Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effect of Solvent Type on Extraction Efficiency
2.2. Effect of Fruit Chilling Prior to Extraction
2.3. Effect of Extract Storage on Stability of TPC and Antioxidant Potential
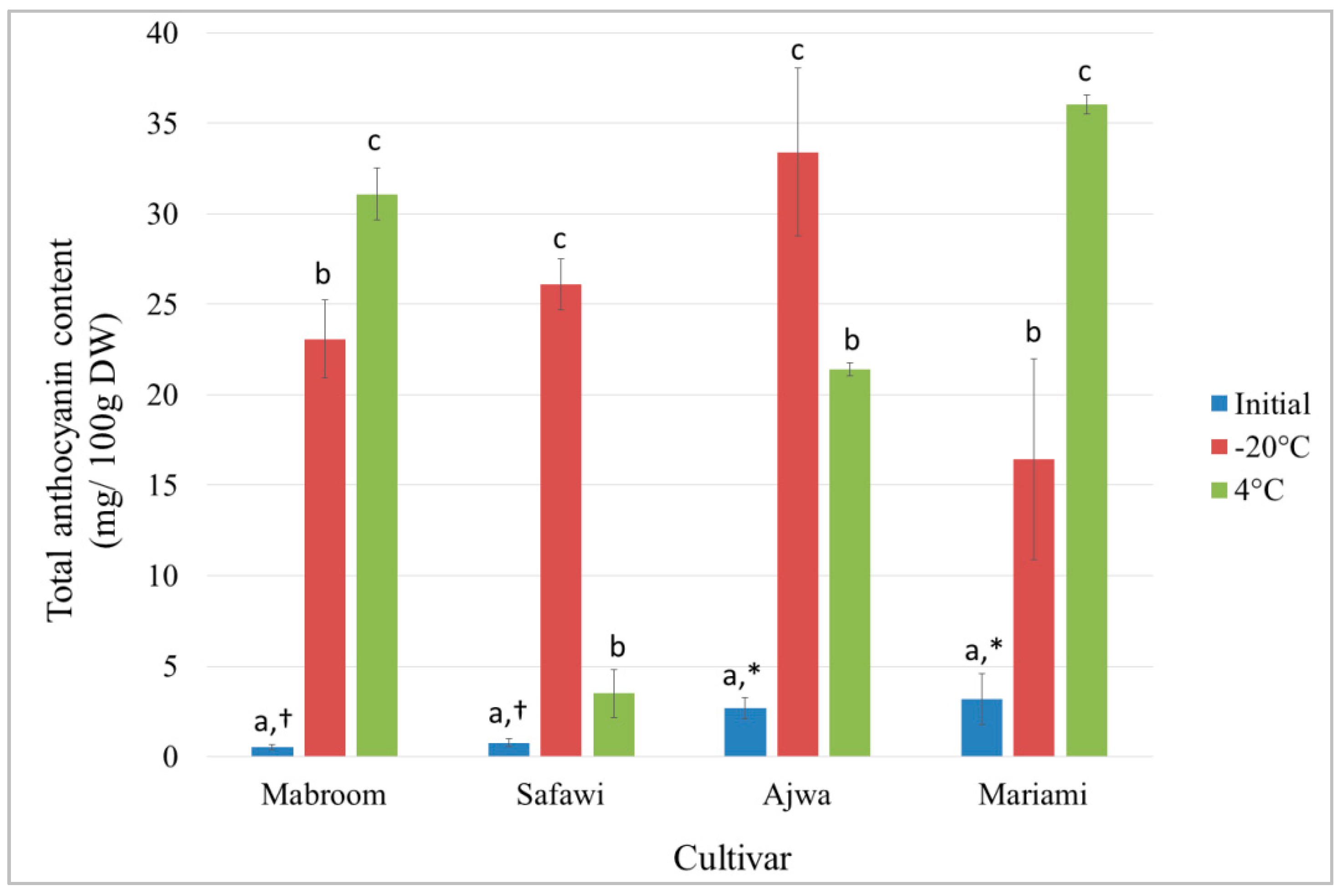
2.4. Total Anthocyanin Content (TAC) and Effect of Extract Storage on Anthocyanin Stability
2.5. Antibacterial Activity
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Quantification of Bioactive Compounds
3.2.1. Determination of Total Phenolic Content (TPC)
3.2.2. DPPH Radical Scavenging Activity
3.2.3. Determination of Total Anthocyanin Content (TAC)
3.3. Determination of Antibacterial Activity
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Treatment (Storage Temperature) | pH | |||
|---|---|---|---|---|
| Mabroom | Safawi | Ajwa | Mariami | |
| Initial | 6.40 | 6.32 | 6.38 | 6.46 |
| −20 °C | 5.86 | 5.75 | 5.83 | 5.96 |
| 4 °C | 1.69 | 1.76 | 1.59 | 1.62 |
References
- Hadrami, I.E.; Hadrami, A.E. Breeding Date palm. In Breeding Plantation Tree Crops: Tropical Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer New York: New York, NY, USA, 2009; pp. 191–216. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Crop Production and Trade Data; Available online: http://faostat.fao.org/site/339/default.aspx (accessed on 15 January 2016).
- Eid, N.M.S.; Al-Awadi, B.; Vauzour, D.; Oruna-Concha, M.J.; Spencer, J.P.E. Effect of cultivar type and ripening on the polyphenol content of date palm fruit. J. Agric. Food Chem. 2013, 61, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Amira, E.; Behija, S.E.; Beligh, M.; Lamia, L.; Manel, I.; Mohamed, H.; Lotfi, A. Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. J. Agric. Food Chem. 2012, 60, 10896–10902. [Google Scholar] [CrossRef] [PubMed]
- Al-Daihan, S.; Bhat, R.S. Antibacterial activities of extracts of leaf, fruit, seed and bark of Phoenix dactylifera. Afr. J. Biotechnol. 2012, 11, 10021–10025. [Google Scholar] [CrossRef]
- Jassim, S.A.A.; Naji, M.A. In vitro evaluation of the antiviral activity of an extract of date palm (Phoenix dactylifera L.) pits on a pseudomonas phage. Evid.-Based Complement. Altern. Med. 2010, 7, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Pehrsson, E.C.; Blaser, M.J.; Sandhu, K.; Gao, Z.; Wang, B.; Magris, M.; Hidalgo, G.; Contreras, M.; Noya-Alarcon, O.; et al. The microbiome of uncontacted Amerindians. Sci. Adv. 2015, 1, e1500183–e1500183. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Sadek, P.C. The HPLC Solvent Guide; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Shams Ardekani, M.R.; Khanavi, M.; Hajimahmoodi, M.; Jahangiri, M.; Hadjiakhoondi, A. Comparison of antioxidant activity and total phenol contents of some date seed varieties from Iran. Iran. J. Pharm. Res. 2010, 9, 141–146. [Google Scholar] [PubMed]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Anokwuru, C.P.; Anyasor, G.N.; Ajibaye, O.; Fakoya, O.; Okebugwu, P. Effect of extraction solvents on phenolic, flavonoid and antioxidant activities of three nigerian medicinal plants. Nat. Sci. 2011, 9, 53–61. [Google Scholar]
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007, 102, 1233–1240. [Google Scholar] [CrossRef]
- Koffi, E.; Sea, T.; Dodehe, Y.; Soro, S. Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. J. Anim. Plant Sci. 2010, 5, 550–558. [Google Scholar]
- Kallithraka, S.; Garciaviguera, C.; Bridle, P.; Bakker, J. Survey of solvents for the extraction of grape seed phenolics. Phytochem. Anal. 1995, 6, 265–267. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Kajdzanoska, M.; Petreska, J.; Stefova, M. Comparison of different extraction solvent mixtures for characterization of phenolic compounds in strawberries. J. Agric. Food Chem. 2011, 59, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Hislop, E.C.; Hoad, G.V.; Archer, S.A. Fungal Pathogenicity and the Plant’s Response; Cutting, C.V., Ed.; Academic Press: Cambridge, MA, USA, 1973; pp. 87–117. [Google Scholar]
- Tauber, H.; Petit, E.L. Convenient methods for preparing crystalline catalase from cow liver. J. Biol. Chem. 1952, 195, 703–706. [Google Scholar] [PubMed]
- Hong, Y.J.; Tomas-Barberan, F.A.; Kader, A.A.; Mitchell, A.E. The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix dactylifera). J. Agric. Food Chem. 2006, 54, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J. Nutr. Sci. 2014, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.J.; Oldoni, T.L.C.; Alencar, S.M.D.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kchaou, W.; Abbes, F.; Attia, H.; Besbes, S. In vitro antioxidant activities of three selected dates from Tunisia (Phoenix dactylifera L.). J. Chem. 2014. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef] [PubMed]
- Regnaultroger, C.; Hadidane, R.; Biard, J.F.; Boukef, K. High-performance liquid and thin-layer chromatographic determination of phenolic-acids in palm (Phoenix, Dactilifera) products. Food Chem. 1987, 25, 61–71. [Google Scholar] [CrossRef]
- Ritenour, M.A.; Ahrens, M.J.; Saltveit, M.E. Effects of temperature on ethylene-induced phenylalanine ammonia-lyase activity and russet spotting in harvested iceberg lettuce. J. Am. Soc. Hortic. Sci. 1995, 120, 84–87. [Google Scholar]
- Ritenour, M.A.; Saltveit, M.E. Identification of a phenylalanine ammonia-lyase inactivating factor in harvested head lettuce (Lactuca sativa). Physiol. Plant. 1996, 97, 327–331. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, M.W.; Kang, Y.H. Effects of ethylene and Ca2+ on activity of phenylalanine ammonia-lyase in glucan-treated Daucus carota. J. Plant Biol. 1994, 37, 263–269. [Google Scholar]
- MartinezTellez, M.A.; Lafuente, M.T. Effect of high temperature conditioning on ethylene, phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase activities in flavedo of chilled ‘Fortune’ mandarin fruit. J. Plant Physiol. 1997, 150, 674–678. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Maier, V.P.; Metzler, D.M. Phenolic constituents of the date (Phoenix dactylifera) and their relation to browning. First Int. Congr. Food Sci. Technol. 1964. [Google Scholar]
- Stewart, R.J.; Sawyer, B.J.B.; Bucheli, C.S.; Robinson, S.P. Polyphenol oxidase is induced by chilling and wounding in pineapple. Funct. Plant Biol. 2001, 28, 181–191. [Google Scholar] [CrossRef]
- He, J. Isolation of Anthocyanin Mixtures from Fruits and Vegetables and Evaluation of Their Stability, Availability and Biotransformation in the Gastrointestinal Tract. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2008. [Google Scholar]
- Shen, W.; Nada, K.; Tachibana, S. Effect of cold treatment on enzymic and nonenzymic antioxidant activities in leaves of chilling-tolerant and chilling-sensitive cucumber (Cucumis sativus L.) cultivars. J. Jpn. Soc. Hortic. Sci. 1999, 68, 967–973. [Google Scholar] [CrossRef]
- Wise, R.R.; Naylor, A.W. Chilling-enhanced photooxidation—Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987, 83, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, W.; Abbes, F.; Blecker, C.; Attia, H.; Besbes, S. Effects of extraction solvents on phenolic contents and antioxidant activities of Tunisian date varieties (Phoenix dactylifera L.). Ind. Crops Prod. 2013, 45, 262–269. [Google Scholar] [CrossRef]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flores, S.H.; Rios, A.D.; de Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014, 33, 111–116. [Google Scholar] [CrossRef]
- Kondo, S.; Kittikorn, M.; Kanlayanarat, S. Preharvest antioxidant activities of tropical fruit and the effect of low temperature storage on antioxidants and jasmonates. Postharvest Biol. Technol. 2005, 36, 309–318. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Koyama, H.; Tawata, S. Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. Food Chem. 2007, 104, 1648–1653. [Google Scholar]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Ahmadkhaniha, R. Effect of frozen storage on the anthocyanins and phenolic components of pomegranate juice. J. Food Sci. Technol. Mysore 2014, 51, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, I.; Malecka, M.; Szlachta, M.; Gliszczynska-Swiglo, A. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Hawken, P.; Talcott, S.T. Phytochemical, antioxidant and pigment stability of ACAI (Euterpe oleracea Mart.) as affected by clarification, ascorbic acid fortification and storage. Food Res. Int. 2007, 40, 620–628. [Google Scholar] [CrossRef]
- Liolios, C.C.; Sotiroudis, G.T.; Chinou, I. Fatty acids, sterols, phenols and antioxidant activity of phoenix theophrasti fruits growing in Crete, Greece. Plant Foods Hum. Nutr. 2008, 64, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.S.; Lohmann, D.H.; Russell, K.E. The kinetics of reaction of 2,2-diphenyl-1-picrylhydrazyl with phenols. Can. J. Chem. 1961, 39, 1588–1594. [Google Scholar] [CrossRef]
- Miller, N.J.; Diplock, A.T.; Rice-Evans, C.A. Evaluation of the total antioxidant activity as a marker of the deterioration of apple juice on storage. J. Agric. Food Chem. 1995, 43, 1794–1801. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purees. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- Lohachoompol, V.; Mulholland, M.; Srzednicki, G.; Craske, J. Determination of anthocyanins in various cultivars of highbush and rabbiteye blueberries. Food Chem. 2008, 111, 249–254. [Google Scholar] [CrossRef]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.; Hager, T.J.; Hager, A.; Howard, L. Dietary black raspberry anthocyanins do not alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Cabrita, L.; Andersen, O.M. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chem. 1998, 63, 435–440. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Nutritional and functional properties of dates: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Embarek, G.; Kokkalou, E.; Kefalas, P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem. 2005, 89, 411–420. [Google Scholar] [CrossRef]
- Francis, F.J. Food colorants—Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Winterhalter, P. A novel synthetic route to substituted pyranoanthocyanins with unique colour properties. Tetrahedron Lett. 2003, 44, 7583–7587. [Google Scholar] [CrossRef]
- Al-Seeni, M.N. Minerals content and antimicrobial efficacy of date extracts against some pathogenic bacteria. Life Sci. J. Acta Zhengzhou Univ. Overseas Ed. 2012, 9, 504–508. [Google Scholar]
- Bhat, R.S.; Al-Daihan, S. Antibacterial properties of different cultivars of Phoenix dactylifera L. and their corresponding protein content. Ann. Biol. Res. 2012, 3, 4751–4757. [Google Scholar]
- Shakiba, M.; Kariminik, A.; Parsia, P. Antimicrobial activity of different parts of Phoenix dactylifera. Int. J. Mol. Clin. Microbiol. 2011, 1, 107–111. [Google Scholar]
- Riaz, M.; Kumar, V.; Mansoury, E.; Al-Kandari, F.; Al-Kandari, E.; Al-Attar, E.; Al-Ameer, F. Pink rot of inflorscence: A new disease of date palm in Kuwait. Mycopathologia 2009, 7, 1–4. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 2001, 49, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Gawron-Gzella, A.; Dudek-Makuch, M.; Matlawska, I. DPPH radical scavenging activity and phenolic compound content in different leaf extracts from selected blackberry species. Acta Biol. Crac. Ser. Bot. 2012, 54, 32–38. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). Biomed. Res. Int. 2015, 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A. Thin-layer densitometry as an alternative tool in the quantitative evaluation of the free radical scavenging activity of natural antioxidants. Z. Naturforschung Sect. B J. Chem. Sci. 2005, 60, 1212–1218. [Google Scholar]
- Lachman, J.; Orsák, M.; Pivec, V. Antioxidant contents and composition in some vegetables and their role in human nutrition. Zahrad. (Hortic. Sci.) 2000, 27, 103–117. [Google Scholar]
- Ponmozhi, P.; Geeta, M.; Saravana Kumar, M.; Suganya Devi, P. Extraction of anthocyanin and analyzing its antioxidant properties from Pithecellobium Dulce fruit pericarp. Asian J. Pharm. Clin. Res. 2011, 4, 41–45. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

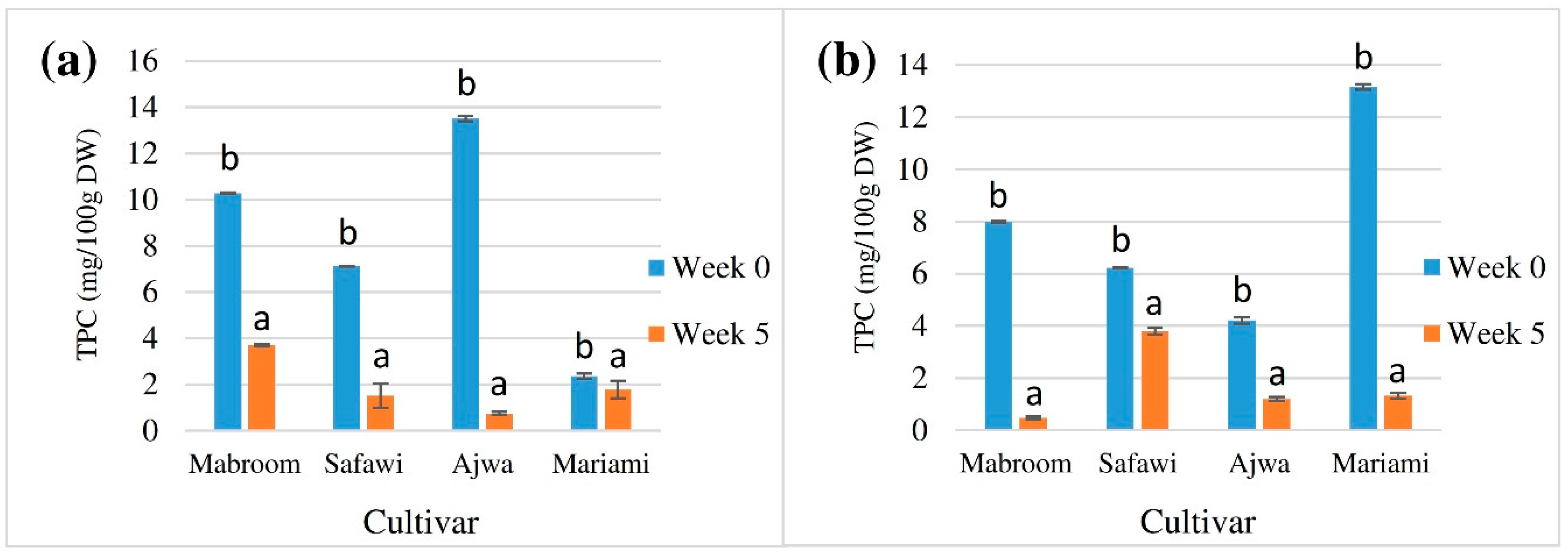
| Cultivar | Solvent | TPC (mg GAE/ 100 g DW) | |
|---|---|---|---|
| Week 0 | Week 8 | ||
| Mabroom | Methanol | 16.48 ± 0.07 b | 10.28 ± 0.04 a |
| Acetone | 2.85 ± 0.11 a | 7.99 ± 0.36 b | |
| Safawi | Methanol | 5.76 ± 0.10 a | 7.12 ± 0.02 b |
| Acetone | 1.96 ± 0.07 a | 6.23 ± 0.00 b | |
| Ajwa | Methanol | 5.78 ± 0.03 a | 13.51 ± 0.12 b |
| Acetone | 2.69 ± 0.08 a | 4.21 ± 0.02 b | |
| Mariami | Methanol | 24.53 ± 0.74 b | 2.36 ± 0.11 a |
| Acetone | 8.25 ± 0.20 a | 13.17 ± 0.36 b | |
| Cultivar | Solvent | IC50 (μg/mL) | |
|---|---|---|---|
| Week 0 | Week 8 | ||
| Mabroom | Methanol | 2282.88 ± 43.19 c | 1379.24 ± 40.75 b |
| Acetone | 1512.68 ± 42.76 c | 1323.17 ± 74.63 b | |
| Safawi | Methanol | 2306.90 ± 12.07 b | 2179.67 ± 157.36 b |
| Acetone | 3552.33 ± 98.38 c | 451.67 ± 11.67 b | |
| Ajwa | Methanol | 1722.09 ± 103.75 c | 1377.33 ± 66.74 b |
| Acetone | 1970.97 ± 2.65 c | 53.80 ± 1.31 a | |
| Mariami | Methanol | 1860.76 ± 9.79 c | 1188.95 ± 2.79 b |
| Acetone | 1250.79 ± 17.06 c | 130.77 ± 2.62 b | |
| Cultivar | Solvent | IC50 (μg/mL) | |
|---|---|---|---|
| Week 0 | Week 5 | ||
| Mabroom | Methanol | 1379.24 ± 40.75 b | 1412.74 ± 47.35 b |
| Acetone | 1323.17 ± 74.63 b | 2476.12 ± 1.00 c | |
| Safawi | Methanol | 2179.67 ± 157.36 c | 152.66 ± 3.53 b |
| Acetone | 451.67 ± 11.67 b | 577.20 ± 51.95 c | |
| Ajwa | Methanol | 1377.33 ± 66.74 c | 1121.67 ± 27.98 b |
| Acetone | 53.80 ± 1.31 a | 1385.50 ± 8.89 c | |
| Mariami | Methanol | 1188.95 ± 2.79 b | 1190.28 ± 56.00 b |
| Acetone | 130.77 ± 2.62 b | 2280.08 ± 5.33 c | |
| Gram | Bacteria | Treatment | Diameter of Inhibition Zone (mm) | |||
|---|---|---|---|---|---|---|
| Mabroom | Safawi | Ajwa | Mariami | |||
| Positive | S. aureus | dH2O | ND | ND | ND | ND |
| Amp | 15.8 ± 0.3 d | 6.3 ± 0.3 a | 6.5 ± 0.3 c | 16.3 ± 0.9 c | ||
| A | 0 | 0 | 0 | 0 | ||
| Ma | 0.3 ± 0.3 a | 0 | 0 | 0 | ||
| Mb | 3.3 ± 1.7 b | 0 | 0 | 0 | ||
| Mc | 4.2 ± 0.8 b | 0 | 1.0 ± 0.3 a | 2.7 ± 2.7 a | ||
| Md | 10.0 ± 0.6 c | 0 | 2.0 ± 0.7 a | 9.5 ± 0.3 b | ||
| Me | 15.3 ± 0.3 d | 0 | 4.0 ± 1.5 b | 17.5 ± 0.3 c | ||
| B. cereus | dH2O | ND | ND | ND | ND | |
| Amp | 7.3 ± 0.3 a | 9.0 ± 0.5 b | 6.8 ± 0.6 b | 6.7 ± 0.3 a | ||
| A | 0 | 0 | 0 | 0 | ||
| Ma | 0 | 0 | 0 | 0 | ||
| Mb | 0 | 0 | 0 | 0 | ||
| Mc | 0 | 0 | 0 | 0 | ||
| Md | 0 | 1.3 ± 0.6 a | 1.0 ± 0.3 a | 0 | ||
| Me | 13.5 ± 1.5 b | 1.3 ± 0.6 a | 2.0 ± 0.7 a | 5.0 ± 5.0 a | ||
| Negative | E. coli | dH2O | ND | ND | ND | ND |
| Tet | 11.0 ± 1.7 | 5.7 ± 0.7 b | 7.3 ± 0.3 b | 10.7 ± 1.2 | ||
| A | 0 | 0 | 0 | 0 | ||
| Ma | 0 | 0 | 0 | 0 | ||
| Mb | 0 | 0 | 0 | 0 | ||
| Mc | 0 | 0 | 0 | 0 | ||
| Md | 0 | 1.0 ± 0.0 a | 1.0 ± 0.3 a | 0 | ||
| Me | 0 | 1.0 ± 0.3 a | 1.0 ± 0.3 a | 0 | ||
| S. marcescens | dH2O | ND | ND | ND | ND | |
| Tet | 10.0 ± 0.0 | 8.3 ± 0.9 a | 12.7 ± 1.5 b | 15.7 ± 9.1 | ||
| A | 0 | 0 | 0 | 0 | ||
| Ma | 0 | 0 | 0 | 0 | ||
| Mb | 0 | 0 | 0 | 0 | ||
| Mc | 0 | 0 | 3.0 ± 0.0 a | 0 | ||
| Md | 0 | 0 | 5.5 ± 0.3 a | 0 | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samad, M.A.; Hashim, S.H.; Simarani, K.; Yaacob, J.S. Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars. Molecules 2016, 21, 419. https://doi.org/10.3390/molecules21040419
Samad MA, Hashim SH, Simarani K, Yaacob JS. Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars. Molecules. 2016; 21(4):419. https://doi.org/10.3390/molecules21040419
Chicago/Turabian StyleSamad, Muhammad Azizan, Siti Hajar Hashim, Khanom Simarani, and Jamilah Syafawati Yaacob. 2016. "Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars" Molecules 21, no. 4: 419. https://doi.org/10.3390/molecules21040419
APA StyleSamad, M. A., Hashim, S. H., Simarani, K., & Yaacob, J. S. (2016). Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars. Molecules, 21(4), 419. https://doi.org/10.3390/molecules21040419





