Abstract
A novel series of sulfonamide derivatives (14 compounds) bearing thiourea moieties were efficiently synthesized and evaluated for their possible in vitro anticancer activity against four human tumor cell lines. The results indicated that compound 6 was the most potent, showing effectiveness on all the tested cell lines. Compounds 7 and 10 also showed promising results.
1. Introduction
Cancer and carcinogenesis are complicated and multifactor processes that results in several biochemical alterations in a single cell. All diseases can most likely be attributed to multifactorial effects, and these factors may interact with each other, hence inducing biological changes in a single cell over a period of time. The interaction of the multifactorial effects aggravates the cancer. To solve the extraordinary complexity of cancer and provide excellent therapeutic drugs, several researchers have focused their work on discovering new multitarget drugs which are able to interact with multiple altered pathways [1,2]. In the efforts to find new drugs with these capabilities, scientists have focused on many different characteristics of cancer biology during their research. Among the anticancer drugs discovered recently, various sulfonamides possess potent anticancer properties [3,4] which is attributed to their ability to inhibit carbonic anhydrase enzymes [5]. Carbonic anhydrases is a family of Zn-based metalloenzymes that catalyze the interconversion between carbon dioxide and bicarbonate with generation of protons. The carbonic anhydrase isozyme IX (CA IX) is reported to be associated with tumorigenesis being highly expressed in hypoxic tumors with limited expression in normal tissues [6]. Thiourea derivatives represent one of the most promising classes of anticancer agents with a wide range of activities against various leukemia and solid tumors [7,8,9]. In previous work, combinations of sulfonamide and thiourea derivatives have produced promising anticancer agents [10,11,12]. The importance of this paper lies in that the next generation sulfonamide-thiourea derivatives might be more efficacious as anticancer agents. Since one of the common methods for drug design in medicinal chemistry is varying substituents, we report herein the synthesis and in vitro anticancer evaluation of a novel series of thioureido-sulfonamide derivatives as a continuation of our ongoing research project concerned with the synthesis of novel heterocyclic compounds as anticancer agents, where we have identified novel classes of pyrrole, pyrrolopyrimidine, 4-aminopyridine, quinolines and sulfonamides and these new derivatives showed significant anticancer activity on different biological targets and human cancer cell lines [13,14,15,16,17].
2. Results
2.1. Chemistry
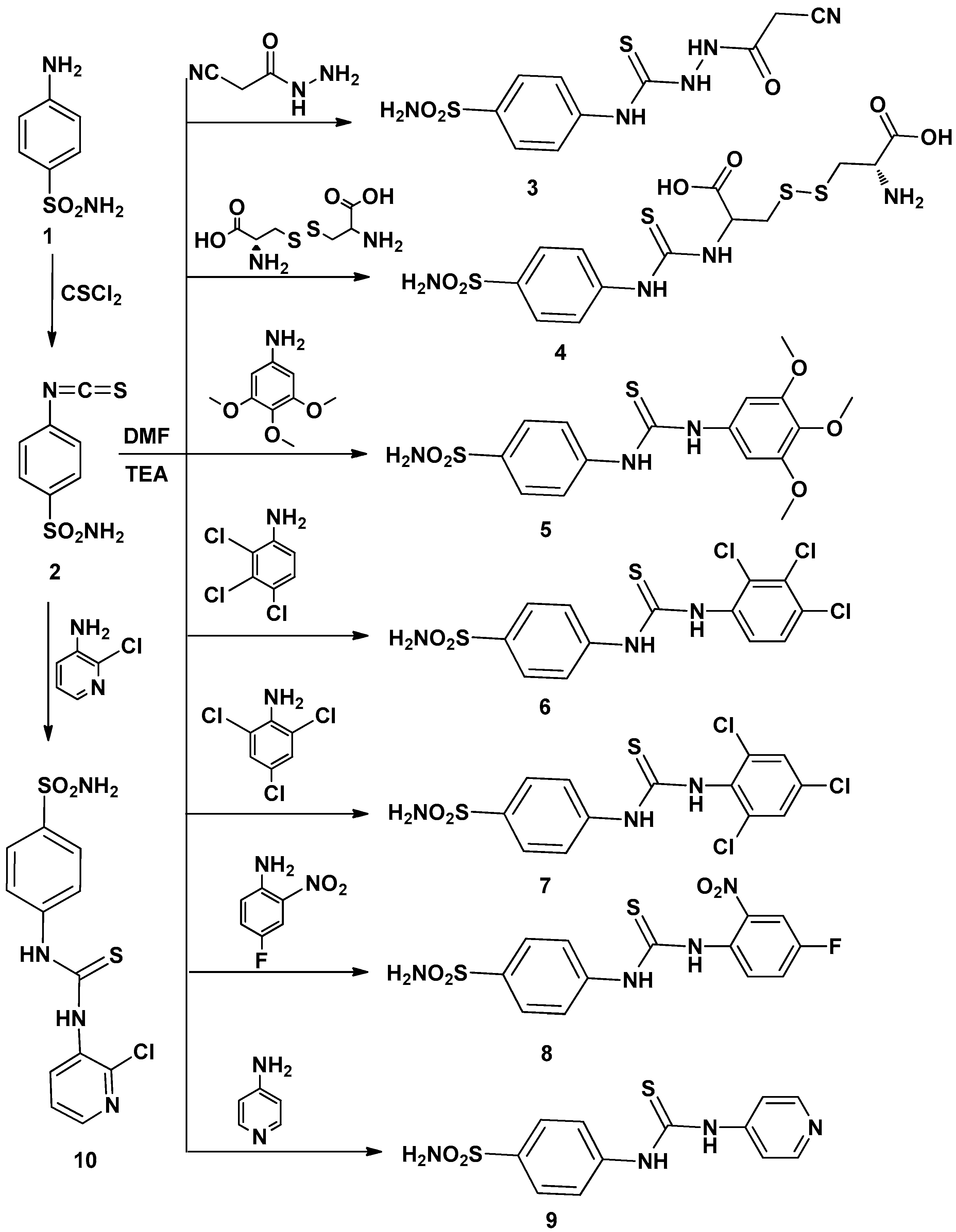
The aim of this work was to design and synthesize a novel series of thioureido-sulfonamide derivatives and examine their potential anticancer activity starting with the reported isothiocyanatobenzenesulfonamide 2 which was prepared by the reaction of sulfanilamide with thiophosgene following a reported method [18].
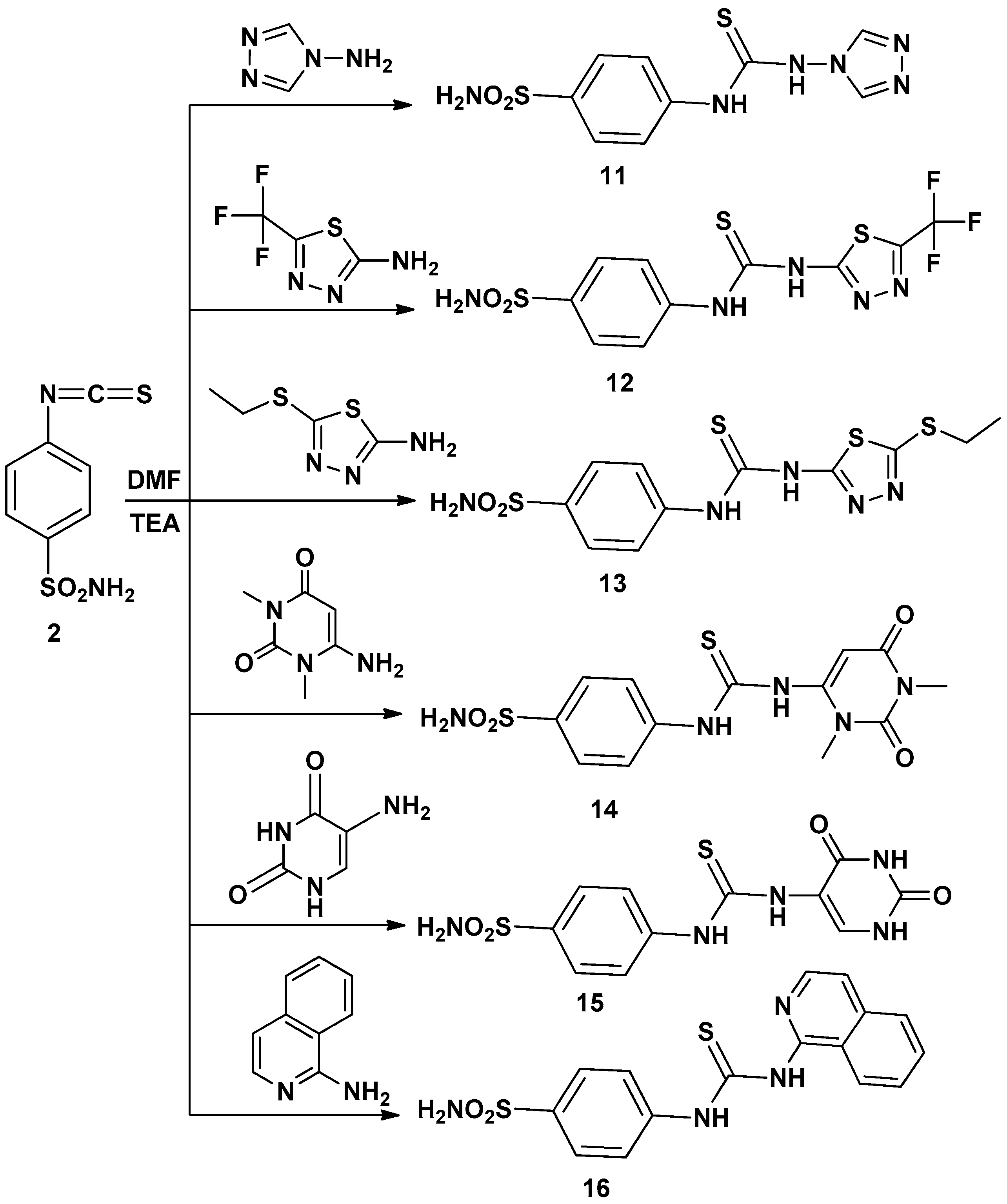
Thus, interaction of compound 2 with several amines in dry DMF containing triethylamine as catalyst afforded the corresponding sulfonamide derivatives 3–16 (Scheme 1 and Scheme 2) according to the reported methods [11,12,19]. The structures of the obtained compounds were established on the basis of elemental analyses and spectral data. The IR spectra of compounds 3–16 showed the absence of N=C=S groups and presence of absorption bands for (NH), (CH arom.), (CH aliph.), (C=S) and (SO2).
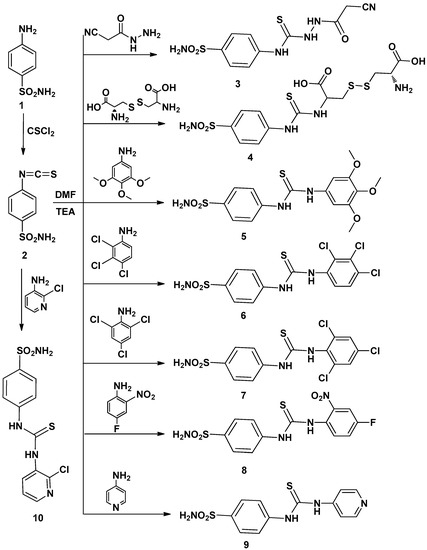
Scheme 1.
Synthetic pathways for compounds 2–10.
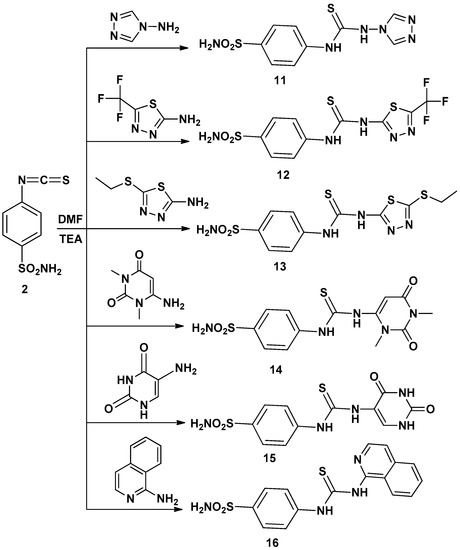
Scheme 2.
Synthetic pathways for compounds 11–16.
The 1H-NMR spectra of compounds 3–16 exhibited a singlet at 9.3–14.4 ppm assigned to the 2NH groups of thiourea which were exchanged upon deuteration, in addition to the corresponding protons assigned to the introduced aromatic and heterocyclic moieties.
The 13C-NMR spectra of compounds 3–16 exhibited additional signals for the introduced C=S group of thiourea falling in the range of δ 163.7–188.9 ppm, which is in conformity with the assigned structures. The mass spectra of compound 3–16 showed molecular ion peaks at their respective m/z values, supported by the elemental analyses data which were found within the limit of 0.4% of theoretical values for all the synthesized compounds.
2.2. In-Vitro Anticancer Evaluation
The synthesized compounds were evaluated for their in vitro anticancer activity against human lung cancer cell line (A549), cervical (HeLa) cancer cell line, colorectal cell line (LoVo) and breast cancer cell line (MDA-MB-231) using doxorubicin as reference drug. Doxorubicin (CAS, 25316-40-9), an anthracycline intercalating agent, is the reference compound used in this study is a potent anticancer drug. It works by intercalating DNA and thus inhibiting macromolecular biosynthesis. It is mostly used against of a wide range of cancers, including acute leukemia’s Hodgkin’s disease, and other lymphomas and cancers of the breast, adrenal cortex, colorectal, cervical, endometrium, lung, ovary, colon, liver and other sites. By Plotting the relationship between surviving fraction and drug concentration we obtained the survival curve of cancer cell lines and calculated the IC50 value, which corresponds to the concentration required for 50% inhibition of cell viability. The results are presented in Table 1, where some compounds exhibit fair activity compared to doxorubicin as reference drug. In case of human lung cancer cell line (A549) compounds 6, 9, and 10 were the most potent on this cell line with lower IC50 values than doxorubicin ranging from 171.4–272.1 µM. Moreover, the most potent compounds on HeLa cell lines were the sulfonamide derivatives 4, 6, 10 and 12 (IC50 range 137.5–259.6 µM). In case of the colorectal cell line (LoVo), four compounds (6, 7, 10 and 16) exhibited better activity than doxorubicin. Mild activity was observed for the synthesized compounds against breast cancer cell line (MDA-MB-231) where, the most potent compounds were compounds 2, 6, 7, 12, 13 and 16 which are found to be less active than doxorubicin. Generally, the breast cancer cell line (MDA-MB-231) was the most sensitive to the synthesized compounds. Considering broad spectrum anticancer activity, closer examination of the data presented in Table 1, revealed that compound 6 was the most active members of this study, showing effectiveness toward the four cell lines. By examining the SAR of the synthesized compounds, the starting isothiocyanate derivative 2 showed low activity on the tested cell lines, and findings for compounds 3–5, 8, 9 and 11–16 were similar. Meanwhile, introduction of a trichlorobenzene moiety in compound 6 significantly increased the activity against the four cell lines. We also concluded that the 2,3,4-position of chlorine atoms on the benzene ring was better than the 2,4,6-position in compound 7 which showed slightly lower activity towards the tested cell lines. In addition, introduction of a chloropyridine moiety in compound 10 also resulted in high activity from which we can suggest the importance of aryl/heteroaryl chlorine derivatives in these anticancer agents.

Table 1.
In vitro anticancer screening of the synthesized compounds against four cell lines.
3. Experimental Section
3.1. General Information
Melting points (uncorrected) were determined in open capillary on a Gallen Kamp melting point apparatus (Sanyo Gallen Kamp, Southborough, UK). Precoated silica gel plates (Kieselgel 0.25 mm, 60 F254, Merck, Darmstadt, Germany) were used for thin layer chromatography. A developing solvent system of chloroform/methanol (8:2) was used and the spots were detected by ultraviolet light. IR spectra (KBr disc) were recorded using an FT-IR spectrometer (Perkin Elmer, Norwalk, CT, USA). Mass spectra were recorded on a 600 GC/MS (Clarus, Middletown, CT, USA) and TQ 320 GC/MS/MS mass spectrometers (Varian, West Sussex, UK), 1H-NMR spectra were scanned on a NMR spectrometer (Bruker AXS Inc., Flawil, Switzerland), operating at 500 MHz for 1H-NMR and 125.76 MHz for 13C-NMR. Chemical shifts are expressed in δ-values (ppm) relative to TMS as an internal standard, using DMSO-d6 as a solvent. Elemental analyses were done on a model 2400 CHNSO analyser (Perkin Elmer, Norwalk, CT, USA). All the values were within ±0.4% of the theoretical values. All reagents used were of AR grade. The starting material 4-chloro-2-phenylquinazoline was purchased from Sigma (St. Louis, MO, USA) and was directly used for the preparation of target compounds.
3.2. Chemistry: General Procedure for the Synthesis of Sulfonamide Derivatives 3–16
A mixture of 4-isothiocyanato benzenesulfonamide 2 (2.14 g, 0.01 mol) and an appropriate amine (0.012 mol) in dry dimethylformamide (15 mL) containing trimethylamine (0.3 mL) was refluxed for 24 h., then left to cool. The solid product formed upon pouring onto ice/water was collected by filtration and recrystallized from ethanol-dimethylformamide to give 3–16, respectively.
2-(2-Cyanoacetyl)-N-(4-sulfamoylphenyl)hydrazine carbothioamide (3): Yield, 86%; m.p. 153.6 °C. IR (KBr, cm−1): 3312, 3214 (NH, NH2), 3099 (CH arom.), 2954, 2853 (CH aliph.), 1655 (C꞊O), 1387, 1157 (SO2), 1250 (C=S). 1H-NMR (DMSO-d6): 3.3 [s, 2H, CH2], 6.4–8.0 [m, 6H, Ar-H + SO2NH2], 9.3, 10.4, 13.0 [3s, 3NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 25.3, 120.3 (2), 126.1, 127.8 (2), 139.2, 141.5, 152.3, 163.7. MS m/z (%): 313 (M+) (13.44), 156 (100). Anal. Calcd. for C10H11N5O3S2 (313): C, 38.33; H, 3.54; N, 22.35. Found: C, 38.09; H, 3.19; N, 22.64.
2-Amino-3-(2-carboxy-2-(3-(4-sulfamoylphenyl)thioureido)ethyl)disulfanyl)propanoic acid (4): Yield, 68%; m.p. 252.6 °C. IR (KBr, cm−1): 3354 (OH), 3253, 3185 (NH, NH2), 3030 (CH arom.), 2964, 2858 (CH aliph.), 1783 (2C=O), 1388, 1162 (SO2). 1H-NMR (DMSO-d6): 3.0, 3.5 [m, 6H, CH-CH2-S-S-CH2-CH], 7.0–7.8 [m, 6H, Ar-H + SO2NH2], 8.0 [s, 2H, NH2, exchangeable with D2O], 10.9 [s, 2H, 2NH, exchangeable with D2O], 13.9 [s, 2H, 2OH, exchangeable with D2O], 13C-NMR (DMSO-d6): 40.2, 40.3, 66.8 (2), 123.6 (2), 126.7 (2), 140.9, 142.1, 157.3, 168.1, 178.5. MS m/z (%): 455 (M+) (18.48), 152 (100). Anal. Calcd. for C13H18N4O6S4 (455): C, 34.35; H, 3.99; N, 12.33. Found: C, 34.65; H, 4.33; N, 12.59.
4-(3-(3,4,5-Trimethoxyphenyl)thioureido)benzenesulfonamide (5): Yield, 78%; m.p. 203.7 °C. IR (KBr, cm−1): 3345, 3231, 3155 (NH, NH2), 3100 (CH arom.), 2967, 2829 (CH aliph.), 1328, 1180 (SO2), 1258 (C=S). 1H-NMR (DMSO-d6): 3.8 [s, 9H, 3OCH3], 6.6–7.7 [m, 8H, Ar-H + SO2NH2], 9.9 [s, 2H, 2NH, exchangeable with D2O].13C-NMR (DMSO-d6):56.3 (2), 60.5, 102.2 (2), 123.3 (2), 127.7 (2), 130.5, 135.0, 135.4, 139.6, 153.7 (2), 179.7. MS m/z (%): 397 (M+) (9.23), 229 (100). Anal. Calcd. for C16H19N3O5S2 (397): C, 48.35; H, 4. 82; N, 10.57. Found: C, 48.71; H, 4.56; N, 10.27.
4-(3-(2,3,4-Trichlorophenyl)thioureido)benzensulfonamide (6): Yield, 89%; m.p. 231.3 °C. IR (KBr, cm−1): 3353, 3245, 3211(NH), 3010 (CH arom.), 1330, 1159 (SO2), 1268 (C=S), 725 (C–Cl).1H-NMR (DMSO-d6): 6.6–7.7 [m, 8H, Ar-H + SO2NH2,], 10.3[s, 2H, 2NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 123.0 (2), 126.5, 128.8 (2), 129.5, 130.5, 131.6, 137.6, 139.8, 140.1, 142.6, 180.0. MS m/z (%): 411 (M+) (36.71), 74 (100). Anal. Calcd. for C13H10Cl3N3O2S2 (411): C, 38.02; H, 2.45; N, 10.23. Found: C, 38.32; H, 2.18; N, 10.54.
4-(3-(2,4,6-Trichlorophenyl)thiouredo)benzenesulfonamide (7): Yield, 76%; m.p. 225.2 °C. IR (KBr, cm−1): 3425, 3369, 3327, 3249 (NH, NH2), 3080 (CH arom.), 1393, 1181 (SO2), 1299 (C=S), 854 (C–Cl).1H-NMR (DMSO-d6):7.3–7.8 [m, 8H, Ar-H + SO2NH2], 10.3 [s, 2H, 2NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 118.8 (2), 127.9, 128.7 (2), 133.1 (2), 136.2, 139.8, 140.1 (2), 141.0, 181.0. MS m/z (%): 411 (M+) (12.71), 93 (100). Anal. Calcd. for C13H10Cl3N3O2S2 (411): C, 38.02; H, 2.45; N, 10.23. Found: C, 37.82; H, 2.76; N, 9.91.
4-(3-(4-Fluoro-2-nitrophenyl)thioureido)benzenesulfonamide (8): Yield, 77%; m.p. 206.3 °C. IR (KBr, cm−1): 3482, 3359, 3244 (NH, NH2), 3105 (CH arom.), 1377, 1182 (SO2), 1273 (C=S). 1H-NMR (DMSO-d6): 7.0–7.8 [m, 9H, Ar-H + SO2NH2], 10.3 [s, 2H, +2NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 110.1, 121.4, 123.2 (2), 125.3, 126.7, 129.1 (2), 139.8, 142.8, 143.9, 153.1, 180.1. MS m/z (%): 370 (M+) (8.76), 139 (100). Anal. Calcd. for C13H11 FN4O4S2 (370): C, 42.16; H, 2.99; N, 15.13. Found: C, 42.51; H, 2.63; N, 15.41.
4-(3-Pyridin-4-ylthioureido)benzenesulfonamide (9): Yield, 81%; m.p. 295.6 °C. IR (KBr, cm−1): 3411, 3332, 3215 (NH, NH2), 3100 (CH arom.), 1590 (C=N), 1332, 1155 (SO2), 1245 (C=S). 1H-NMR (DMSO-d6): 6.3–8.3 [m, 10H, Ar-H + SO2NH2], 10.1 [s, 2H, 2NH, exchangeable with D2O].13C-NMR (DMSO-d6): 109.2 (2), 127.1 (2), 127.3 (2), 141.1 (2), 149.7 (2), 159.8, 178.3. MS m/z (%): 308 (M+) (15.18), 77 (100). Anal. Calcd. for C12H12N4O2S2 (308): C, 46.74; H, 3.92; N, 18.17. Found: C, 46.46; H, 3.64; N, 18.49.
4-(3-(2-Chloropyridin-3-yl)thioureido)benzenesulfonamide (10): Yield, 74%; m.p. 245.3 °C. IR (KBr, cm−1): 3454, 3363, 3243 (NH, NH2), 3102 (CH arom.), 1623 (C=N), 1381, 1155 (SO2), 1253 (C=S), 794 (C–Cl). 1H-NMR (DMSO-d6): 7.4–8.3 [m, 9H, Ar-H + SO2NH2], 10.3 [s, 2H, 2NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 117.7 (2), 122.2, 122.9, 127.4 (2), 137.9, 140.0, 141.6, 143.2, 146.2, 182.3. MS m/z (%): 343 (M+) (1.89), 112 (100). Anal. Calcd. for C12H11ClN4O2S2 (343): C, 42.04; H, 3.23; N, 16.34. Found: C, 42.29; H, 3.53; N, 16.05.
4-(3-4H-1,2,4-Triazol-4-ylthioureido)benzenesulfonamide (11): Yield, 66%; m.p. 247.8 °C. IR (KBr, cm−1): 3346, 33235, 3175 (NH, NH2), 3064 (CH arom.), 1593 (C=N), 1407, 1156 (SO2), 1262 (C=S). 1H-NMR (DMSO-d6): 7.3–8.8 [m, 8H, Ar-H + SO2NH2], 10.1 [s, 2H, 2NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 125.2 (2), 127.5 (2), 137.9, 140.4, 142.2, 145.3, 180.6. MS m/z (%): 298(M+) (4.64), 68 (100). Anal. Calcd. for C9H10N6O2S2 (298): C, 36.23; H, 3.38; N, 28.17. Found: C, 36.51; H, 3.07; N, 27.85.
4-(3-(5-(Trifluoromethyl)-1,3,4-thiadiazol-2-yl)thioureido)benzenesulfonamide (12): Yield, 59%; m.p. 234.5 °C. IR (KBr, cm−1): 3352, 3243, 3185 (NH, NH2), 3009 (CH arom.), 1586 (C=N), 1407, 1181 (SO2), 1290 (C=S).1H-NMR (DMSO-d6): 7.3–7.9 [m, 6H, Ar-H + SO2NH2], 10.3 [s, 2H, 2NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 116.4, 123.3 (2), 126.7 (2), 139.8, 142.8, 151.8, 158.6, 180.1. MS m/z (%): 383 (M+) (14.63), 169 (100). Anal. Calcd. for C10H8F3N5O2S3 (383): C, 31.33; H, 2.10; N, 18.27. Found: C, 31.62; H, 2.42; N, 18.55.
4-(3-(5-(Ethylthio)-1,3,4-thiadiazol-2-yl)thioureido)benzenesulfonamide (13): Yield, 89%; m.p. 232.2° C. IR (KBr, cm−1): 3352, 3220, 3182 (NH, NH2), 3078 (CH arom.), 2945, 2853 (CH aliph.), 1595 (C=N), 1402, 1182 (SO2), 1243 (C=S). 1H-NMR (DMSO-d6): 1.3 [t, 3H, CH3], 3.2 [q, 2H, CH2], 7.2–7.9 [m, 6H, Ar-H + SO2NH2], 10.3, 10.7 [2s, 2H, 2NH, exchangeable with D2O]. 13C-NMR (DMSO-d6):15.2, 28.3, 122.2 (2), 127.8 (2), 139.8, 142.8, 154.6, 164.5, 180.0. MS m/z (%): 376 (M+) (17.26), 145 (100). Anal. Calcd. for C11H13N5O2S4 (376): C, 35.18; H, 3.49; N, 18.65. Found: C, 35.49; H, 3.18; N, 18.36.
4-(3-(1,3-Dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-yl)thioureido)benzene-sulfonamide (14): Yield, 90%; m.p. 247.4 °C. IR (KBr, cm−1): 3398, 3254, 3225 (NH, NH2), 3100 (CH arom.), 2946, 2880 (CH aliph.), 1692 (2C=O), 1381, 1183 (SO2), 1235 (C=S).1H-NMR (DMSO-d6): 3.1, 3.2 [2s, 6H, 2NCH3], 6.7 [s, 1H, CH], 7.3–7.9 [m, 6H, Ar-H + SO2NH2], 10.3, 14.4 [2s, 2H, 2NH,exchangeable with D2O]. 13C-NMR (DMSO-d6): 27.5, 29.6, 75.3, 123.2 (2), 126.0 (2), 141.4, 142.5, 152.0, 161.9, 163.8, 188.9. MS m/z (%): 369 (M+) (9.28), 138 (100). Anal. Calcd. for C13H15N5O4S2 (369): C, 42.27; H, 4. 09; N, 18.96. Found: C, 42.51; H, 4.33; N, 18.62.
4-(3-(2,4-Dioxo-1,2,3,6-tetrahydropyrimidin-5-yl)thioureido)benzenesulfonamide (15): Yield, 67%; m.p. >360 °C. IR (KBr, cm−1): 3367, 3286, 3155 (NH, NH2), 3013 (CH arom.), 1698, 1671 (2C=O), 1316, 1184 (SO2), 1275 (C=S). 1H-NMR (DMSO-d6): 6.8–8.1 [m, 7H, Ar-H + SO2NH2], 9.3, 10.0, 10.7, 11.0 [4s, 4H, 4NH, exchangeable with D2O]. 13C-NMR (DMSO-d6):112.9, 122.4 (2), 126.5 (2), 127.9, 139.7, 142.9, 150.2, 161.9, 162.1, 180.4. MS m/z (%): 341 (M+) (2.77), 111 (100). Anal. Calcd. for C11H11N5O4S2 (341): C, 38.70; H, 3.25; N, 20.52. Found: C, 38.37; H, 3.56; N, 20.24.
4-(3-Isoquinolin-1-ylthioureido)benzenesulfonamide (16): Yield, 87%; m.p. 217.1 °C. IR (KBr, cm−1): 3313, 3286, 3174 (NH, NH2), 3087 (CH arom.), 1635 (C=N), 1397, 1189 (SO2), 1213 (C=S). 1H-NMR (DMSO-d6): 7.4–8.8 [m, 10H, Ar-H + SO2NH2], 10.8, 11.8 [2s, 2H, 2NH, exchangeable with D2O].13C-NMR (DMSO-d6): 111.6, 115.2, 119.7 (2), 122.2, 125.1, 126.6, 127.2 (2), 129.4, 135.6, 137.4, 140.4, 141.0, 170.0, 179.7. MS m/z (%): 358 (M+) (47.82), 129 (100). Anal. Calcd. for C16H14N4O2S2 (358): C, 53.61; H, 3.94; N, 15.63. Found: C, 53.36; H, 3.62; N, 15.36.
3.3. In-Vitro Anticancer Evaluation
3.3.1. Cell Culture
Human cancer cell lines HeLa (cervical), A549 (lungs) and LoVo (colorectal) were grown in DMEM + GlutaMax (Invitrogen, Carlsbad, CA, USA), and MDA-MB-231 (breast) were grown in DMEM-F12 + GlutaMax) medium (Invitrogen), supplemented with 10% heat-inactivated bovine serum (Gibco, Gaithersburg, MD, USA) and 1x penicillin-streptomycin (Gibco) at 37 °C in a humified chamber with 5% CO2 supply.
3.3.2. Cytotoxicity Assay
The in vitro anticancer screening was done at pharmacognosy Department, College of Pharmacy, King Saud University Riyadh Saudi Arabia. Cells were seeded (105 cells/well) in 96-well flat-bottom plates (Becton-Dickinson Labware, Franklin Lakes, NJ, USA) a day before treatment and grown overnight. Compounds were dissolved in dimethyl sulfoxide (DMSO; Sigma) and finally prepared as 1.0 mg/mL stocks, respectively in the culture media. The final concentration of DMSO never exceeded 0.1% in the treatment doses. Six different doses of compounds (400, 200, 100, 50, 25 and 10 µM) were further prepared by diluting the stocks in culture media, and cells were treated (in triplicate/dose). Doxorubicin was included as standard reference drug (positive control) and untreated culture was considered as negative control. The treated cultures were further incubated for 48 h. At 48 h post-treatment, cell viability test was performed using TACS MTT Cell Proliferation and Viability Assay Kit (TACS, Abcam, Cambridge, MA, USA) as per manufacturer’s instructions. The optical density (OD) was recorded at 570 nm in a microplate reader (EL × 800, BioTek, Winooski, VT, USA) and cell survival fraction was determined. The cell survival fraction was calculated as [(A − B)/A], where A and B are the OD of untreated and of treated cells, respectively. The relation between surviving fraction and drug concentration is plotted to get the survival curve of each tumor cell line after the specified time. The concentration required for 50% inhibition of cell viability (IC50) was calculated and compared with the reference drug doxorubicin. Compounds that fail to inhibit 50% of cell viability are considered to be inactive. The results are given in Table 1.
4. Conclusions
The present work describes the synthesis of a novel series of thioureido-sulfonamide derivatives. 4-(3-(2,3,4-Trichlorophenyl)thioureido)benzensulfonamide (6) 4-(3-(2,4,6-trichlorophenyl)-thio-ureido)benzenesulfonamide (7) and 4-(3-(2-chloropyridin-3-yl)thioureido)benzenesulfonamide (10) were the most potent candidates in the in vitro anticancer screening study, showing higher activity than doxorubicin. However, further investigation relating the structure and the activity of the sulfonamide and thiourea derivatives as well as their stability under biological conditions is required. These detailed investigations could be helpful in designing more potent anticancer agents for therapeutic use.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding of this research through the Research Group Project No. RGP-VPP-302.
Author Contributions
M.M.G. suggested the research idea, contributed in the experimental work and in writing the paper. M.S.AlSaid contributed in the experimental work, the biological activity and in writing the paper. M.S.Al-Dosari, A.H.A. contributed in the biological activity. M.G.E contributed in writing the paper and performed the docking study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antonello, A.; Tarozzi, A.; Morroni, F.; Cavalli, A.; Rosini, M.; Hrelia, P.; Bolognesi, M.L.; Melchiorre, C. Multitarget-directed drug design strategy: A novel molecule designed to block epidermal growth factor receptor (EGFR) and to exert proapoptotic effects. J. Med. Chem. 2006, 49, 6642–6645. [Google Scholar]
- Rachid, Z.; Brahimi, F.; Qiu, Q.; Williams, C.; Hartley, J.M.; Hartley, J.A.; Jean-Claude, B.J. Novel nitrogen mustard-armed combi-molecules for the selective targeting of epidermal growth factor receptor overexperessing solid tumors: Discovery of an unusual structure-activity relationship. J. Med. Chem. 2007, 50, 2605–2608. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.M.; Enyedy, E.A.; Supuran, C.T.; Krupenko, N.I.; Krupenko, S.A.; Santos, M.A. Pteridine-sulfonamide conjugates as dual inhibitors of carbonic anhydrases and dihydrofolate reductase with potential antitumor activity. Bioorg. Med. Chem. 2010, 18, 5081–5089. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr. Cancer Drug Targets 2002, 2, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Inhibition of carbonic anhydrase IX as a novel anticancer mechanism. World J. Clin. Oncol. 2012, 3, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases as drug targets. Curr. Pharm. Des. 2008, 14, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Koca, I.; Özgür, A.; Coşkun, K.A.; Tutar, Y. Synthesis and anticancer activity of acyl thioureas bearing pyrazole moiety. Bioorg. Med. Chem. 2013, 21, 3859–3865. [Google Scholar] [CrossRef] [PubMed]
- Madabhushi, S.; Mallu, K.K.R.; Vangipuram, V.S.; Kurva, S.; Poornachandra, Y.; Kumar, C.G. Synthesis of novel benzimidazole functionalized chiral thioureas and evaluation of their antibacterial and anticancer activities. Bioorg. Med. Chem. Lett. 2014, 24, 4822–4825. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, Y.; Yang, Y.S.; Zhang, F.; Zhang, Y.B.; Wang, X.L.; Tang, J.F.; Zhong, W.Q.; Zhu, H.L. Design, modification and 3D QSAR studies of novel naphthalin-containing pyrazoline derivatives with/without thiourea skeleton as anticancer agents. Bioorg. Med. Chem. 2013, 21, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ceruso, M.; Alsaid, M.S.; Nissan, Y.M.; Arafa, R.K.; Supuran, C.T. Novel sulfonamides bearing pyrrole and pyrrolopyrimidine moieties as carbonic anhydrase inhibitors: Synthesis, cytotoxic activity and molecular modeling. Eur. J. Med. Chem. 2014, 87, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; El-Gazzar, M.G.; El-Gazzar, M.G. Synthesis, in vitro anticancer screening and radiosensitizing evaluation of some new N-(quinoxalin-2-yl) benzenesulfonamide derivatives. Arzneim. Forsch. 2012, 62, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; El-Gazzar, M.G.; El-Gazzar, M.G. Synthesis, in vitro anticancer screening and radiosensitizing evaluation of some new 4-[3(substituted) thioureido]-N-(quinoxalin-2-yl)-benzenesulfonamide derivatives. Acta. Pharm. 2011, 61, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; El-Gazzar, M.G.; Zahran, S.S. Synthesis, anticancer and radiosensitizing evaluation of some novel sulfonamide derivatives. Eur. J. Med. Chem. 2015, 92, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; Ceruso, M.; Nissan, Y.M.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis, molecular docking, cytotoxic and inhibition of the human carbonic anhydrase isoforms I, II, IX, XII with novel benzenesulfonamides incorporating pyrrole, pyrrolopyrimidine and fused pyrrolopyrimidine moieties. Bioorg. Med. Chem. 2014, 14, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; El-Gazzar, M.G.; Alsaid, M.S. Synthesis and anti-Breast cancer evaluation of novel N-(guanidinyl)-benzenesulfonamides. Int. J. Mol. Sci. 2014, 15, 5582–5595. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; El-Gazzar, M.G.; Alsaid, M.S. Synthesis, characterization and anti-breast cancer activity of new 4-aminoantipyrine-based heterocycles. Int. J. Mol. Sci. 2014, 15, 7539–7553. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, M.S.; Ghorab, M.M.; Alsaid, M.S.; Nissan, Y.M.; Ahmed, A.B. Synthesis and anticancer activity of some novel trifluoromethylquinolines carrying a biologically active benzenesulfonamide moiety. Eur. J. Med. Chem. 2013, 69, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Scozzafava, A.; Mincione, F.; Menabuoni, L.; Ilies, M.A.; Supuran, C.T. Carbonic anhydrase inhibitors: Water soluble 4-sulfamoylphenylthioureas as topical intraocular pressure lowering agents with long lasting effects. J. Med. Chem. 2000, 43, 4884–4892. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Alquasoumi, S.I.; Alafeefy, A.M.; Aboulmagd, S.A. Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur. J. Med. Chem. 2010, 45, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).