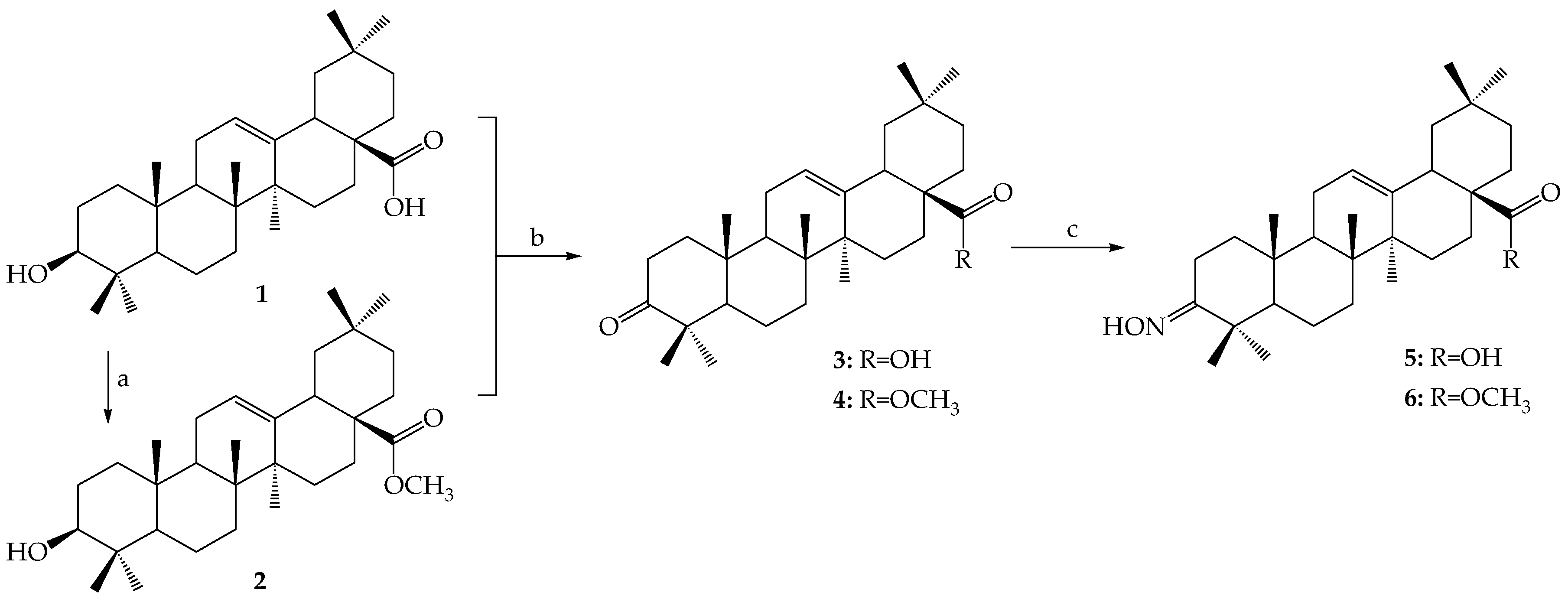
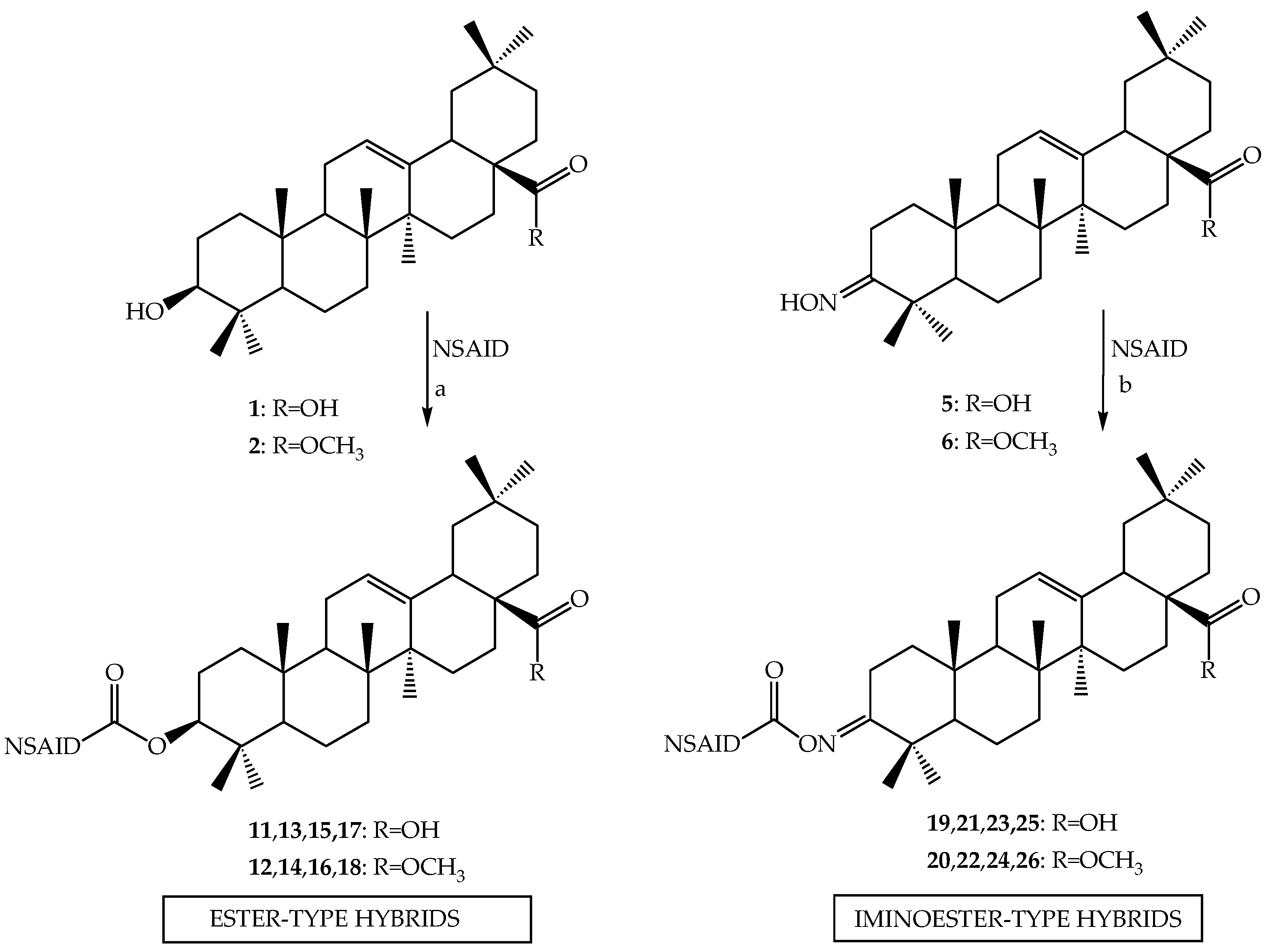
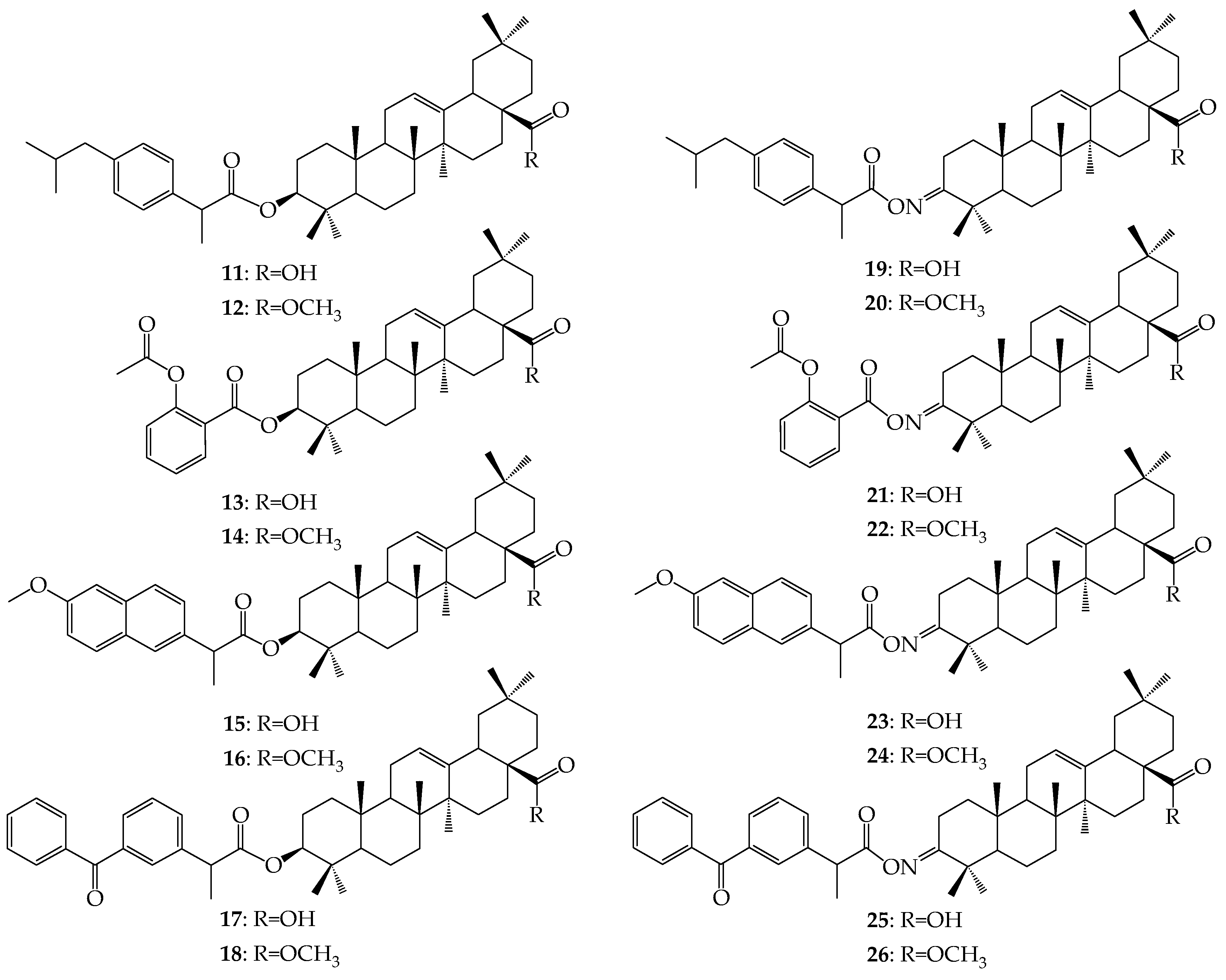
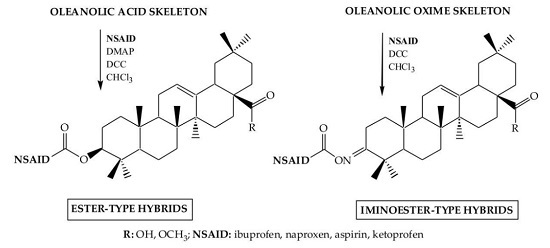
3.2.2. Synthesis of Ester-Type Hybrid Compounds 11–18
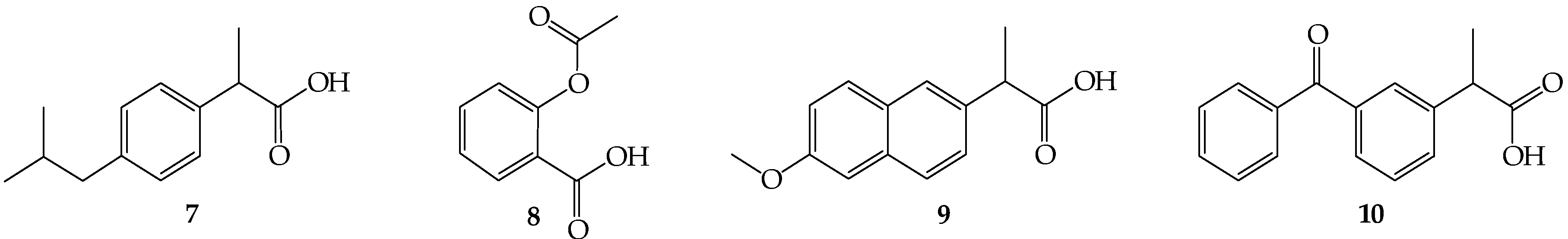
Oleanolic acid (1) or oleanolic acid methyl ester (2) (1 mmol), DCC (1.03 g, 5 mmol), DMAP (0.37 g, 3 mmol) and anti-inflammatory drugs: ibuprofen, aspirin, naproxen, or ketoprofen (4 mmol) were mixed at 0 °C and then stirred at room temperature in dry CHCl3 (12 mL) for 2 h. Hexane (8 mL) was added to the reaction mixture and cooled. Next, the resulting precipitate of dicyclohexylurea was filtered. The organic phase was washed with 5% aqueous hydrochloric acid, 5% aqueous sodium bicarbonate and water and dried over anhydrous sodium sulfate. After removal of the drying agent and solvent evaporation, the crude new esters (11–18) were purified with column chromatography on silica gel using a hexane–ethyl acetate (4:1, v/v) mixture as an eluent.
Oleanoyl ibuprofenate (
11) [
4]: after purification by silica gel column chromatography yield: 0.56 g (87%) of
11 (R
f = 0.48, hexane
/EtOAc 4:1) as colorless solid, mp: 228–230 °C; IR ν
max (KBr, cm
−1) = 3300–3420 (COOH), 1710 (C=O, ester ibu-olean), 1695 (COOH), 1170 (C–O, ester ibu-olean);
1H-NMR (CDCl
3): Oleanoyl moiety: δ = 11.01 (1H, s, COOH), 5.26 (1H, t,
J = 3.4, CH-12), 4.43 (1H, m,
CH
-3), 2.80 (1H, dd,
J = 14.1, 3.4, CH-18), 1.11–0.54 (21H, s, 7 × CH
3); Ibuprofen moiety: δ = 7.20 (2H, d,
J = 8.4, CH-arom), 7.07 (2H, d,
J = 8.0, CH-arom), 3.69 (1H, m, CH), 2.44 (2H, d,
J = 4.0, CH
2), 2.25 (1H, m, CH), 1.49 (3H, d,
J = 7.0, CH
3), 0.87 (3H, s, CH
3), 0.86 (3H, s, CH
3);
13C-NMR (CDCl
3): Oleanoyl moiety: δ = 183.50 (C-28), 143.58 (C-13), 122.53 (C-12), 80.85 (C-3), 55.30 (C-5), 47.60 (C-9), 46.72 (C-17), 45.85 (C-19), 41.72 (C-14), 41.30 (C-18), 39.50 (C-8), 38.10 (C-1), 37.90 (C-4), 36.91 (C-10), 33.88 (C-21), 33.15 (C-29), 32.70 (C-7), 32.52 (C-22), 30.70 (C-20), 29.81 (C-15), 27.96 (C-23), 27.80 (C-2), 25.98 (C-27), 23.66 (C-30), 23.55 (C-16), 23.45 (C-11), 18.20 (C-6), 17.88 (C-26), 16.85 (C-24), 15.30 (C-25); Ibuprofen moiety: δ = 174.35 (C=O ester ibu-olean), 140.39, 137.74, 137.48, 129.25, 127.33, 127.10 (6 × C-arom), 44.93 (CH
2), 41.59 (CHCOO), 27.55 (CH(CH
3)), 22.30, 22.20, 19.60 (3 × CH
3); DEPT: 10 × CH
3, 11 × CH
2, 11 × CH; MS (EI)
m/z = 645.0 [M]
+ (C
43H
64O
4).
Oleanoyl ibuprofenate methyl ester (
12) [
4]: after purification by silica gel column chromatography yield: 0.53 g (80%) of
12 (R
f = 0.85 (hexane
/EtOAc 4:1) as colorless solid, mp:139–141 °C; IR ν
max (KBr, cm
−1) = 1720 (C=O, ester ibu-olean), 1700 (COOCH
3), 1170 (C–O, ester ibu-olean);
1H-NMR (CDCl
3): Oleanoyl moiety: δ = 5.27 (1H, t,
J = 3.4, CH-12), 4.42 (1H, m, CH-3), 3.67 (3H, s, OCH
3), 2.85 (1H, dd,
J = 13.6, 3.3, CH-18), 1.11–0.54 (21H, s, 7 × CH
3); Ibuprofen moiety: δ = 7.20 (2H, d,
J = 8.4, CH-arom), 7.07 (2H, d,
J = 8.0, CH-arom), 3.67 (1H, m, CH), 2.43 (2H, d,
J = 4.0, CH
2), 2.26 (1H, m, CH), 1.50 (3H, d,
J = 7.0, CH
3), 0.87 (3H, s, CH
3), 0.86 (3H, s, CH
3);
13C-NMR (CDCl
3): Oleanoyl moiety: δ = 178.39 (C-28), 143.70 (C-13), 122.39 (C-12), 80.90 (C-3), 55.63 (C-5), 51.59 (COOCH
3), 47.77 (C-9), 46.70 (C-17), 45.87 (C-19), 41.70 (C-14), 41.20 (C-18), 39.51 (C-8), 38.20 (C-1), 37.24 (C-4), 36.70 (C-10), 33.75 (C-21), 33.01 (C-29), 32.75 (C-7), 31.40 (C-22), 30.23 (C-20), 28.45 (C-15), 27.90 (C-23), 27.45 (C-2), 25.80 (C-27), 23.78 (C-30), 23.55 (C-16), 23.48 (C-11), 18.29 (C-6), 17.40 (C-26), 16.90 (C-24), 15.30 (C-25); Ibuprofen moiety: δ = 174.36 (C=O ester ibu-olean), 140.37, 137.73, 137.48, 129.10 127.25 (6 × C-arom), 127.08, 44.92 (CH
2), 41.60 (CHCOO), 27.56 (CH(CH
3)), 22.32, 22.30, 19.29 (3 × CH
3); DEPT: 11 × CH
3, 11 × CH
2, 11 × CH; MS (EI)
m/z = 658.6 [M]
+ (C
44H
66O
4).
Oleanoyl aspirinate (13): after purification by silica gel column chromatography yield: 0.38 g (62%) of 13 (Rf = 0.45, hexane/EtOAc 4:1) as colorless solid, mp: 182–184 °C; IR νmax (KBr, cm−1) = 3350 (COOH), 1710 (C=O, ester asp-olean), 1700 (COOH), 1650 (C=O asp), 1175 (C–O, ester asp-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 11.01 (1H, COOH), 5.28 (1H, t, J = 3.4, CH-12), 4.49 (1H, m, CH-3), 2.86 (1H, dd, J = 14.1, 3.4, CH-18), 1.12–0.56 (21H, s, 7 × CH3); Aspirin moiety: δ = 7.50, 7.43, 7.22, 7.09 (4H, m, CH-arom), 2.33 (3H, s, CH3); 13C-NMR (CDCl3): Oleanoyl fragment: δ = 180.83 (C-28), 143.76 (C-13), 122.30 (C-12), 82.17 (C-3), 52.44 (C-5), 47.67 (C-9), 46.79 (C-17), 45.33 (C-19), 41.97 (C-14), 41.23 (C-18), 39.00 (C-8), 38.11 (C-1), 37.79 (C-4), 36.98 (C-10), 33.81 (C-21), 33.10 (C-29), 32.56 (C-22), 32.12 (C-7), 30.75 (C-20), 27.98 (C-15), 27.69 (C-23), 27.54 (C-2), 25.79 (C-27), 23.60 (C-30), 23.51 (C-16), 23.34 (C-11), 18.20 (C-6), 16.82 (C-26), 16.73 (C-24), 15.36 (C-25); Aspirin moiety: δ = 170.96 (C=O ester aspir-olean), 169.58 (COOCH3), 150.63, 137.33, 130.38, 125.80, 121.92, 116.13 (6 × Carom), 21.12 (CH3); DEPT: 8 × CH3, 10 × CH2, 9 × CH; MS (EI) m/z = 618. 9 [M]+ (C39H54O6).
Oleanoyl aspirinate methyl ester (14): after purification by silica gel column chromatography yield: 0.45 g (72%) of 14 (Rf = 0.65 (hexane/EtOAc 4:1) as colorless solid, mp: 223–226 °C; IR νmax (KBr, cm−1) = 1725 (C=O, ester asp-olean), 1710 (COOCH3), 1660 (C=O asp), 1175 (C–O, ester asp-olean); 1H-NMR (CDCl3): Oleanoyl moiety: 5.28 (1H, t, J = 3.4, CH-12), 4.48 (1H, m, CH-3), 3.62 (3H, s, COOCH3), 2.85 (1H, dd, J = 13.6, 4.04, CH-18), 1.12–0.58 (21H, s, 7 × CH3); Aspirin moiety: δ = 7.52, 7.31, 7.21, 7.00 (4H, m, CH-arom), 2.06 (3H, s, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.31 (C-28), 143.78 (C-13), 122.25 (C-12), 80.91 (C-3), 55.27 (C-5), 51.52 (COOCH3), 47.52 (C-9), 46.71 (C-17), 45.81 (C-19), 41.60 (C-14), 41.26 (C-18), 39.25 (C-8), 38.07 (C-1), 37.66 (C-4), 36.90 (C-10), 33.82 (C-21), 33.08 (C-29), 32.56 (C-7), 31.36 (C-22), 30.68 (C-20), 28.00 (C-15), 27.65 (C-23), 27.52 (C-2), 25.88 (C-27), 23.62 (C-30), 23.50 (C-16), 23.38 (C-11), 18.19 (C-6), 16.81 (C-26), 16.67 (C-24), 15.34 (C-25); Aspirin moiety: δ = 170.31 (C=O ester asp-olean), 170.04 (COOCH3), 150.04, 137.92, 131.38, 127.80, 121.99, 117.03 (6 × C-arom), 21.15 (CH3); DEPT: 9 × CH3, 10 × CH2, 9 × CH; MS (EI) m/z = 632.9 [M]+ (C40H56O6).
Oleanoyl naproxenate (
15) [
4]: after purification by silica gel column chromatography yield: 0.58 g (89%) of
15 (R
f = 0.44, hexane
/EtOAc 4:1) as colorless solid, mp: 257–258 °C; IR ν
max (KBr, cm
−1) = 3300–3420 (COOH), 1725 (C=O, ester napx-olean), 1690 (COOH), 1175 (C–O, ester napx-olean);
1H-NMR (CDCl
3): Oleanoyl moiety: δ = 10.95 (1H, COOH), 5.25 (1H, t,
J = 3.4, CH-12), 4.47 (1H, m, CH-3), 2.80 (1H, dd,
J = 13.6, 4.0, CH-18), 1.11–0.57 (21H, s, 7 × CH
3); Naproxen moiety: δ = 7.70 (1H, d,
J = 8.8, CH-arom), 7.68 (1H, s, CH-arom), 7.64 (1H, d,
J = 8.8, CH-arom), 7.42 (1H, d,
J = 8.4, CH-arom), 7.40 (1H, d,
J = 8.4, CH-arom), 7.14 (1H, d,
J = 11, CH-arom), 3.91 (3H, s, OCH
3), 3,84 (1H, m, CH), 1.57 (3H, d,
J = 7.0, CH
3);
13C-NMR (CDCl
3): Oleanoyl moiety: δ = 183.24 (C-28), 143.53 (C-13), 122.51 (C-12), 81.06 (C-3), 55.26 (C-5), 47.46 (C-9), 46.49 (C-17), 45.79 (C-19), 41.56 (C-14), 40.95 (C-18), 39.21 (C-8), 37.98 (C-1), 37.84 (C-4), 36.89 (C-10), 33.76 (C-21), 33.02 (C-29), 32.48 (C-7), 32.39 (C-22), 30.63 (C-20), 29.72 (C-15), 27.68 (C-23), 27.61 (C-2), 25.85 (C-27), 23.53 (C-30), 23.49 (C-16), 23.35 (C-11), 18.17 (C-6), 17.99 (C-26), 16.52 (C-24), 15.27 (C-25); Naproxen moiety: δ = 174.25 (C=O ester napx-olean), 157.51, 136.02, 133.60, 129.23, 128.90, 127.46, 126.38, 125.99, 118.85, 105.56 (10 × C-arom), 55.3 (OCH
3), 45.9 (CH), 17.04 (CH
3); DEPT: 9 × CH
3, 10 × CH
2, 12 × CH; MS (EI)
m/z = 668.5 [M]
+ (C
44H
60O
5).
Oleanoyl naproxenate methyl ester (
16) [
4]: after purification by silica gel column chromatography yield: 0.53 g (78%) of
16 (R
f = 0.60, hexane
/EtOAc 4:1) as colorless solid, mp: 158–160 °C; IR ν
max (KBr, cm
−1) = 1725 (C=O, ester napx-olean), 1700 (COOCH
3), 1185 (C–O, ester napx-olean);
1H-NMR (CDCl
3): Oleanoyl moiety: δ = 5.26 (1H, t,
J = 3.6, CH-12), 4.46 (1H, m, CH-3), 3.61 (3H, s, COOCH
3), 2.84 (1H, dd,
J = 13.6, 4.0, CH-18), 1.10–0.56 (21H, s, 7 × CH
3); Naproxen moiety: δ = 7.70 (1H, d,
J = 8.8, CH-arom), 7.68 (1H, s, CH-arom), 7.67 (1H, d,
J = 8.8, CH-arom), 7.42 (1H, d,
J = 8.4, CH-arom), 7.40 (1H, d,
J = 8.4, CH-arom), 7.14 (1H, d,
J = 11, CH-arom), 3.91 (3H, s, OCH
3), 3.83 (1H, m, CH), 1.58 (3H, d,
J = 7.0, CH
3);
13C-NMR (CDCl
3): Oleanoyl moiety: δ = 178.30 (C-28), 143.77 (C-13), 122.23 (C-12), 81.04 (C-3), 55.27 (C-5), 51.51 (COOCH
3), 47.46 (C-9), 46.69 (C-17), 45.96 (C-19), 41.59 (C-14), 41.25 (C-18), 39.22 (C-8), 38.00 (C-1), 37.84 (C-4), 36.86 (C-10), 33.82 (C-21), 33.08 (C-29), 32.51 (C-7), 32.35 (C-22), 30.67 (C-20), 29.69 (C-15), 27.67 (C-23), 27.63 (C-2), 25.87 (C-27), 23.62 (C-30), 23.50 (C-16), 23.39 (C-11), 18.15 (C-6), 16.78 (C-26), 16.54 (C-24), 15.27 (C-25); Naproxen moiety: δ = 174.25 (C=O ester napx-olean), 157.49, 136.02, 133.58, 129.24, 128.88, 126.94, 126.39, 125.98, 118.84, 105.53 (10 × C-arom), 55.20 (OCH
3), 45.80 (CH), 18.04 (CH
3); DEPT: 10 × CH
3, 10 × CH
2, 12 × CH; MS (EI)
m/z = 682.7 [M]
+ (C
45H
62O
5).
Oleanoyl ketoprofenate (17): after purification by silica gel column chromatography yield: 0.27 g (79%) of 17 (Rf = 0.32, hexane/EtOAc 4:1) as colorless resin (with a tendency to crystallize); IR νmax (KBr, cm−1) = 3300–3420 (COOH), 1725 (C=O, ester ketopr-olean), 1690 (COOH), 1175 (C–O, ester ketopr-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 10.95 (1H, COOH), 5.27 (1H, t, J = 3.4, CH-12), 4.47 (1H, m, CH-3), 2.80 (1H, dd, J = 13.6, 3.6, CH-18), 1.21–0.61 (21H, s 7 × CH3); Ketoprofen moiety: δ = 7.80 (1H, d, J = 8.0, CH-arom), 7.73 (1H, s, CH-arom), 7.64 (1H, d, J = 7.2, CH-arom), 7.58 (1H, d, J = 6.8, CH-arom), 7.57 (1H, d, J = 6.0, CH-arom), 7.53 (1H, d, J = 6.4, CH-arom), 7.49 (1H, d, J = 7.6, CH-arom), 7.47 (1H, d, J = 8.8, CH-arom), 7.43 (1H, d, J = 7.6, CH-arom), 3.78 (2H, m, CH), 1.59 (3H, d, J = 7.2, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.28 (C-28), 143.79 (C-13), 122.22 (C-12), 81.34 (C-3), 55.22 (C-5), 47.50 (C-9), 46.70 (C-17), 45.95 (C-19), 41.61 (C-18), 41.28 (C-14), 39.26 (C-8), 37.85 (C-1), 37.74 (C-4), 36.62 (C-10), 33.84 (C-21), 33.08 (C-29), 32.54 (C-7), 32.36 (C-22), 30.66 (C-20), 28.43 (C-15), 27.72 (C-23), 27.65 (C-2), 25.88 (C-27), 23.47 (C-30), 23.38 (C-16), 23.05 (C-11), 18.09 (C-6), 18.03 (C-26), 16.62 (C-24), 16.53 (C-25); Ketoprofen moiety: δ = 196.57 (C=O), 173.57 (C=O, ester ketopr-olean), 141.22, 140.95, 137.88, 137.57, 130.00, 129.26, 128.83, 128.44, 128.39, 128.28 (12 × C-arom), 45.83 (CH), 15.27 (CH3); DEPT: 8 × CH3, 10 × CH2, 15 × CH; MS (EI) m/z = 692.9 [M]+ (C46H60O5).
Oleanoyl ketoprofenate methyl ester (18): after purification by silica gel column chromatography yield: 0.31 g (88%) of 18 (Rf = 0.50, hexane/EtOAc 4:1) as colorless resin (with a tendency to crystallize); IR νmax (KBr, cm−1) = 1725 (C=O, ester ketopr-olean), 1700 (COOCH3), 1185 (C–O, ester ketopr-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 5.27 (1H, t, J = 3.6, CH-12), 4.46 (1H, m, CH-3), 3.62 (3H, s, COOCH3), 2.81 (1H, dd, J = 13.6, 3.6, CH-18), 1.21–0.61 (21H, s 7 × CH3); Ketoprofen moiety: δ = 7.80 (1H, d, J = 8.0, CH-arom), 7.73 (1H, s, CH-arom), 7.64 (1H, d, J = 7.2, CH-arom), 7.58 (1H, d, J = 6.8, CH-arom), 7.57 (1H, d, J = 6.0, CH-arom), 7.53 (1H, d, J = 6.4, CH-arom), 7.49 (1H, d, J = 7.6, CH-arom), 7.47 (1H, d, J = 8.8, CH-arom), 7.43 (1H, d, J = 7.6, CH-arom), 3.78 (2H, m, CH), 1.59 (3H, d, J = 7.2, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.28 (C-28), 143.79 (C-13), 122.22 (C-12), 81.34 (C-3), 55.22 (C-5), 51.49 (COOCH3), 47.50 (C-9), 46.70 (C-17), 45.95 (C-19), 41.61 (C-18), 41.28 (C-14), 39.26 (C-8), 37.85 (C-1), 37.74 (C-4), 36.62 (C-10), 33.84 (C-21), 33.08 (C-29), 32.54 (C-7), 32.36 (C-22), 30.66 (C-20), 28.43 (C-15), 27.72 (C-23), 27.65 (C-2), 25.88 (C-27), 23.47 (C-30), 23.38 (C-16), 23.05 (C-11), 18.09 (C-6), 18.03 (C-26), 16.62 (C-24), 16.53 (C-25); Ketoprofen moiety: δ = 196.57 (C=O), 173.57 (C=O, ester ketopr-olean), 141.22, 140.95, 137.88, 137.57, 130.00, 129.26, 128.83, 128.44, 128.39, 128.28 (12 × C-arom), 45.83 (CH), 15.27 (CH3); DEPT: 9 × CH3, 10 × CH2, 15 × CH; MS (EI) m/z = 706.9 [M]+ (C47H62O5).
3.2.3. Synthesis of Iminoester-Type Hybrid Compounds 19–26
Oleanolic acid oxime (5) or its methyl ester (6) (1 mmol), DCC (1.03 g, 5 mmol) and anti-inflammatory drugs: ibuprofen, aspirin, naproxen, or ketoprofen (4 mmol), were mixed at 0 °C and then stirred at room temperature in dry CHCl3 (12 mL) for 2 h. Hexane (8 mL) was added to the reaction mixture and cooled. Next, the resulting precipitate of dicyclohexylurea was filtered. The organic phase was washed with 5% aqueous hydrochloric acid, 5% aqueous sodium bicarbonate and water and dried over anhydrous sodium sulfate. After removal of the drying agent and solvent evaporation, the crude new iminoesters 19–26 were purified with column chromatography on silica gel using hexane–ethyl acetate (4:1, v/v) mixture as a eluent.
Oleanoyl oxime ibuprofenate (19): after purification by silica gel column chromatography yield: 0.46 g (74%) of 19 (Rf = 0.45; hexane/EtOAc 4:1) as colorless solid, mp: 163–165 °C; IR νmax (KBr, cm−1) = 3300–3420 (COOH), 1715 (C=O, iminoester ibu-olean), 1695 (COOH), 1170 (C–O, iminoester ibu-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 11.17 (1H, COOH), 5.53 (1H, t, J = 3.4, CH-12), 2.84 (1H, dd, J = 13.4, 3.4, CH-18), 1.35–0.72 (21H, s, 7 × CH3); Ibuprofen moiety: δ = 7.18 (2H, d, J = 8.4, CH-arom), 7.08 (2H, d, J = 8.0, CH-arom), 3.85 (1H, m, CH), 2.43 (2H, d, J = 7.0, CH2), 2.25 (1H, m, CH), 1.55 (3H, d, J = 7.0, CH3), 0.89 (3H, s, CH3), 0.88 (3H, s CH3); 13C-NMR (CDCl3): δ = Oleanoyl moiety: δ = 178.30 (C-28), 172.60 (C-3), 143.53 (C-13), 126.60 (C-12), 55.30 (C-5), 49.50 (C-9), 47.61 (C-17), 45.89 (C-19), 41.74 (C-14), 41.30 (C-18), 39.58 (C-8), 38.10 (C-1), 37.92 (C-4), 36.92 (C-10), 33.15 (C-29), 32.54 (C-22), 33.86 (C-21), 32.72 (C-7), 30.73 (C-20), 29.82 (C-15), 27.95 (C-23), 27.80 (C-2), 25.95 (C-27), 23.65 (C-30), 23.58 (C-16), 23.43 (C-11), 18.20 (C-6), 17.80 (C-26), 16.86 (C-24), 15.30 (C-25); Ibuprofen moiety: δ = 174.76 (C=O iminoester ibu-olean), 140.52, 138.40, 137.48, 128.98, 126.88, 126.69 (6 × C-arom), 44.59 (CH2), 40.18 (CHCOO), 27.56 (CH(CH3)), 22.32 , 18.58, 15.09 (3 × CH3); DEPT: 10 × CH3, 11 × CH2, 10 × CH; MS (EI) m/z = 657.6 [M]+ (C43H63NO4).
Oleanoyl oxime ibuprofenate methyl ester (20): after purification by silica gel column chromatography yield: 0.51 g (77%) of 20 (Rf = 0.67, hexane/EtOAc 4:1) as colorless resin; IR νmax (KBr, cm−1) = 1710 (C=O, iminoester ibu-olean), 1170 (C–O, iminoester ibu-olean); 1700 (COOCH3); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 5.28 (1H, t, J = 3.4, CH-12), 3.62 (3H, s, OCH3), 2.84 (1H, dd, J = 13.7, 3.4, CH-18), 1.49–0.72 (21H, s, 7 × CH3); Ibuprofen moiety: δ = 7.23 (2H, d, J = 8.0, CH-arom), 7.08 (2H, d, J = 8.0, CH-arom), 3.85 (1H, m, CH), 2.45 (2H, d, J = 7.0, CH2), 2.25 (1H, m, CH), 1.55 (3H, d, J = 7.0, CH3), 0.88 (3H, s, CH3), 0.87 (3H, s, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.30 (C-28), 172.44 (C-3), 143.90 (C-13), 122.30 (C-12), 55.30 (C-5), 51.59 (COOCH3), 49.51 (C-9), 47.65 (C-17), 45.90 (C-19), 41.72 (C-14), 40.19 (C-18), 39.58 (C-8), 38.19 (C-1), 37.90 (C-4), 36.69 (C-10), 33.10 (C-29), 32.51 (C-22), 33.81 (C-21), 32.78 (C-7), 30.27 (C-20), 29.68 (C-15), 27.99 (C-23), 27.58 (C-2), 25.69 (C-27), 23.86 (C-30), 23.59 (C-16), 23.45 (C-11), 18.29 (C-6), 17.38 (C-26), 16.88 (C-24), 15.39 (C-25); Ibuprofen moiety: δ = 175.78 (C=O iminoester ibu-olean), 140.56, 137.62, 137.48, 129.22, 127.35, 127.10, 44.50 (CH2), 40.19 (CHCOO), 27.52 (CH(CH3)), 22.30, 18.59, 15.20 (3 × CH3); DEPT: 11 × CH3, 11 × CH2, 10 × CH; MS (EI) m/z = 671.6 [M]+ (C44H65NO4).
Oleanoyl oxime aspirinate (21): after purification by silica gel column chromatography yield: 0.39 g (62%) of 21 (Rf = 0.39, hexane/EtOAc 4:1) as colorless solid, mp: 104–107 °C; IR νmax (KBr, cm−1) = 3350 (COOH), 1750 (C=O iminoester asp-olean), 1710 (COOH), 1695 (C=O asp.), 1175 (C–O, iminoester asp-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 10.80 (1H, COOH), 5.32 (1H, t, J = 3.4, CH-12), 3.31 (1H, m, CH-18), 1.33–0.83 (21H, s, 7 × CH3); Aspirin moiety: δ = 7.50, 7.43, 7.22, 7.10 (4H, m, CH-arom), 2.35 (3H, s, CH3). 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.26 (C-28), 176.40 (C-3), 143.96 (C-13), 122.13 (C-12), 55.39 (C-5), 47.63 (C-9), 46.76 (C-17), 45.83 (C-19), 41.75 (C-14), 41.39 (C-18), 40.95 (C-8), 38.19 (C-1), 37.99 (C-4), 36.98 (C-10), 33.11 (C-29), 32.55 (C-22), 33.82 (C-21), 32.71 (C-7), 30.72 (C-20), 29.89 (C-15), 27.92 (C-23), 27.80 (C-2), 25.90 (C-27), 23.69 (C-30), 23.58 (C-16), 23.45 (C-11), 18.29 (C-6), 17.89 (C-26), 16.81 (C-24), 15.32 (C-25); Aspirin moiety: δ = 171.12 (C=O iminoester asp-olean), 165.54 (COOCH3), 154.04, 131.38, 129.58, 127.79, 125.92, 121.90 (6 × C-arom), 21.30 (CH3); DEPT: 8 × CH3, 10 × CH2, 8 × CH; MS (EI) m/z = 631. 9 [M]+ (C39H53NO6).
Oleanoyl oxime aspirinate methyl ester (22): after purification by silica gel columnchromatography yield: 0.42 g (67%) of 22 (Rf = 0.47, hexane/EtOAc 4:1) as colorless solid, mp: 179–180 °C; IR νmax (KBr, cm−1) = 1750 (C=O asp), 1710 (COOCH3), 1650 (C=O asp), 1175 (C-O iminoester asp-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 5.28 (1H, t, J = 3.4, CH-12), 3.63 (3H, s, COOCH3), 2.99 (1H, dd, J = 13.7, 3.4, CH-18), 1.20–0.77 (21H, s, 7 × CH3); Aspirin moiety: δ = 7.97, 7.57, 7.32, 7.14 (4H, m, CH-arom), 2.35 (3H, s, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.26 (C-28), 176.40 (C-3), 143.96 (C-13), 122.13 (C-12), 55.78 (C-5), 51.59 (COOCH3), 47.14 (C-9), 46.72 (C-17), 45.79 (C-19), 41.73 (C-14), 41.61 (C-18), 41.32 (C-8), 39.29 (C-1), 38.74 (C-4), 36.99 (C-10), 33.82 (C-21), 33.09 (C-29), 32.32 (C-22), 31.67 (C-7), 30.68 (C-20), 30.36 (C-15), 27.64 (C-23), 27.12 (C-2), 26.07 (CH3), 25.85 (C-27), 24.32 (C-30), 23.61 (C-16), 23.45 (C-11), 19.88 (C-6), 18.96 (C-26), 16.80 (C-24), 15.11 (C-25); Aspirin moiety: δ = 171.12 (C=O iminoester asp-olean), 165.54 (COOCH3), 154.04, 131.39, 129.60, 127.80, 125.97, 121.99 (6 × Carom), 21.08 (CH3); DEPT: 9 × CH3, 10 × CH2, 8 × CH; MS (EI) m/z = 645. 9 [M]+ (C40H55NO6).
Oleanoyl oxime naproxenate (23): after purification by silica gel column chromatography yield: 0.55 g (81%) of 23 (Rf = 0.36, hexane/EtOAc 4:1) as colorless solid, mp: 160–162 °C; IR νmax (KBr, cm−1) = 3300–3420 (COOH), 1725 (C=O, iminoester napx-olean), 1690 (COOH), 1175 (C–O, iminoester napx-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 9.78 (1H, COOH), 5.59 (1H, t, J = 3.4, CH-12), 2.50 (1H, dd, J = 13.6, 4.0, CH-18), 1.32–0.57 (21H, s, 7 × CH3); Naproxen moiety: δ = 7.70 (1H, d, J = 8.8, CH-arom), 7.68 (1H, s, CH-arom), 7.64 (1H, d, J = 8.8, CH-arom), 7.38 (2H, dd, J = 8.4, 8.4 CH-arom), 7.15 (1H, d, J = 11, CH-arom), 3.91 (3H, s, OCH3), 3.85 (1H, m, CH), 1.52 (3H, d, J = 7.0, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.25 (C-28), 175.60 (C-3), 143.5 (C-13), 125.65 (C-12), 55.68 (C-5), 49.92 (C-9), 46.51 (C-17), 45.83 (C-19), 41.55 (C-14), 41.05 (C-18), 39.26 (C-8), 38.02 (C-1), 37.87 (C-4), 36.94 (C-10), 33.80 (C-21), 33.14 (C-29), 32.66 (C-7), 32.38 (C-22), 30.44 (C-20), 29.10 (C-15), 26.28 (C-23), 26.12 (C-2), 25.38 (C-27), 24.60 (C-30), 23.89 (C-16), 23.39 (C-11), 18.03 (C-6), 17.10 (C-26), 16.56 (C-24), 15.32 (C-25); Naproxen moiety: δ = 174.18 (C=O iminoester napx-olean), 154.06, 136.69, 133.51, 129.12, 128.92, 127.46, 125.99, 125.65, 119.07, 105.52 (10 × C-arom), 55.28 (OCH3); 45.52 (CH), 18.12 (CH3); DEPT: 9 × CH3, 10 × CH2, 11 × CH; MS (EI) m/z = 681.9 [M]+ (C44H59NO5).
Oleanoyl oxime naproxenate methyl ester (24): after purification by silica gel column chromatography yield: 0.52 g (75%) of 24 (Rf = 0.51, hexane/EtOAc 4:1) as colorless solid, mp: 110–112 °C; IR νmax (KBr, cm−1) = 1710 (C=O, iminoester ibu-olean), 1695 (COOCH3), 1170 (C–O, iminoester ibu-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 5.27 (1H, t, J = 3.6, CH-12), 3.62 (3H, s, OCH3), 2.86 (1H, dd, J = 13.6, 4.2, CH-18), 1.23–0.73 (21H, s, 7 × CH3); Naproxen moiety: δ = 7.70 (1H, d, J = 8.8, CH-arom), 7.69 (1H, s, CH-arom), 7.42 (1H, d, J = 8.4, CH-arom), 7.39 (1H, d, J = 8.4, CH-arom), 7.14 (1H, d, J = 11, CH-arom), 3.91 (3H, s, OCH3), 4.04 (1H, m, CH), 1.59 (3H, d, J = 7.0, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.26 (C-28), 172.51 (C-3), 143.93 (C-13), 122.23 (C-12), 55.77 (C-5), 51.59 (COOCH3), 49.91 (C-17), 46.69 (C-9), 45.79 (C-19), 41.45 (C-14), 41.30 (C-18), 39.24 (C-8), 38.60 (C-1), 37.90 (C-4), 36.9 (C-10), 33.80 (C-29), 33.08 (C-22), 32.90 (C-21), 32.42 (C-7), 32.31 (C-23), 31.16 (C-20), 30.68 (C-15), 27.60 (C-2), 26.93 (C-27), 25.31 (C-30), 23.60 (C-16), 22.99 (C-11), 18.87 (C-6), 18.76 (C-26), 16.76 (C-24), 15.01 (C-25); Naproxen moiety: δ = 175.73 (C=O iminoester napx-olean), 157.59, 136.74, 133.64, 129.16, 128.96, 127.49, 126.38, 125.99, 118.85, 105.58 (10 × C-arom), 55.30 (OCH3), 45.59 (CH), 18.75 (CH3); DEPT: 10 × CH3, 10 × CH2, 11 × CH; MS (EI) m/z = 695.9 [M]+ (C45H61NO5).
Oleanoyl oxime ketoprofenate (25): after purification by silica gel column chromatography yield: 0.26 g (73%) of 25 (Rf = 0.18; hexane/EtOAc 4:1) as colorless resin; IR νmax (KBr, cm−1) = 3300–3420 (COOH), 1715 (C=O, iminoester ketopr-olean), 1695 (COOH), 1170 (C–O, iminoester ketopr-olean); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 11.05 (1H, COOH), 5.28 (1H, s, CH-12), 2.84 (1H, dd, J = 13.4, 3.4, CH-18), 1.25–0.74 (21H, s, 7 × CH3); Ketoprofen moiety: δ = 7.80 (1H, d, J = 8.0, CH-arom.), 7.75 (1H, s, CH-arom.), 7.64 (1H, d, J = 7.2, CH-arom.), 7.58 (1H, d, J = 6.8, CH-arom.), 7.57 (1H, d, J = 6.0, CH-arom.), 7.53 (1H, d, J = 6.4, CH-arom.), 7.49 (1H, d, J = 7.6, CH-arom), 7.47 (1H, d, J = 8.8, CH-arom.), 7.43 (1H, d, J = 7.6, CH-arom.), 3.75 (2H, m, CH), 1.60 (3H, d, J = 7.2, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.25 (C-28), 171.94 (C-3), 143.90 (C-13), 122.00 (C-12), 55.77 (C-5), 47.07 (C-9) 46.72 (C-17), 44.46 (C-19), 41.70 (C-18), 41.32 (C-14), 39.27 (C-8), 38.66 (C-1), 38.61 (C-4), 36.93 (C-10), 33.83 (C-21), 33.08 (C-29), 32.61 (C-7), 32.32 (C-22), 30.68 (C-20), 27.62 (C-23), 26.96 (C-15), 25.83 (C-2), 24.67 (C-27), 23.62 (C-30), 23.42 (C-16), 23.02 (C-11), 19.44 (C-6), 18.41 (C-26), 16.78 (C-24), 15.15 (C-25); Ketoprofen moiety: δ = 196.46 (C=O), 175.93 (C=O, ester ketopr-olean), 140.66, 140.59, 137.89, 137.48, 132.49, 131.59, 130.04, 129.35, 129.21, 128.99, 128.52, 128.31 (12 × C-arom), 45.52 (CH), 15.05 (CH3); DEPT: 8 × CH3, 10 × CH2, 14 × CH; MS (EI) m/z = 705.9 [M]+ (C46H59NO5).
Oleanoyl oxime ketoprofenate methyl ester (26): after purification by silica gel column chromatography yield: 0.30 g (84%) of 26 (Rf = 0.38, hexane/EtOAc 4:1) as colorless resin; IR νmax (KBr, cm−1) = 1710 (C=O, iminoester ketopr-olean), 1170 (C–O, iminoester ketopr-olean); 1700 (COOCH3); 1H-NMR (CDCl3): Oleanoyl moiety: δ = 5.28 (1H, s, CH-12), 3.62 (3H, s, OCH3), 2.84 (1H, dd, J = 13.4, 3.3, CH-18), 1.27–0.74 (21H, s, 7 × CH3); Ketoprofen moiety: δ = 7.80 (1H, d, J = 8.0, CH-arom.), 7.75 (1H, s, CH-arom.), 7.64 (1H, d, J = 7.2, CH-arom.), 7.58 (1H, d, J = 6.8, CH-arom.), 7.57 (1H, d, J = 6.0, CH-arom.), 7.53 (1H, d, J = 6.4, CH-arom.), 7.49 (1H, d, J = 7.6, CH-arom), 7.47 (1H, d, J = 8.8, CH-arom.), 7.43 (1H, d, J = 7.6, CH-arom.), 3.75 (2H, m, CH), 1.60 (3H, d, J = 7.2, CH3); 13C-NMR (CDCl3): Oleanoyl moiety: δ = 178.25 (C-28), 171.84 (C-3), 143.92 (C-13), 122.02 (C-12), 55.77 (C-5), 51.52 (COOCH3), 47.07 (C-9) 46.72 (C-17), 44.46 (C-19), 41.70 (C-18), 41.32 (C-14), 39.27 (C-8), 38.66 (C-1), 38.61 (C-4), 36.93 (C-10), 33.83 (C-21), 33.08 (C-29), 32.61 (C-7), 32.32 (C-22), 30.68 (C-20), 27.62 (C-23), 26.96 (C-15), 25.83 (C-2), 24.67 (C-27), 23.62 (C-30), 23.42 (C-16), 23.02 (C-11), 19.44 (C-6), 18.41 (C-26), 16.78 (C-24), 15.05 (C-25); Ketoprofen moiety: δ = 196.46 (C=O), 175.93 (C=O, ester ketopr-olean), 140.66, 140.59, 137.89, 137.48, 132.49, 131.59, 130.04, 129.35, 129.21, 128.99, 128.52, 128.31 (12 × C-arom), 45.82 (CH), 15.02 (CH3); DEPT: 9 × CH3, 10 × CH2, 14 × CH; MS (EI) m/z = 719.9 [M]+ (C47H61NO5).