Research on the Composition and Distribution of Organic Sulfur in Coal
Abstract
:1. Introduction
2. Results and Discussion
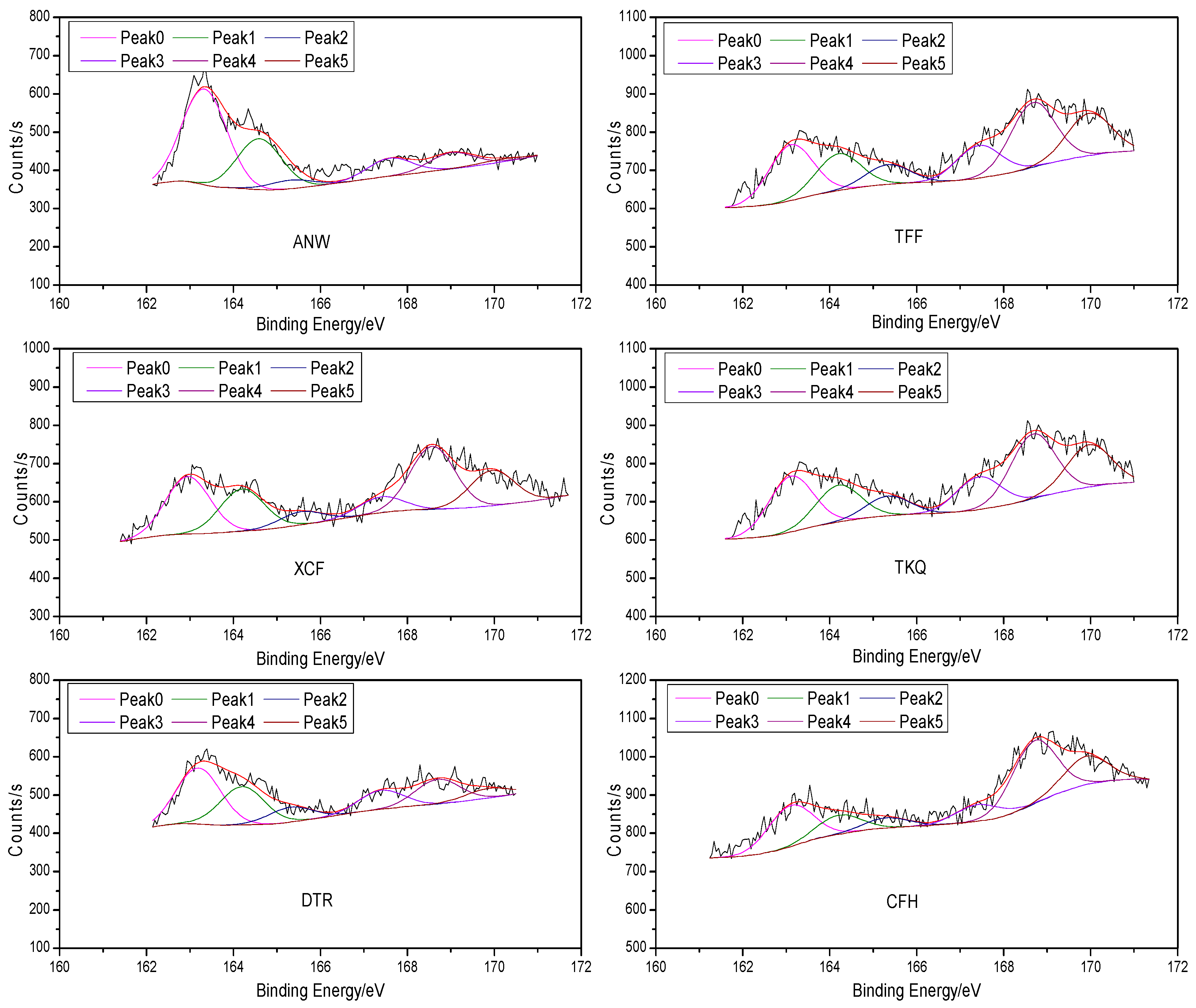
2.1. XPS Test Results and Analysis
2.2. GC/MS Results and Analysis of THF Extract
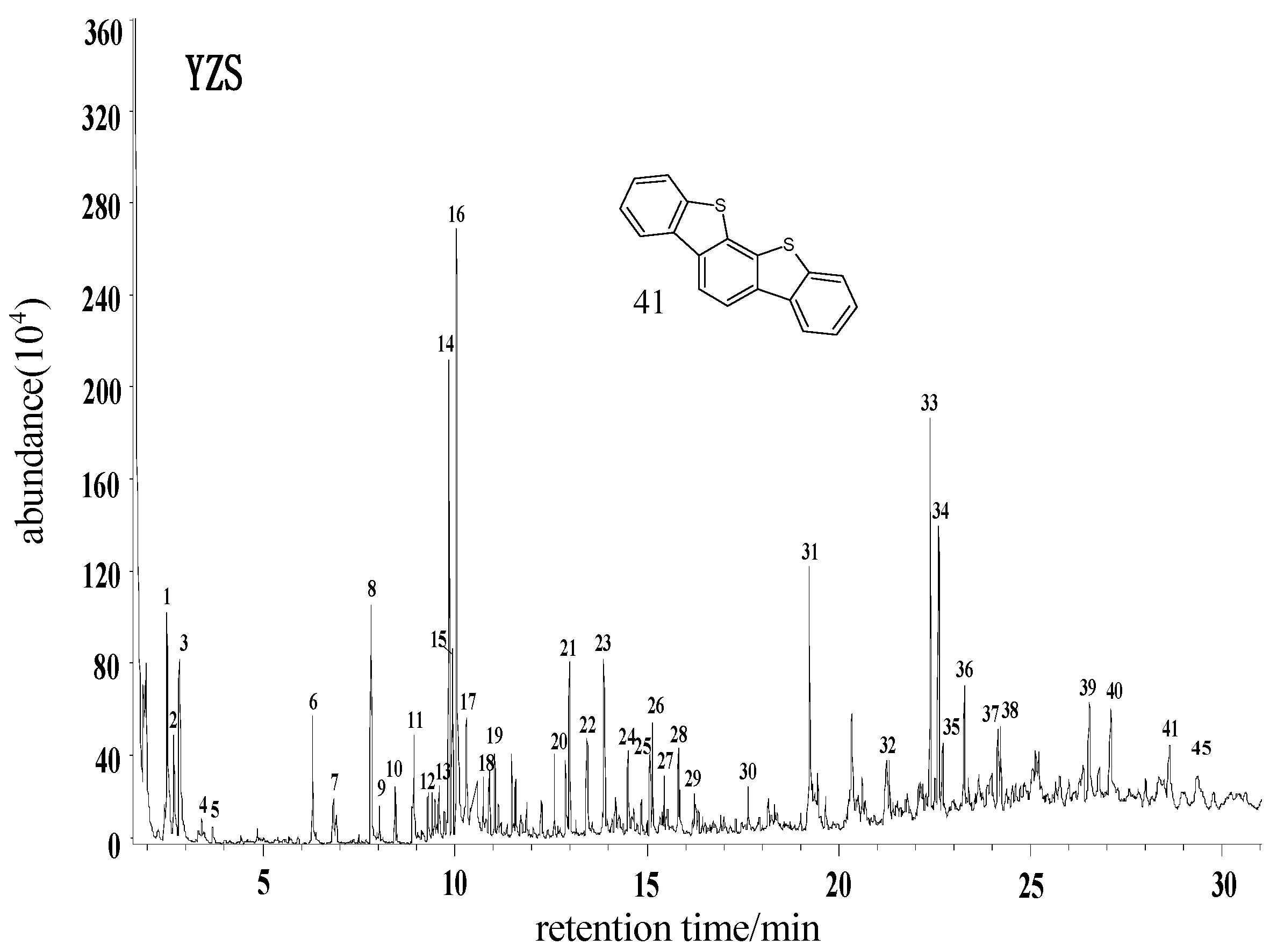
2.2.1. Yangzhuang Lean Coal (YZS) from Huaibei
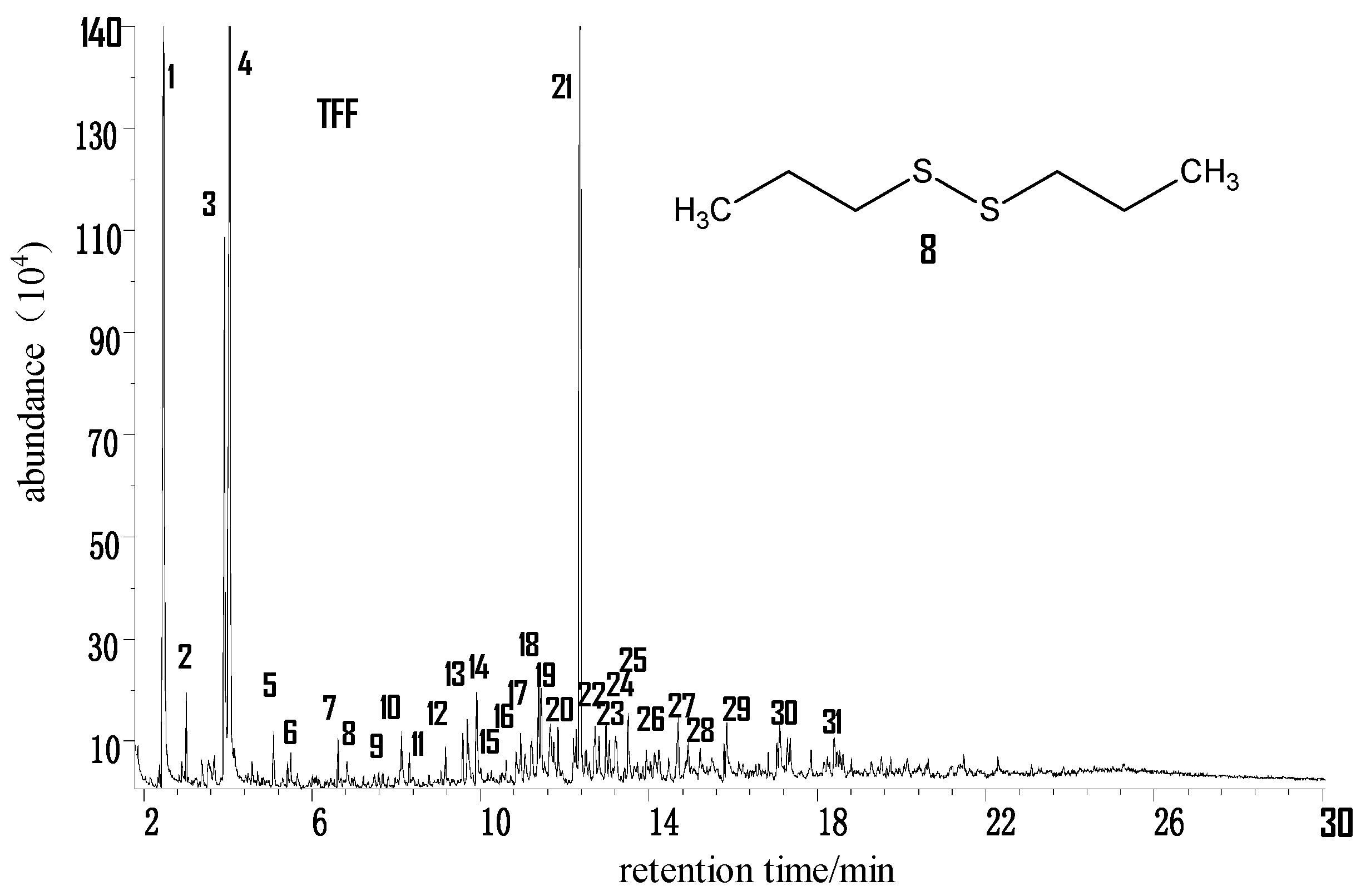
2.2.2. Taifeng Fat Coal (TFF) from Guizhou
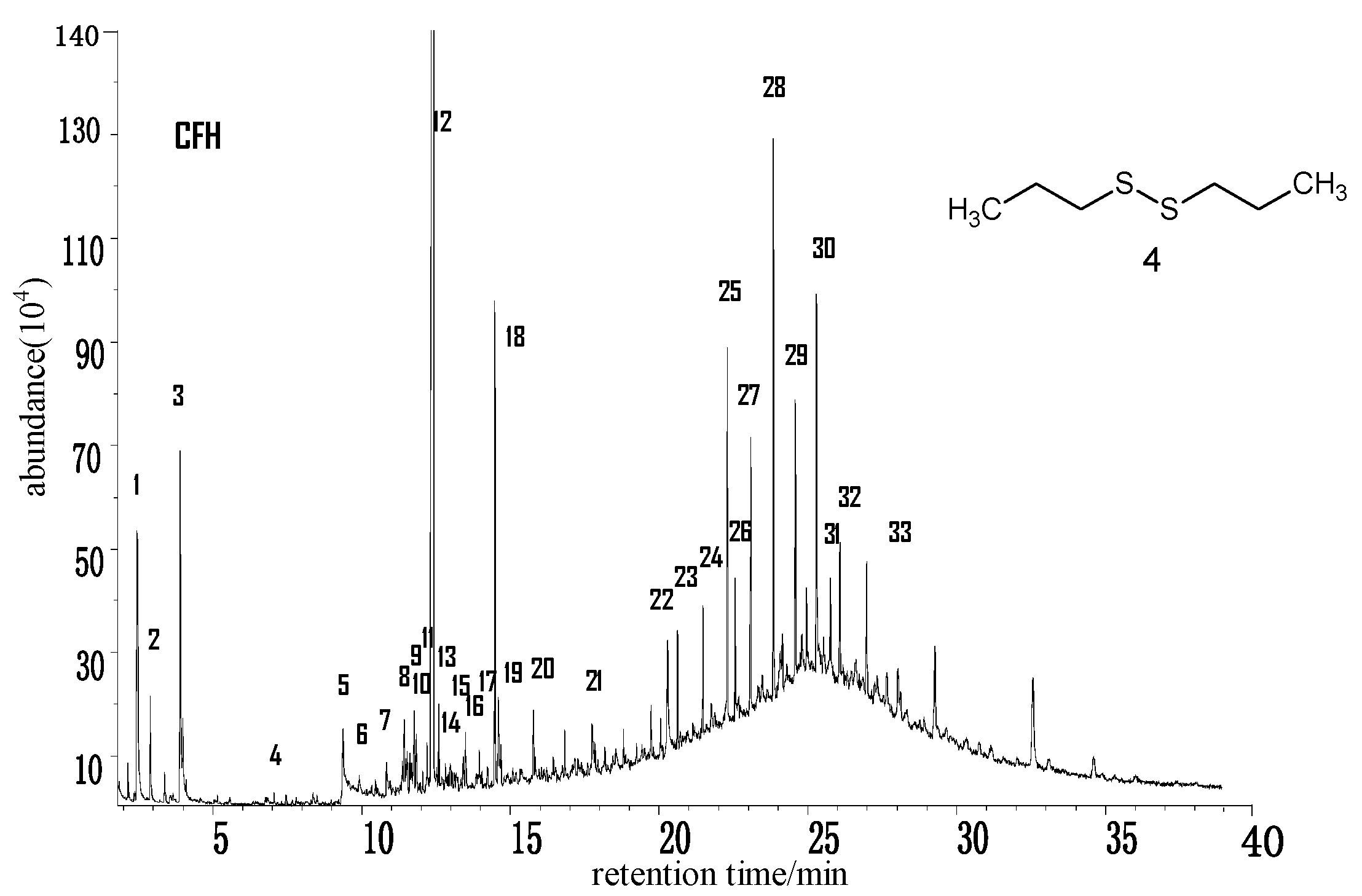
2.2.3. Chifeng Lignite (CFH) from Inner Mongolia
2.3. Discussion
3. Experimental Section
3.1. XPS Experimental Method and Coal Sample Selection
3.2. THF Solvent Extraction Experimental Methods and Procedures
4. Conclusions
- (1)
- Among the organic sulfur in coal samples, the contents of sulfide (mercaptan and thioether) sulfur, thiophenic sulfur and sulfonate are higher, while the contents of sulfoxide and sulfone are lower. The change rule of sulfide sulfur (mercaptan and thioether) and pyrite content with the degree of metamorphosis of coal is not obvious (due to the interference of pyrite); thiophenic sulfur content is reduced with decreasing metamorphic degree of coal; sulfoxide and sulfone content shows no obvious change rule; sulfonate content is raised with the decrease of metamorphic degree; and the content of sulfate sulfur is rarely related with metamorphic degree.
- (2)
- The distribution and content of free organic sulfur small molecules in coal are closely related to the degree of metamorphosis of coal. That is, with a higher metamorphic degree of a coal, the content ratio as well as the kinds of aromatic hydrocarbons in its extracts increase correspondingly, while the relative content of aliphatic hydrocarbons drops; the chemical structure of extracts is simple, with a small number of substituent groups; the content and kinds of heteroatomic compounds are lower. The content of aliphatic hydrocarbon compounds in extracts has a direct link to the metamorphic degree of coal, too. That is, with a lower degree of coal metamorphosis, the ratio of high-carbon number alkanes in the extract increases. The free organic sulfur small molecules in coal of low metamorphic degree are mainly composed of aliphatic sulfides, while those in coals of medium and high metamorphic degree are mainly thiophenes. Besides, the aromatization degree of organic sulfur small molecules rises with rising degree of coalification.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calkins, W.H. The chemical forms of sulfur in coal: A review. Fuel 1994, 73, 475–484. [Google Scholar] [CrossRef]
- Mukherjee, S. Demineralization and desulfurization of high-sulfur Assam coal with alkali treatment. Energy Fuels 2003, 17, 559–564. [Google Scholar] [CrossRef]
- Sugawara, K. Organic sulfur removal from coal by rapid pyrolysis with alkali leaching and density separation. Fuel Energy Abstr. 1996. [Google Scholar] [CrossRef]
- Borah, D.; Baruah, M.K.; Haque, I. Oxidation of high sulphur coal. Part 1. Desulphurisation and evidence of the formation of oxidised organic sulphur species. Fuel 2001, 80, 501–507. [Google Scholar] [CrossRef]
- Attar, A. Chemistry, thermodynamics and kinetics of reactions of sulphur in coal-gas reactions: A review. Fuel 1978, 57, 201–212. [Google Scholar] [CrossRef]
- Kelemen, S.R.; George, G.N.; Gorbaty, M.L. Direct determination and quantification of sulphur forms in heavy petroleum and coals: 1. The X-ray photoelectron spectroscopy (XPS) approach. Fuel 1990, 69, 939–944. [Google Scholar] [CrossRef]
- Oka, N.; Sojar, H.T.; Hamada, N.; Genco, R.J.; Gong, B.; Pigram, P.J.; Lamb, R.N.; Oka, N.; Sojar, H.T.; Hamada, N. Surface studies of low-temperature oxidation of bituminous coal vitrain bands using XPS and SIMS. Fuel 1998, 77, 1081–1087. [Google Scholar]
- Zhang, L.; Li, Z.; Li, J.; Zhou, Y.; Yang, Y.; Tang, Y. Studies on the Low-Temp Oxidation of Coal Containing Organic Sulfur and the Corresponding Model Compounds. Molecules 2015, 20, 22241–22256. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, Z.; Peng, Y.; Yang, Y.; Tang, Y.; Liu, Z. Pore structures and methane sorption characteristics of coal after extraction with tetrahydrofuran. J. Nat. Gas Sci. Eng. 2014, 19, 287–294. [Google Scholar] [CrossRef]
- Acharya, C.; Kar, R.N.; Sukla, L.B. Bacterial removal of sulphur from three different coals. Fuel 2001, 80, 2207–2216. [Google Scholar] [CrossRef]
- Olsson, G.; Pott, B.-M.; Larsson, L.; Holst, O.; Karlsson, H.T. Microbial desulfurization of coal by Thiobacillus ferrooxidans and thermophilic archaea. Fuel Process. Technol. 1994, 40, 277–282. [Google Scholar] [CrossRef]
- Runnion, K.; Combie, J.D. Organic sulfur removal from coal by microorganisms from extreme environments. FEMS Microbiol. Rev. 1993, 11, 139–144. [Google Scholar] [CrossRef]
- Pietrzak, R.; Grzybek, T.; Wachowska, H. XPS study of pyrite-free coals subjected to different oxidizing agents. Fuel 2007, 86, 2616–2624. [Google Scholar] [CrossRef]
- Perry, D.L.; Grint, A. Application of XPS to coal characterization. Fuel 1983, 62, 1024–1033. [Google Scholar] [CrossRef]
- Gong, B.; Buckley, A.; Lamb, R.; Nelson, P. XPS determination of the forms of nitrogen in coal pyrolysis chars. Surf. Interface Anal. 1999, 28, 126–130. [Google Scholar] [CrossRef]
- Gorbaty, M.L.; Kelemen, S.R. Characterization and reactivity of organically bound sulfur and nitrogen fossil fuels. Fuel Process. Technol. 2001, 71, 71–78. [Google Scholar] [CrossRef]
- Marinov, S.P.; Tyuliev, G.; Stefanova, M.; Carleer, R.; Yperman, J. Low rank coals sulphur functionality study by AP-TPR/TPO coupled with MS and potentiometric detection and by XPS. Fuel Process. Technol. 2004, 85, 267–277. [Google Scholar] [CrossRef]
- Palacios, J.M.; Vairavamurthy, A.; Olivella, M.A. A study of sulfur functionalities in fossil fuels using destructive-(ASTM and Py-GC-MS) and non-destructive-(SEM-EDX, XANES and XPS) techniques. Fuel 2002, 81, 405–411. [Google Scholar]
- Sample Availability: Not Available.
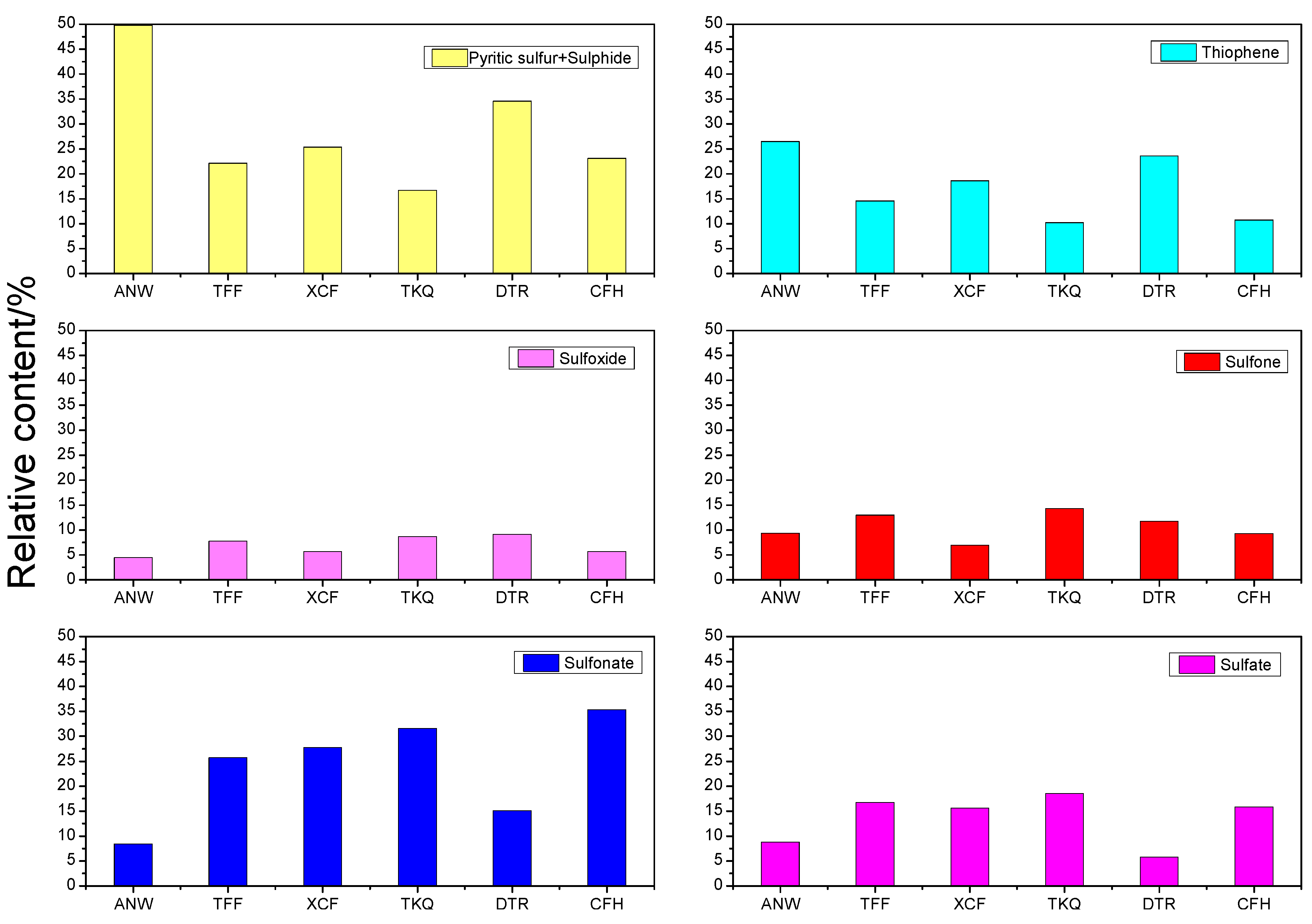
| Sample | Results | Pyritic Sulfur and Sulphide Sulfur | Thiophene | Sulfoxide | Sulfone | Sulfonate | Sulfate |
|---|---|---|---|---|---|---|---|
| ANW | Peak Area | 320.05 | 170.15 | 28.88 | 60.36 | 53.99 | 8.80 |
| W(%) | 49.83 | 26.49 | 4.50 | 9.40 | 8.41 | 1.37 | |
| TFF | Peak Area | 186.07 | 122.45 | 65.42 | 109.03 | 216.06 | 141.01 |
| W(%) | 22.15 | 14.58 | 7.79 | 12.98 | 25.72 | 16.79 | |
| XCF | Peak Area | 191.86 | 141.07 | 43.48 | 52.70 | 210.35 | 118.01 |
| W(%) | 25.33 | 18.62 | 5.74 | 6.96 | 27.77 | 15.58 | |
| TKQ | Peak Area | 76.03 | 46.69 | 39.59 | 65.24 | 144.27 | 84.52 |
| W(%) | 16.66 | 10.23 | 8.68 | 14.30 | 31.61 | 18.52 | |
| DTR | Peak Area | 187.32 | 127.96 | 49.37 | 63.53 | 81.91 | 31.63 |
| W(%) | 34.58 | 23.62 | 9.11 | 11.73 | 15.12 | 5.84 | |
| CFH | Peak Area | 137.34 | 63.72 | 34.08 | 55.09 | 210.18 | 94.24 |
| W(%) | 23.10 | 10.72 | 5.73 | 9.26 | 35.35 | 15.85 |
| Number | Name | Molecular Formula |
|---|---|---|
| 1 | 1,4-Dimethylbenzene | C8H10 |
| 2 | Ethenylbenzene | C8H8 |
| 3 | γ-Butyrolactone | C4H6O2 |
| 4 | 1,2,3-Trimethylbenzene | C9H12 |
| 5 | 1,2,4-Trimethylbenzene | C9H12 |
| 6 | Naphthalene | C10H8 |
| 7 | 1-(1,4-Dimethylcyclohex-3-en-1-yl)ethanone | C10H16O |
| 8 | 1-Methylnaphthalene | C11H10 |
| 9 | 2-Methylnaphthalene | C11H10 |
| 10 | 2,6-Di-tert-butyl-4,4-dimethylcyclohexa-2,5-dien-1-one | C16H26O |
| 11 | Biphenyl | C12H10 |
| 12 | 1,6-Dimethylnaphthalene | C12H12 |
| 13 | Diphenylmethane | C13H12 |
| 15 | 2,6-Di-tert-Butylcyclohexa-2,5-diene-1,4-dione | C14H20O2 |
| 16 | (2aR,4aR,7aS,7bR)-2,2a,4a,7a-Tetramethyl-2a,3,4,4a,6,7,7a,7b-octahydro-5H-cyclopenta[cd]inden-5-one | C15H22O |
| 17 | 4-Methylbiphenyl | C13H12 |
| 19 | 2,6-Di-tert-butyl-4-ethylphenol | C16H26O |
| 19-1 | 9H-Fluorene | C13H10 |
| 19-2 | 4-tert-Butyl-2,6-di(propan-2-yl)phenyl acetate | C18H28O2 |
| 20 | 2,4,6-Tri-tert-butylphenol | C18H30O |
| 21 | 3,5-Di-tert-butylbenzene-1,2-diol | C14H22O2 |
| 22 | 2,6-Di-tert-butyl-4-(hydroxymethyl)phenol | C15H24O2 |
| 23 | Phenanthrene | C14H10 |
| 24 | Butyl 2-methylpropyl benzene-1,2-dicarboxylate | C16H22O4 |
| 25 | 1-Methylphenanthrene | C15H12 |
| 26 | 2-Methylphenanthrene | C15H12 |
| 29 | 2-Phenylnaphthalene | C16H12 |
| 30 | Hexadecanamide | C16H33C18H35NO |
| 31 | Octadec-9-enamide | C18H35NO |
| 31-1 | Triphenylene | C18H12 |
| 32 | 2-Methyltriphenylene | C19H14 |
| 33 | 4,4′-Ethane-1,2-diylbis(2,6-di-tert-butylphenol) | C30H46O2 |
| 34 | Bis(2-ethylhexyl) decanedioate | C26H50O4 |
| 35 | Benzo[pqr]tetraphene | C20H12 |
| 36 | Benzo[j]fluoranthene | C20H12 |
| 37 | 10-Methylbenzo[pqr]tetraphene | C21H14 |
| 38 | 3-Methylperylene | C21H14 |
| 39 | 5,5′,6,6′,7,7′,8,8′-Octahydro-2,2′-binaphthalene | C20H22 |
| 40 | Naphtho[7,8,1,2,3-nopqr]tetraphene | C22H12 |
| 41 | Dibenzo[d,d′]benzo[1,2-b:5,4-b′]bisthiophene | C18H10S2 |
| 42 | Naphtho[1,2,3,4-pqr]tetraphene | C24H14 |
| Number | Name | Molecular Formula |
|---|---|---|
| 1 | Tetrahydro-2-furanol | C4H8O2 |
| 2 | 1,1,3-Trimethylcyclopentane | C8H16 |
| 3 | Dihydrofuran-2(3H)-one | C4H6O2 |
| 4 | Tetrahydro-2-furanmethanol | C5H10O2 |
| 5 | 1,3,5-Trimethylbenzene | C9H12 |
| 6 | 1,2,4-Trimethylbenzene | C9H12 |
| 7 | Undecane | C11H24 |
| 8 | Dipropyl disulfide | C6H14S2 |
| 9 | Naphthalene | C10H8 |
| 10 | Dodecane | C12H26 |
| 11 | Undecane,4,5-dimethyl- | C13H28 |
| 12 | Tridecane | C13H28 |
| 13 | 2-Methylnaphthalene | C11H10 |
| 14 | 1-Methylnaphthalene | C11H10 |
| 15 | 2-Ethenylnaphthalene | C12H10 |
| 16 | Tetradecane | C14H30 |
| 17 | 2-Ethylnaphthalene | C12H12 |
| 18 | 2,3-Dimethylnaphthalene | C12H12 |
| 19 | 1,7-Dimethylnaphthalene | C12H12 |
| 20 | 1,4-Dimethylnaphthalene | C12H12 |
| 21 | 2,6-di-tert-Butyl-4-methylphenol | C15H24O |
| 22 | 2,3,6-Trimethylnaphthalene | C13H14 |
| 23 | 1,6,7-Trimethylnaphthalene | C13H14 |
| 24 | 1,4,6-Trimethylnaphthalene | C13H14 |
| 25 | 1,4,5-Trimethylnaphthalene | C13H14 |
| 26 | Hexadecane | C16H34 |
| 27 | Heptadecane | C17H36 |
| 28 | 2-Methyl-9H-fluorene | C14H12 |
| 29 | Phenanthrene | C14H10 |
| 30 | 1-Methylphenanthrene | C15H12 |
| 31 | 2,5-Dimethylphenanthrene | C16H14 |
| Number | Name | Molecular Formula |
|---|---|---|
| 1 | Tetrahydro-2-furanol | C4H8O2 |
| 2 | 2-Methyl-2-pentenal | C6H10O |
| 3 | Dihydrofuran-2(3H)-one | C4H6O2 |
| 4 | Dipropyl disulfide | C6H14S2 |
| 5 | 3-Methylbenzoic acid | C8H8O2 |
| 6 | 1-Methylnaphthalene | C11H10 |
| 7 | Biphenyl | C12H10 |
| 8 | 2,3-Dimethylnaphthalene | C12H12 |
| 10 | 2,6-Di-tert-butylcyclohexa-2,5-diene-1,4-dione | C14H20O2 |
| 12 | 2,6-Di-tert-butyl-4-methylphenol | C15H24O |
| 13 | 4,4,6,7-Tetramethyl-3,4-dihydronaphthalen-1(2H)-one | C14H18O |
| 14 | 1,4,6-Trimethylnaphthalene | C13H14 |
| 15 | 1-Methyl-7-(propan-2-yl)naphthalene | C14H16 |
| 16 | 1,4,5-Trimethylnaphthalene | C13H14 |
| 17 | 5-tert-Butyl-1,1-dimethyl-2,3-dihydro-1H-indene | C15H22 |
| 18 | 1,6-Dimethyl-4-(propan-2-yl)naphthalene | C15H18 |
| 19 | 1,4-Dimethyl-6-(propan-2-yl)naphthalene | C15H18 |
| 21 | 3,8-Dimethyl-5-(propan-2-yl)naphthalen-2-ol | C15H18O |
| 22 | N-phenylnaphthalen-2-amine | C16H13N |
| 23 | Tricosane | C23H48 |
| 24 | Tetracosane | C24H50 |
| 25 | Pentacosane | C25H52 |
| 26 | 1-(2-{1-[(2-Ethylhexyl)oxy]ethenyl}phenyl)ethenol | C18H26O2 |
| 27 | Hexacosane | C26H54 |
| 28 | Heptacosane | C27H56 |
| 29 | Octacosane | C28H58 |
| 30 | Nonacosane | C29H60 |
| 31 | 1,1′:4′,1′′:4′′,1′′′-Quaterphenyl | C24H18 |
| 32 | Triacontane | C30H62 |
| 33 | Hentriacontane | C31H64 |
| Sample | Proximate Analysis (wt % as Received) | Ultimate Analysis(wt % Daf) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Odaf | Cdaf | Hdaf | Ndaf | |
| ANW | 1.84 | 7.91 | 6.90 | 83.35 | 2.90 | 91.05 | 3.23 | 1.34 |
| TFF | 1.22 | 17.38 | 29.69 | 51.71 | 5.55 | 83.59 | 5.29 | 1.42 |
| XCF | 1.39 | 16.61 | 32.94 | 49.06 | 7.11 | 83.11 | 5.47 | 1.43 |
| TKQ | 2.32 | 9.83 | 34.85 | 53.00 | 10.21 | 82.24 | 5.18 | 1.63 |
| DTR | 2.86 | 7.90 | 21.80 | 67.44 | 8.94 | 85.59 | 4.08 | 0.74 |
| CFH | 15.32 | 13.89 | 31.88 | 38.91 | 18.58 | 74.63 | 4.47 | 0.86 |
| Sample | St.d/% | Sp.d/% | Ss.d/% | So.d/% | |||
|---|---|---|---|---|---|---|---|
| Absolute | Absolute | Relative | Absolute | Relative | Absolute | Relative | |
| ANW | 1.37 | 0.65 | 47.45 | 0.01 | 0.73 | 0.70 | 51.09 |
| TFF | 3.42 | 1.13 | 33.04 | 0.02 | 0.58 | 2.27 | 66.37 |
| XCF | 2.39 | 1.07 | 44.77 | 0.03 | 1.26 | 1.29 | 53.97 |
| TKQ | 0.67 | 0.03 | 4.48 | 0.00 | 0.00 | 0.64 | 95.52 |
| DTR | 0.60 | 0.04 | 6.67 | 0.04 | 6.67 | 0.51 | 85.00 |
| CFH | 1.25 | 0.40 | 32.00 | 0.05 | 4.00 | 0.80 | 64.00 |
| Sample | Proximate Analysis (wt % as Received) | Ultimate Analysis (wt % Daf) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Odaf | Cdaf | Hdaf | Ndaf | |
| YZS | 1.58 | 46.16 | 5.30 | 40.86 | 2.50 | 89.0 | 4.12 | 3.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, Z.; Yang, Y.; Zhou, Y.; Li, J.; Si, L.; Kong, B. Research on the Composition and Distribution of Organic Sulfur in Coal. Molecules 2016, 21, 630. https://doi.org/10.3390/molecules21050630
Zhang L, Li Z, Yang Y, Zhou Y, Li J, Si L, Kong B. Research on the Composition and Distribution of Organic Sulfur in Coal. Molecules. 2016; 21(5):630. https://doi.org/10.3390/molecules21050630
Chicago/Turabian StyleZhang, Lanjun, Zenghua Li, Yongliang Yang, Yinbo Zhou, Jinhu Li, Leilei Si, and Biao Kong. 2016. "Research on the Composition and Distribution of Organic Sulfur in Coal" Molecules 21, no. 5: 630. https://doi.org/10.3390/molecules21050630
APA StyleZhang, L., Li, Z., Yang, Y., Zhou, Y., Li, J., Si, L., & Kong, B. (2016). Research on the Composition and Distribution of Organic Sulfur in Coal. Molecules, 21(5), 630. https://doi.org/10.3390/molecules21050630





