verticillioides. J. Essent. Oil Bear. Plants 2015, 18, 359–367. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Kfoury, M.; Sahraoui, A.L.-H.; Bourdon, N.; Laruelle, F.; Fontaine, J.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem. 2016, 196, 518–525. [Google Scholar] [CrossRef] [PubMed]
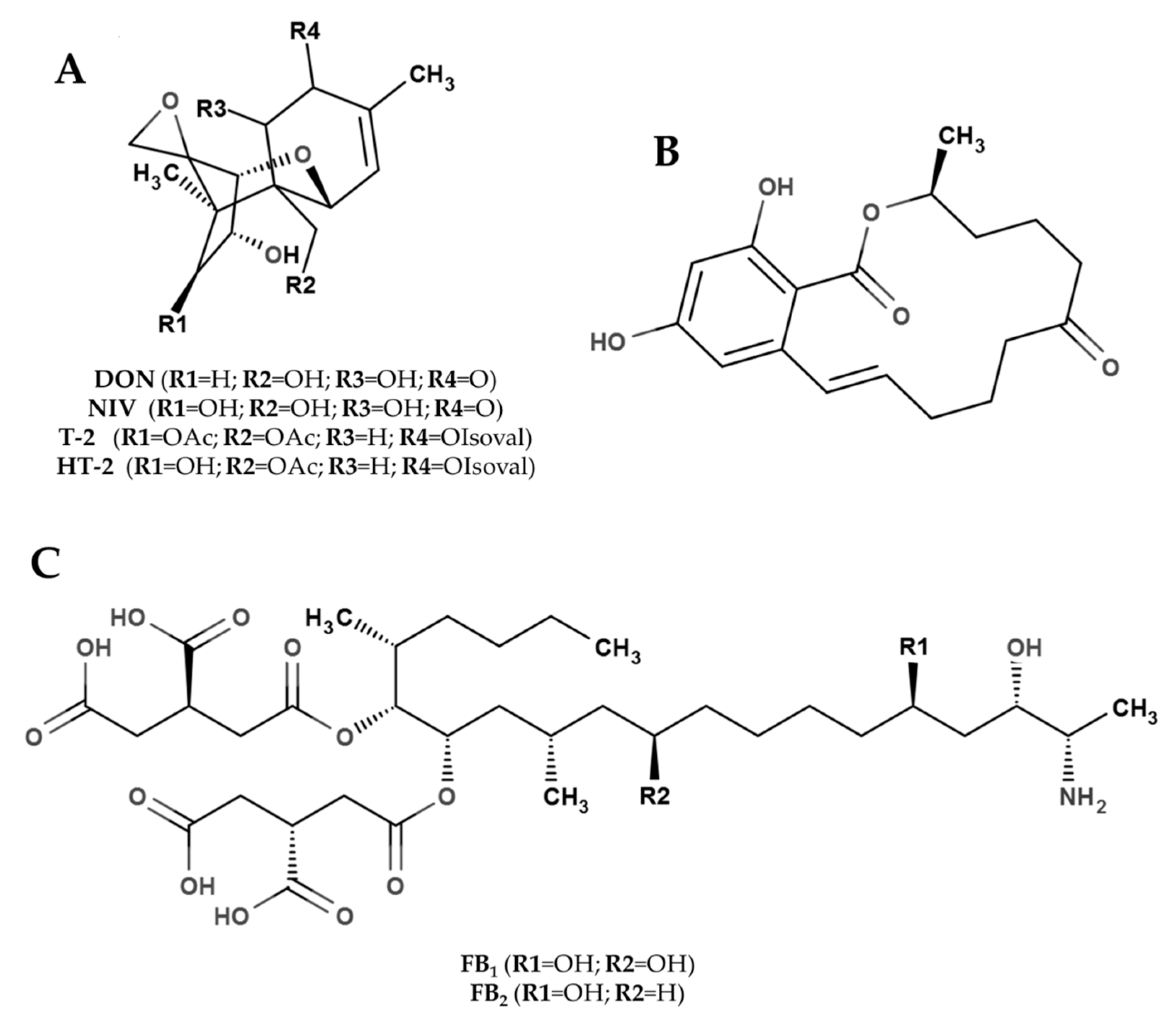
| Country | Cereal | Contamination Range (ppb) | Samples | Incidence (%) | Samples Over Limits | Ref. |
|---|---|---|---|---|---|---|
| Argentina | Maize | n.d.–3600 | 3246 | 1.1 | +(n.a.) | [46] |
| Brazil | Wheat | 183–2150 | 150 | 97 | +(3.3) | [47] |
| Canada | Durum wheat | n.d.–4700 | 54 | 75 | +(n.a.) | [48] |
| China | Maize | 3.3–834.4 | 132 | 77 | - | [49] |
| Wheat | 2.4–1130 | 672 | 91.5 | - | [50] | |
| Croatia | Maize | 215–2942 | 63 | 71 | +(6%) | [51] |
| Wheat | 115–278 | 51 | 65 | - | ||
| Finland | Barley | n.a.–1180 | 34 | 82.4 | - | [52] |
| Oat | n.a.–23,800 | 31 | 100 | +(32%) | ||
| Wheat | n.a.–5510 | 30 | 96.7 | +(23%) | ||
| Italy | Durum wheat | n.d.–14,452 | 240 | 76.5 | +(n.a.) | [53] |
| Maize | 3–428 | 140 | 21.4 | - | [54] | |
| Morocco | Wheat | 121–1480 | 80 | 5 | - | [55] |
| Poland | Maize | n.d.–90 | 30 | 66.6 | - | [56] |
| Sweden | Wheat | n.a.–6460 | 125 | 82 | +(2.4%) | [57] |
| Syria | Wheat | 9–550 | 40 | 22.5 | - | [58] |
| Tanzania | Maize | 68–2196 | 60 | 63 | +(5%) | [59] |
| Country | Cereal | Contamination Range (ppb) | Samples | Incidence (%) | Samples Over Limits | Ref. |
|---|---|---|---|---|---|---|
| Croatia | Maize | 5–42 * | 63 | 57 | - | [51] |
| Wheat | 6–18 * | 51 | 25 | - | ||
| Finland | Barley | n.a–18.1 * | 34 | 20.6 | - | [52] |
| n.a.–39.5 ** | 35.3 | |||||
| Oat | n.a.–548 * | 31 | 61.3 | +(3.2%) | ||
| n.a.–1830 ** | 74.2 | |||||
| Wheat | 1.4–5.4 * | 30 | 46.7 | - | ||
| 3.0–15.9 ** | 63.3 | |||||
| Italy | Durum wheat | n.d.–212 | 340 | 26.5 | +(n.a.) | [53] |
| UK | Oat | n.a.–2321 * | 303 | 84 | +(10%) | [60] |
| n.a.–6480 ** | 79 | |||||
| Tanzania | Maize | 15–25 ** | 60 | 25 | - | [59] |
| Country | Cereal | Contamination Range (ppb) | Samples | Incidence (%) | Samples Over Limits | Ref. | |
|---|---|---|---|---|---|---|---|
| Argentina | Maize | n.d.–10,000 | 3246 | 2.7% | +(n.a.) | [46] | |
| Brazil | Wheat | 20.4–233 | 150 | 32 | +(4%) | [47] | |
| China | Wheat | 1.13–3048 | 180 | 12.8 | +(n.a) | [61] | |
| Croatia | Maize | 10–611 | 63 | 78 | +(6%) | [51] | |
| Wheat | 7–107 | 51 | 69 | - | |||
| Egypt | Maize | 0.8–3.5 | 50 | 70 | - | [62] | |
| Finland | Barley | n.a.–17 | 34 | 5.9 | - | [52] | |
| Oat | n.a.–675 | 31 | 41.9 | +(3.2%) | |||
| Wheat | n.a.–234 | 30 | 46.7 | +(3.3%) | |||
| Italy | Maize | n.d–53 | 140 | 0.7 | - | [54] | |
| Poland | Maize | n.d.–59.9 | 30 | 43.3 | - | [56] | |
| Sweden | Wheat | n.d.–678 | 125 | 46 | +(n.a) | [57] | |
| Syria | Wheat | 4.–34 | 40 | 25 | - | [58] | |
| Tanzania | Maize | 73–1464 | 60 | 5 | +(3.3%) | [59] | |
| Tunisia | Durum wheat | n.d.–560 | 155 | 79.3 | +(23%) | [63] | |
| Country | Cereal | Contamination Range (ppb) | Samples | Incidence (%) | Samples Over Limits | Ref. |
|---|---|---|---|---|---|---|
| Argentina | Durum wheat | 0.15–1304 * | 40 | 77 | - | [64] |
| Maize | n.d.–498,212 | 3246 | 97.6 | +(n.a.) | [46] | |
| Wheat | 0.16–680 * | 135 | 97 | - | [64] | |
| Brazil | Cereal mix | n.d.–1876 * | 105 | 83.8 | +(2%) | [65] |
| Maize | 66–7832 * | 232 | 46.6 | +(n.a.) | [66] | |
| China | Maize | n.d.–22,362 | 146 | 39.7 | +(1.4%) | [67] |
| Wheat products | 0.3–34.6 * | 362 | 6.4 | - | [68] | |
| Croatia | Maize | n.d.–4438 | 63 | 90 | +(1.6%) | [51] |
| Wheat | n.d.–203 | 51 | 39 | - | ||
| Egypt | Maize | 59–1915 * | 20 | 100 | - | [62] |
| Guatemala | Maize | 10–17100 * | 640 | 98 | +(20%) | [69] |
| Italy | Maize | n.d.–21007 | 140 | 97.8 | +(25.6%) | [22] |
| Poland | Maize | 59–1190 * | 30 | 100 | - | [56] |
| Syria | Wheat | n.d.–6 * | 40 | 10 | - | [58] |
| South Africa | Maize | 10–33,260 | 288 | 30 | +(16.6%) | [70] |
| Tanzania | Maize | 16–18184 * | 60 | 73 | +(15%) | [59] |
| Deoxynivalenol in Food [89] | |
| Commodity | Maximum Level (ppb) |
| Unprocessed cereals (excluding durum wheat, oats and maize) | 1250 |
| Unprocessed durum wheat and oats | 1750 |
| Unprocessed maize | 1750 |
| Cereals intended for direct human consumption, cereal flour, bran and germ as end product marketed for direct human consumption | 750 |
| T-2 and HT-2 in Food [93] | |
| Commodity | Maximum Level Sum of T-2 and HT-2 (ppb) |
| Barley (including malting barley) and maize | 200 |
| Oats (with husk) | 1000 |
| Wheat, rye and other cereals | 100 |
| Oats for direct human consumption | 200 |
| Maize for direct human consumption | 100 |
| Other cereals for direct human consumption | 50 |
| Zearalenone in Food [89] | |
| Commodity | Maximum Level (ppb) |
| Unprocessed cereals other than maize | 100 |
| Unprocessed maize | 350 |
| Cereals intended for direct human consumption, cereal flour, bran and germ as end product for direct human consumption | 75 |
| Maize intended for direct human consumption, maize based snacks and maize based breakfast cereals | 100 |
| Fumonisin in Food [89] | |
| Commodity | Maximum Level Sum of B1 and B2 (ppb) |
| Unprocessed maize | 4000 |
| Maize intended for direct human consumption | 1000 |
| Maize based breakfast cereals and maize based snacks (a) | 800 |
| Deoxynivalenol in Feedstuff [155] | |
| Commodity Intended for Animal Feed | Guidance Value (ppm) |
| Cereals and cereal products with the exception of maize by-products | 8 |
| Maize by-products | 12 |
| Complementary and complete feedingstuff | 5 |
| -exception for pigs | 0.9 |
| -exception for calves (<4 months), lambs and kids | 2 |
| T-2 and HT-2 in Feedstuff [156] | |
| Commodity Intended for Animal Feed | Indicative Levels Sum of T-2 and HT-2 (ppm) |
| Oat milling products (husks) | 2 |
| Other cereal products | 0.5 |
| Compound feed, with the exception of feed for cats | 0.25 |
| Zearalenone in Feedstuff [155] | |
| Commodity Intended for Animal Feed | Guidance Value (ppm) |
| Cereals and cereal products with the exception of maize by-products | 2 |
| Maize by-products | 3 |
| Complementary and complete feedingstuff for | |
| -piglets and gilts | 0.1 |
| -sows and fattening pigs | 0.25 |
| -calves, dairy cattle, sheep and goats | 0.5 |
| Fumonisin in Feedstuff [155] | |
| Commodity Intended For Animal Feed | Guidance Value Sum of B1 and B2 (ppm) |
| Maize and maize products | 60 |
| Complementary and complete feedingstuff for | 5 |
| -pigs, horses, rabbits and pet animals -poultry, calves (<4 months), lambs and kids | 20 |
| -adult ruminants (>4 months) and mink | 50 |
| Practice | Small Cereal | Maize | |
|---|---|---|---|
| DON, T-2 and HT-2 | Fumonisin | DON and ZEA | |
| Soil tillage | VH | L | S |
| Crop rotation | VH | L | S |
| Hybrid selection | H | S | VH |
| Planting date | L | H | VH |
| Seed density | L | S | S |
| Weeding | S | L | L |
| Irrigation | L | S | L |
| Balanced fertilization | S | S | S |
| Insecticide treatment | L | VH | L |
| Fungicide treatment | H | L | L |
| Harvest time | S | H | H |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. https://doi.org/10.3390/molecules21050627
Ferrigo D, Raiola A, Causin R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules. 2016; 21(5):627. https://doi.org/10.3390/molecules21050627
Chicago/Turabian StyleFerrigo, Davide, Alessandro Raiola, and Roberto Causin. 2016. "Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management" Molecules 21, no. 5: 627. https://doi.org/10.3390/molecules21050627
APA StyleFerrigo, D., Raiola, A., & Causin, R. (2016). Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules, 21(5), 627. https://doi.org/10.3390/molecules21050627






