Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases
Abstract
:1. Introduction
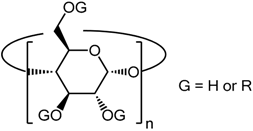
2. Structural Aspects of CDs
3. Modes of Action of CDs
3.1. Direct Action of the CDs on Cell Membranes
3.2. CDs as Drug Delivery Systems
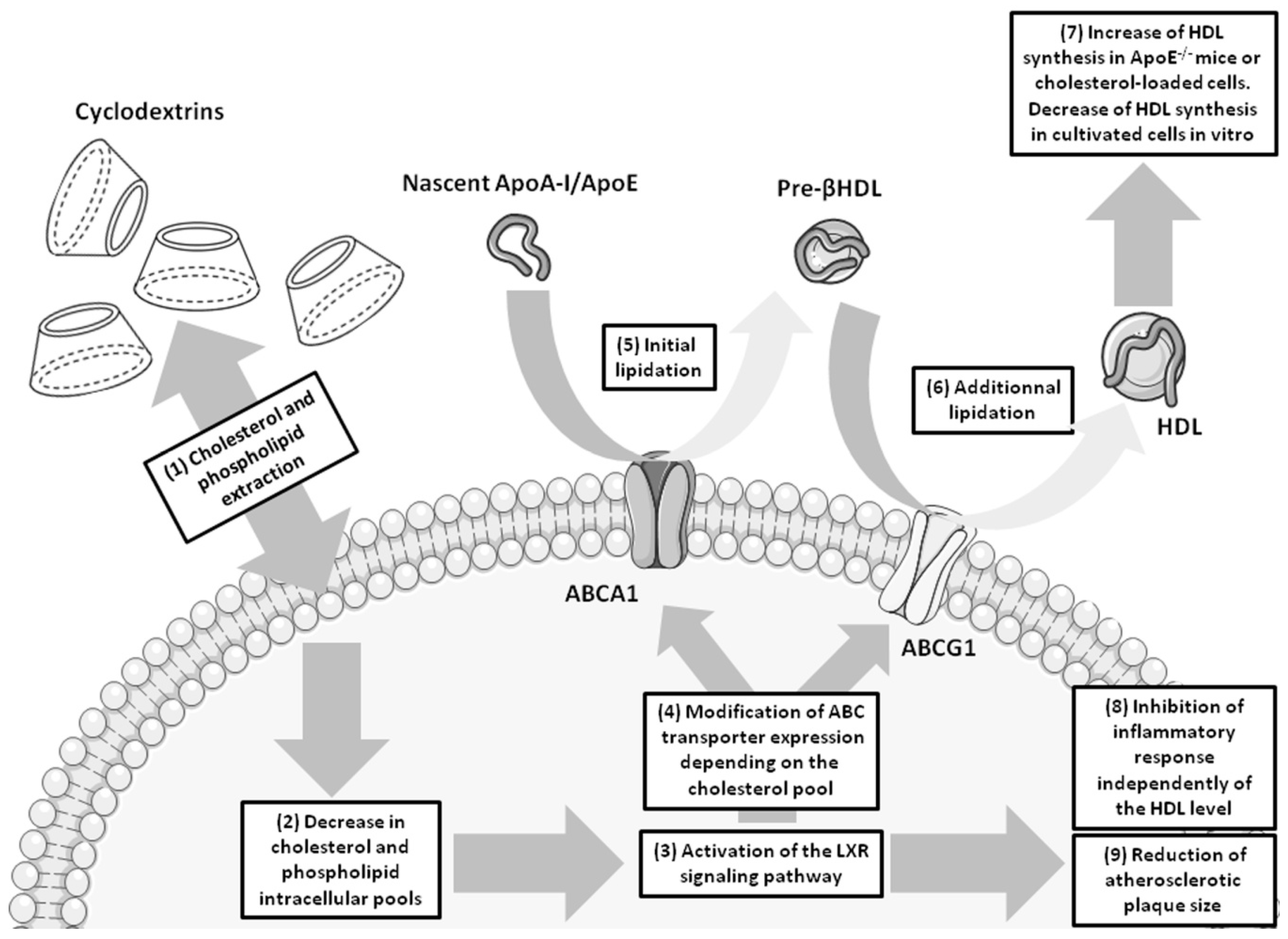
4. Cyclodextrins and Atherosclerosis
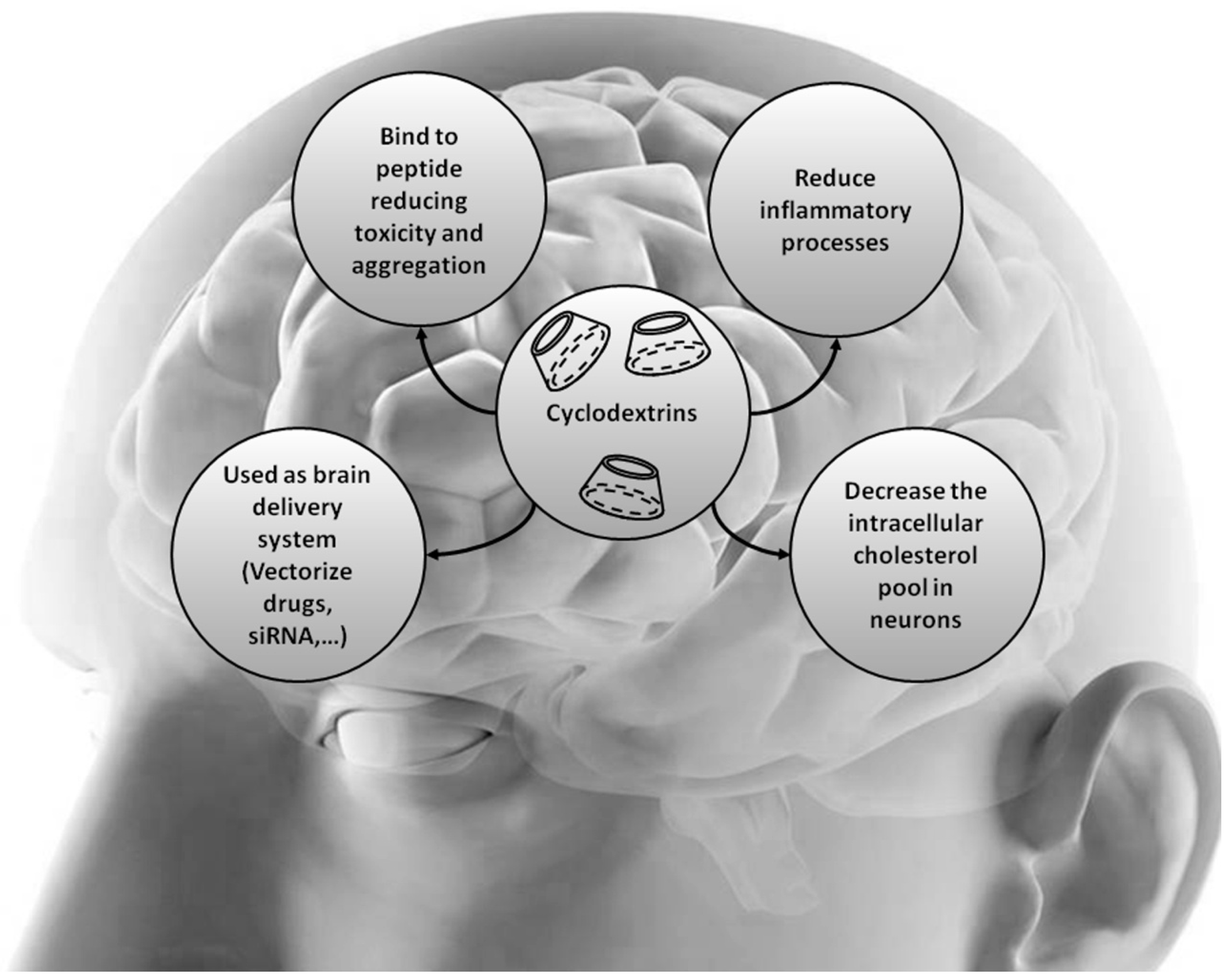
5. Cyclodextrins and Cholesterol-Associated Neurodegenerative Diseases
5.1. Niemann-Pick type C (NPC)
5.2. Alzheimer’s Disease
5.3. Parkinson's Disease (PD)
5.4. Huntington’s Disease
6. Conclusions
Acknowledgments
Conflicts of Interests
References
- Gosselet, F.; Saint-Pol, J.; Fenart, L. Effects of oxysterols on the blood-brain barrier: Implications for Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2014, 446, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Kniesel, U.; Wolburg, H. Tight junctions of the blood-brain barrier. Cell Mol. Neurobiol. 2000, 20, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Regina, A.; Jodoin, J.; Laplante, A.; Dagenais, C.; Berthelet, F.; Moghrabi, A.; Béliveau, R. Drug transport to the brain: Key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul. Pharmacol. 2002, 38, 339–348. [Google Scholar] [CrossRef]
- Chaves, C.; Shawahna, R.; Jacob, A.; Scherrmann, J.M.; Declèves, X. Human ABC transporters at blood-CNS interfaces as determinants of CNS drug penetration. Curr. Pharm. Des. 2014, 20, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Medicinal applications of cyclodextrins. Med. Res. Rev. 1994, 14, 353–386. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Nedley, M.P.; Bhaduri, S.B.; Bredzinski, X.; Boddu, S.H. Masking the bitter taste of injectable lidocaine HCl formulation for dental procedures. AAPS PharmSciTech 2015, 16, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Masson, M.; Jarvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Lenglet, S.; Carbone, F.; Boero, S.; Pelli, G.; Burger, F.; Roth, A.; Bertolotto, M.; Nencioni, A.; Cea, M.; et al. Treatment with KLEPTOSE(R) CRYSMEB reduces mouse atherogenesis by impacting on lipid profile and Th1 lymphocyte response. Vascul. Pharmacol. 2015, 72, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Coisne, C.; Hallier-Vanuxeem, D.; Boucau, M.C.; Hachani, J.; Tilloy, S.; Bricout, H.; Monflier, E.; Wils, D.; Serpelloni, M.; Parissaux, X.; et al. β-Cyclodextrins Decrease Cholesterol Release and ABC-Associated Transporter Expression in Smooth Muscle Cells and Aortic Endothelial Cells. Front. Physiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications? Wiley-VCH: Weinheim, Germany, 2006; p. 507. [Google Scholar]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, P.; Bodor, N. Brain-Targeting Chemical Delivery Systems and Their Cyclodextrin-Based Formulations in Light of the Contributions of Marcus E. Brewster. J. Pharm. Sci. 2016, 105, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Fenyvesi, F.; Bacskay, I.; Váradi, J.; Fenyvesi, E.; Iványi, R.; Szente, L.; Tósaki, A.; Vecsernyés, M. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.W.; Gray, J.E.; Weaver, R.N. Cyclodextrin nephrosis in the rat. Am. J. Pathol. 1976, 83, 367–382. [Google Scholar] [PubMed]
- Perrin, J.H.; Field, F.P.; Hansen, D.A.; Mufson, R.A.; Torosian, G. β-Cyclodextrin as an aid to peritoneal dialysis. Renal toxicity of β-cyclodextrin in the rat. Res. Commun. Chem. Pathol. Pharmacol. 1978, 19, 373–376. [Google Scholar] [PubMed]
- Frijlink, H.W.; Visser, J.; Hefting, N.R.; Oosting, R.; Meijer, D.K.; Lerk, C.F. The pharmacokinetics of β-cyclodextrin and hydroxypropyl- β-cyclodextrin in the rat. Pharm. Res. 1990, 7, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, J.; van Beijstervedt, L.; Meuldermans, W.; Szathmary, S.; Heykants, J. Disposition of HP-b-CD in experimental animals. In Minutes of the 5th International Symposium on Cyclodextrins; Duchene, D., Ed.; Editions de Santé: Paris, France, 1990; pp. 514–517. [Google Scholar]
- Godai, K.; Hasegawa-Moriyama, M.; Kuniyoshi, T.; Kakoi, T.; Ikoma, K.; Isowaki, S.; Matsunaga, A.; Kanmura, Y. Three cases of suspected sugammadex-induced hypersensitivity reactions. Br. J. Anaesth. 2012, 109, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, T.; Tomita, Y.; Yoshida, N.; Tomioka, A.; Horiuchi, T.; Nagata, C.; Orihara, M.; Yamada, M.H.; Saito, S. Three suspected cases of sugammadex-induced anaphylactic shock. BMC Anesthesiol. 2014, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Mahammad, S.; Parmryd, I. Cholesterol depletion using methyl-β-cyclodextrin. Methods Mol. Biol. 2015, 1232, 91–102. [Google Scholar] [PubMed]
- Lopez, C.A.; de Vries, A.H.; Marrink, S.J. Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput. Biol. 2011, 7, e1002020. [Google Scholar] [CrossRef] [PubMed]
- Khuntawee, W.; Wolschann, P.; Rungrotmongkol, T.; Wong-Ekkabut, J.; Hannongbua, S. Molecular Dynamics Simulations of the Interaction of Β Cyclodextrin with Lipid Bilayer. J. Chem. Inf. Model. 2015, 55, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.A.; de Wries, A.H.; Marrink, S.J. Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci Rep. 2013, 3, 2071. [Google Scholar] [CrossRef] [PubMed]
- Legendre, J.Y.; Supersaxo, A. Short-chain phospholipids enhance amphipathic peptide-mediated gene transfer. Biochem. Biophys. Res. Commun. 1995, 217, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Merkus, F.W.; Verhoef, J.C.; Marttin, E.; Romeijn, S.G.; van der Kuy, P.H.; Hermens, W.A.; Schipper, N.G. Cyclodextrins in nasal drug delivery. Adv. Drug Deliv. Rev. 1999, 36, 41–57. [Google Scholar] [CrossRef]
- Hovgaard, L.; Brondsted, H. Drug delivery studies in Caco-2 monolayers. IV. Absorption enhancer effects of cyclodextrins. Pharm. Res. 1995, 12, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Monnaert, V.; Tilloy, S.; Bricout, H.; Fenart, L.; Cecchelli, R.; Monflier, E. Behavior of α-, β-, and γ-cyclodextrins and their derivatives on an in vitro model of blood-brain barrier. J. Pharmacol. Exp. Ther. 2004, 310, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Weickert, C.S.; Garner, B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J. Neurochem. 2008, 104, 1145–1166. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef] [PubMed]
- Gelissen, I.C.; Harris, M.; Rye, K.A.; Quinn, C.; Brown, A.J.; Kockx, M.; Cartland, S.; Packianathan, M.; Kritharides, L.; Jessup, W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Candela, P.; Boucau, M.C.; Fenart, L.; Gosselet, F. Oxysterols decrease apical-to-basolateral transport of Aβ peptides via an ABCB1-mediated process in an in vitro Blood-brain barrier model constituted of bovine brain capillary endothelial cells. Brain Res. 2013, 1517, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, M.; Candela, P.; Saint-Pol, J.; Fenart, L.; Gosselet, F. Bexarotene Promotes Cholesterol Efflux and Restricts Apical-to-Basolateral Transport of Amyloid-β Peptides in an In Vitro Model of the Human Blood-Brain Barrier. J. Alzheimers Dis. 2015, 48, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Salmas, R.E.; Salvador, E.; Roewer, N.; Broscheit, J.; Förster, C. Evaluation of the potential toxicity of unmodified and modified cyclodextrins on murine blood-brain barrier endothelial cells. J. Toxicol. Sci. 2016, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, D.L.; Steen, H.; Adam, R.M.; Garlick, M.; Zurakowski, D.; Gygi, S.P.; Freeman, M.R.; Solomon, K.R. A quantitative proteomic analysis of growth factor-induced compositional changes in lipid rafts of human smooth muscle cells. Proteomics 2005, 5, 4733–4742. [Google Scholar] [CrossRef] [PubMed]
- Shirao, S.; Yoneda, H.; Shinoyama, M.; Sugimoto, K.; Koizumi, H.; Ishihara, H.; Oka, F.; Sadahiro, H.; Nomura, S.; Fujii, M.; et al. A novel trigger for cholesterol-dependent smooth muscle contraction mediated by the sphingosylphosphorylcholine-Rho-kinase pathway in the rat basilar artery: A mechanistic role for lipid rafts. J. Cereb. Blood Flow Metab. 2015, 35, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Possidonio, A.C.; Miranda, M.; Gregoracci, G.B.; Thompson, F.L.; Costa, M.L.; Mermelstein, C. Cholesterol depletion induces transcriptional changes during skeletal muscle differentiation. BMC Genomics 2014, 15, 544. [Google Scholar] [CrossRef] [PubMed]
- Portilho, D.M.; Soares, C.P.; Morrot, A.; Thiago, L.S.; Browne, G.B.; Savino, W.; Costa, M.L.; Mermelstein, C. Cholesterol depletion by methyl-β-cyclodextrin enhances cell proliferation and increases the number of desmin-positive cells in myoblast cultures. Eur. J. Pharmacol. 2012, 694, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Dehouck, B.; Descamps, L.; Fenart, L.; Buée-Scherrer, V.V.; Duhem, C.; Lundquist, S.; Rentfel, M.; Torpier, G.; Dehouck, M.P. In vitro model for evaluating drug transport across the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 165–178. [Google Scholar] [CrossRef]
- Monnaert, V.; Betbeder, D.; Fenart, L.; Bricout, H.; Lenfant, A.M.; Landry, C.; Cecchelli, R.; Monflier, E.; Tilloy, S. Effects of γ- and hydroxypropyl-γ-cyclodextrins on the transport of doxorubicin across an in vitro model of blood-brain barrier. J. Pharmacol. Exp. Ther. 2004, 311, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Yunomae, K.; Hirayama, F.; Uekama, K. Contribution of P-glycoprotein to the enhancing effects of dimethyl-β-cyclodextrin on oral bioavailability of tacrolimus. J. Pharmacol. Exp. Ther. 2001, 297, 547–555. [Google Scholar] [PubMed]
- Tilloy, S.; Monnaert, V.; Fenart, L.; Bricout, H.; Cecchelli, R.; Monflier, E. Methylated β-cyclodextrin as P-gp modulators for deliverance of doxorubicin across an in vitro model of blood-brain barrier. Bioorg. Med. Chem. Lett. 2006, 16, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Li, J.; Xiao, H.; Lowe, T.L. Quaternary ammonium β-cyclodextrin nanoparticles for enhancing doxorubicin permeability across the in vitro blood-brain barrier. Biomacromolecules 2009, 10, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Wu, L.; Xu, L.; Lowe, T.L. β-cyclodextrin-poly(β-amino ester) nanoparticles for sustained drug delivery across the blood-brain barrier. Biomacromolecules 2012, 13, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; El-Dakdouki, M.H.; Zhu, D.C.; Abela, G.S.; Huang, X. Synthesis of β-cyclodextrin conjugated superparamagnetic iron oxide nanoparticles for selective binding and detection of cholesterol crystals. Chem. Commun. (Camb) 2012, 48, 3385–3387. [Google Scholar] [CrossRef] [PubMed]
- Takechi-Haraya, Y.; Tanaka, K.; Tsuji, K.; Asami, Y.; Izawa, H.; Shigenaga, A.; Otaka, A.; Saito, H.; Kawakami, K. Molecular complex composed of β-cyclodextrin-grafted Chitosan and pH-sensitive amphipathic peptide for enhancing cellular cholesterol efflux under acidic pH. Bioconjug. Chem. 2015, 26, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Cogny, A.; Kockx, M.; Dean, R.T.; Gaus, K.; Jessup, W.; Kritharides, L. Cyclodextrins differentially mobilize free and esterified cholesterol from primary human foam cell macrophages. J. Lipid Res. 2003, 44, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Atger, V.M.; de la Llera Moya, M.; Stoudt, G.W.; Rodrigueza, W.V.; Phillips, M.C.; Rothblat, G.H. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J. Clin. Investig. 1997, 99, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kritharides, L.; Kus, M.; Brown, A.J.; Jessup, W.; Dean, R.T. Hydroxypropyl-β-cyclodextrin-mediated efflux of 7-ketocholesterol from macrophage foam cells. J. Biol. Chem. 1996, 271, 27450–27455. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Preclinical Reversal of Atherosclerosis by FDA-Approved Compound that Transforms Cholesterol into an Anti-Inflammatory “Prodrug”. Rejuven. Res. 2016, 19, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Nishiyama, R.; Maeda, Y.; Higashi, T.; Kawaguchi, Y.; Futaki, S.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Era, T.; et al. Cholesterol-Lowering Effect of Octaarginine-Appended β-Cyclodextrin in Npc1-Trap-CHO Cells. Biol. Pharm. Bull. 2016, 39, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.D.; Gong, W.; Verot, L.; Mellon, S.H. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 2004, 10, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Lope-Piedrafita, S.; Bi, X.; Hicks, C.; Yao, Y.; Yu, C.; Chaitkin, E.; Howison, C.M.; Weberg, L.; Trouard, T.P.; et al. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. J. Neurosci. Res. 2005, 82, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Langmade, S.J.; Gale, S.E.; Frolov, A.; Mohri, I.; Suzuki, K.; Mellon, S.H.; Walkley, S.U.; Covey, D.F.; Schaffer, J.E.; Ory, D.S. Pregnane X receptor (PXR) activation: A mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc. Natl. Acad. Sci. USA 2006, 103, 13807–13812. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Turley, S.D.; Burns, D.K.; Miller, A.M.; Repa, J.J.; Dietschy, J.M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ramirez, C.M.; Miller, A.M.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 2010, 51, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Aqul, A.; Liu, B.; Ramirez, C.M.; Pieper, A.A.; Estill, S.J.; Burns, D.K.; Liu, B.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 2011, 31, 9404–9413. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.I.; Zhang, G.; Warren, J.D.; Maxfield, F.R. Endocytosis of β-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5477–5482. [Google Scholar] [CrossRef] [PubMed]
- Vite, C.H.; Bagel, J.H.; Swain, G.P.; Prociuk, M.; Sikora, T.U.; Stein, V.M.; Donnell, P.O.; Ruane, T.; Ward, S.; Crooks, A. Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann-Pick type C1 disease. Sci. Transl. Med. 2015, 7, 276ra26. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; Fishman, Y.I.; Puskas, I.; Szemán, J.; Sohajda, T.; McCauliff, L.A.; Sikora, J.; Storch, J.; Vanier, M.T.; Szente, L.; et al. Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann. Clin. Transl. Neurol. 2016, 3, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, S.M.; Jacobs, R.F.; Massarella, J.; Kauffman, R.E.; Bradley, J.S.; Kimko, H.C.; Kearns, G.L.; Shalayda, K.; Curtin, C.; Maldonado, S.D.; et al. Single-dose pharmacokinetics of intravenous itraconazole and hydroxypropyl-β-cyclodextrin in infants, children, and adolescents. Antimicrob. Agents Chemother. 2007, 51, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, P.; Haskins, N.J.; Howlett, D.R. β-Cyclodextrin interacts with the Alzheimer amyloid β-A4 peptide. FEBS Lett. 1994, 341, 256–258. [Google Scholar] [CrossRef]
- Danielsson, J.; Jarvet, J.; Damberg, P.; Graslund, A. Two-site binding of β-cyclodextrin to the Alzheimer Aβ(1–40) peptide measured with combined PFG-NMR diffusion and induced chemical shifts. Biochemistry 2004, 43, 6261–6269. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, A.; Cukalevski, R.; Danielsson, J.; Jarvet, J.; Onagi, H.; Rebek, J., Jr.; Linse, S.; Gräslund, A. Specific binding of a β-cyclodextrin dimer to the amyloid β peptide modulates the peptide aggregation process. Biochemistry 2012, 51, 4280–4289. [Google Scholar] [CrossRef] [PubMed]
- Gavini, E.; Rassu, G.; Haukvik, T.; Lanni, C.; Racchi, M.; Giunchedi, P. Mucoadhesive microspheres for nasal administration of cyclodextrins. J. Drug Target 2009, 17, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.; Bigucci, F.; Corace, G.; Delucca, A.; Cerchiara, T.; Sorrenti, M.; Catenacci, L.; Di Pietra, A.M.; Zecchi, V. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-Alzheimer drug tacrine. Eur. J. Pharm. Sci. 2011, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Tao, E.X.; Meng, Q.H.; Hou, W.X.; Liu, K.; Shang, H.C.; Tang, J.B.; Zhang, W.F. Formulation, optimization, and pharmacodynamic evaluation of chitosan/phospholipid/β-cyclodextrin microspheres. Drug Des. Dev. Ther. 2016, 10, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 2012, 209, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.; Baum, L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.W.; Solinsky, C.M.; Loya, C.M.; Salituro, F.G.; Rodgers, K.E.; Bauer, G.; Rogawski, M.A.; Brinton, R.D. Allopregnanolone preclinical acute pharmacokinetic and pharmacodynamic studies to predict tolerability and efficacy for Alzheimer’s disease. PLoS ONE 2015, 10, e0128313. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, A.; Soddu, E.; Turunc Bayrakdar, E.; Uyanikgil, Y.; Kanit, L.; Armagan, G.; Rassu, G.; Gavini, E.; Giunchedi, P. Neuroprotective Effects of Engineered Polymeric Nasal Microspheres Containing Hydroxypropyl-β-cyclodextrin on β-Amyloid (1–42)-Induced Toxicity. J. Pharm. Sci. 2016, 105, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bakhos, L.; Chang, L.; Holterman, M.J.; Klein, W.L.; Venton, D.L. Per-6-substituted β-cyclodextrin libraries inhibit formation of β-amyloid-peptide (A β)-derived, soluble oligomers. J. Mol. Neurosci. 2002, 19, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Caldera, F.; Cavalli, R.; Soster, M.; Riedo, C.; Biasizzo, M.; Uccello Barretta, G.; Balzano, F.; Brunella, V. Molecularly imprinted cyclodextrin nanosponges for the controlled delivery of L-DOPA: Perspectives for the treatment of Parkinson’s disease. Expert Opin. Drug Deliv. 2016, 13, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzuki, N.; Taniguchi, S.; Oikawa, T.; Nonaka, T.; Iwatsubo, T.; Hisanaga, S.; Goedert, M.; Hasegawa, M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry 2006, 45, 6085–6094. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Karmakar, S.; Bose, A.; Chowdhury, P.K. β-cyclodextrin and curcumin, a potent cocktail for disaggregating and/or inhibiting amyloids: A case study with alpha-synuclein. Biochemistry 2014, 53, 4081–4083. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, K.; Zeng, Y.; Hancock, T.; Segatori, L. Genetic and chemical activation of TFEB mediates clearance of aggregated alpha-synuclein. PLoS ONE 2015, 10, e0120819. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kang, W.; Chun, I.K.; Oh, S.Y.; Lee, Y.H.; Gwak, H.S. Pharmacokinetic evaluation and modeling of formulated levodopa intranasal delivery systems. Eur. J. Pharm. Sci. 2009, 38, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Modi, G.; Antonio, T.; Reith, M.; Dutta, A. Structural modifications of neuroprotective anti-Parkinsonian (−)-N6-(2-(4-(biphenyl-4-yl)piperazin-1-yl)-ethyl)-N6-propyl-4,5,6,7-tetrahydrobe nzo[d]thiazole-2,6-diamine (D-264): An effort toward the improvement of in vivo efficacy of the parent molecule. J. Med. Chem. 2014, 57, 1557–1572. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, P.; Rockenstein, E.; Adame, A.; Ho, G.; Hashimoto, M.; Masliah, E. Effects of the cholesterol-lowering compound methyl-β-cyclodextrin in models of alpha-synucleinopathy. J. Neurochem. 2006, 98, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Trapani, A.; Mandracchia, D.; de Giglio, E.; Cometa, S.; Mangini, V.; Arnesano, F.; Belgiovine, G.; Castellani, S.; Pace, L.; et al. Intranasal delivery of dopamine to the striatum using glycol chitosan/sulfobutylether-β-cyclodextrin based nanoparticles. Eur. J. Pharm. Biopharm. 2015, 94, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, D.; Xifro, X.; Pol, A.; Humbert, S.; Saudou, F.; Canals, J.M.; Alberch, J. Altered cholesterol homeostasis contributes to enhanced excitotoxicity in Huntington’s disease. J. Neurochem. 2010, 115, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.M.; Ogier, J.R.; Darcy, R.; O’Driscoll, C.M.; Cryan, J.F. Self-assembling modified β-cyclodextrin nanoparticles as neuronal siRNA delivery vectors: Focus on Huntington’s disease. Mol. Pharm. 2013, 10, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Hockly, E.; Richon, V.M.; Woodman, B.; Smith, D.L.; Zhou, X.; Rosa, E.; Sathasivam, K.; Ghazi-Noori, S.; Mahal, A.; Lowden, P.A.; Steffan, J.S.; Marsh, J.L.; Thompson, L.M.; et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Francis, G.A. Cholesterol homeostasis and high-density lipoprotein formation in arterial smooth muscle cells. Trends Cardiovasc. Med. 2010, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Kruth, H.S. Lipoprotein cholesterol and atherosclerosis. Curr. Mol. Med. 2001, 1, 633–653. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Soumian, S.; Amey, J.S.; Sardini, A.; Higgins, C.F.; Davies, A.H.; Gibbs, R.G. ABCA1 expression in carotid atherosclerotic plaques. Stroke 2004, 35, 2801–2806. [Google Scholar] [CrossRef] [PubMed]
- Forcheron, F.; Legedz, L.; Chinetti, G.; Feugier, P.; Letexier, D.; Bricca, G.; Beylot, M. Genes of cholesterol metabolism in human atheroma: Overexpression of perilipin and genes promoting cholesterol storage and repression of ABCA1 expression. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Soumian, S.; Gibbs, R.; Davies, A.; Albrecht, C. mRNA expression of genes involved in lipid efflux and matrix degradation in occlusive and ectatic atherosclerotic disease. J. Clin. Pathol. 2005, 58, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Rahmani, M.; Wong, B.W.; Allahverdian, S.; McManus, B.M.; Pickering, J.G.; Chan, T.; Francis, G.A. ATP-binding cassette transporter A1 expression and apolipoprotein A-I binding are impaired in intima-type arterial smooth muscle cells. Circulation 2009, 119, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Kimura-Matsumoto, M.; Murakami, M.; Murakami, M.; Yamamoto, K.; Akasaka, Y.; Uzuki, M.; Yuri, Y.; Inomata, N.; Yokoo, T.; et al. Distribution of smooth muscle cells and macrophages expressing scavenger receptor BI/II in atherosclerosis. J. Atheroscler. Thromb. 2009, 16, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Malanga, M.; Szeman, J.; Fenyvesi, E.; Puskás, I.; Csabai, K.; Gyémánt, G.; Fenyvesi, F.; Szente, L. Back to the Future: A New Look at Hydroxypropyl Β-Cyclodextrins. J. Pharm. Sci. 2016, 105, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Yamamoto, M.; Irie, T.; Irie, T.; Hirayama, F.; Uekama, K. Some pharmaceutical properties of 3-hydroxypropyl- and 2,3-dihydroxypropyl-β-cyclodextrins and their solubilizing and stabilizing abilities. Chem. Pharm. Bull. (Tokyo) 1989, 37, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; McKilligin, E.; Pei, L.; Watson, M.A.; Collins, A.R.; Laffitte, B.A.; Chen, M.; Noh, G.; Goodman, J.; Hagger, G.N. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 7604–7609. [Google Scholar] [CrossRef] [PubMed]
- Feig, J.E.; Pineda-Torra, I.; Sanson, M.; Bradley, M.N.; Vengrenyuk, Y.; Bogunovic, D.; Gautier, E.L.; Rubinstein, D.; Hong, C.; Liu, J.; et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Investig. 2010, 120, 4415–4424. [Google Scholar] [CrossRef] [PubMed]
- Loren, J.; Huang, Z.; Laffitte, B.A.; Molteni, V. Liver X receptor modulators: A review of recently patented compounds (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.T. Niemann-Pick disease type C. Orphanet. J. Rare Dis. 2010, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.T. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis. 2015, 38, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Pentchev, P.G.; Gal, A.E.; Boothe, A.D.; Fouks, J.; Omodeo-Sale, F.; Brady, R.O. A lysosomal storage disorder in mice characterized by the accumulation of several sphingolipids. Birth Defects Orig. Artic. Ser. 1980, 16, 225–230. [Google Scholar] [PubMed]
- Carstea, E.D.; Morris, J.A.; Coleman, K.G.; Loftus, S.K.; Zhang, D.; Cummings, C.; Gu, J.; Rosenfeld, M.A.; Pavan, W.J.; Krizman, D.B.; et al. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 1997, 277, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Loftus, S.K.; Morris, J.A.; Carstea, E.D.; Gu, J.Z.; Cummings, C.; Brown, A.; Ellison, J.; Ohno, K.; Rosenfeld, M.A.; Tagle, D.A.; et al. Murine model of Niemann-Pick C disease: Mutation in a cholesterol homeostasis gene. Science 1997, 277, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Karasinska, J.M.; Rinninger, F.; Lutjohann, D.; Ruddle, P.; Franciosi, S.; Kruit, J.K.; Singaraja, R.R.; Hirsch-Reinshagen, V.; Fan, J.; Brunham, L.R.; et al. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J. Neurosci. 2009, 29, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- McCauliff, L.A.; Xu, Z.; Storch, J. Sterol transfer between cyclodextrin and membranes: Similar but not identical mechanism to NPC2-mediated cholesterol transfer. Biochemistry 2011, 50, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- Camargo, F.; Erickson, R.P.; Garver, W.S.; Hossain, G.S.; Carbone, P.N.; Heidenreich, R.A.; Blanchard, J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001, 70, 131–142. [Google Scholar] [CrossRef]
- Gosselet, F.; Saint-Pol, J.; Candela, P.; Fenart, L. Amyloid-β Peptides, Alzheimer’s Disease and the Blood-brain Barrier. Curr. Alzheimer Res. 2013, 10, 1015–1033. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B. Cholesterol and the biology of Alzheimer’s disease. Neuron 2004, 41, 7–10. [Google Scholar] [CrossRef]
- Puglielli, L.; Friedlich, A.L.; Setchell, K.D.; Nagano, S.; Opazo, C.; Cherny, R.A.; Barnham, K.J.; Wade, J.D.; Melov, S.; Kovacs, D.M.; et al. Alzheimer disease β-amyloid activity mimics cholesterol oxidase. J. Clin. Investig. 2005, 115, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Maulik, M.; Westaway, D.; Jhamandas, J.H.; Kar, S. Role of cholesterol in APP metabolism and its significance in Alzheimer’s disease pathogenesis. Mo. Neurobiol. 2013, 47, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.S.; Seshadri, S.; Beiser, A.; Wilson, P.W.; Kiel, D.P.; Tocco, M.; D'Agostino, R.B.; Wolf, P.A. Plasma total cholesterol level as a risk factor for Alzheimer disease: The Framingham Study. Arch. Intern. Med. 2003, 163, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Refolo, L.M.; Pappolla, M.A.; LaFrancois, J.; Malester, B.; Schmidt, S.D.; Thomas-Bryant, T.; Tint, G.S.; Wang, R.; Mercken, M.; Petanceska, S.S. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2001, 8, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.C.; Chuang, Y.S.; Hsieh, H.M.; Lee, T.C.; Chiu, K.F.; Liu, C.K.; Wu, M.T. Early Statin Use and the Progression of Alzheimer Disease: A Total Population-Based Case-Control Study. Medicine (Baltimore) 2015, 94, e2143. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, K.; Simons, M.; Bergmann, C.; Stroick, M.; Lutjohann, D.; Keller, P.; Runz, H.; Kuhl, S.; Bertsch, T.; von Bergmann, K.; Hennerici, M.; et al. Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ 42 and Aβ 40 in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 5856–5861. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Callaghan, D.; Jones, A.; Walker, D.G.; Lue, L.F.; Beach, T.G.; Sue, L.I.; Woulfe, J.; Xu, H.; Stanimirovic, D.B.; et al. Cholesterol retention in Alzheimer’s brain is responsible for high β- and gamma-secretase activities and Aβ production. Neurobiol. Dis. 2008, 29, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Koldamova, R.P.; Lefterov, I.M.; Staufenbiel, M.; Wolfe, D.; Huang, S.; Glorioso, J.C.; Walter, M.; Roth, M.G.; Lazo, J.S. The liver X receptor ligand T0901317 decreases amyloid β production in vitro and in a mouse model of Alzheimer’s disease. J. Biol. Chem. 2005, 280, 4079–4088. [Google Scholar] [CrossRef] [PubMed]
- Terwel, D.; Steffensen, K.R.; Verghese, P.B.; Kummer, M.P.; Gustafsson, J.Å.; Holtzman, D.M.; Heneka, M.T. Critical role of astroglial apolipoprotein E and liver X receptor-alpha expression for microglial Aβ phagocytosis. J. Neurosci. 2011, 31, 7049–7059. [Google Scholar] [CrossRef] [PubMed]
- Koldamova, R.; Staufenbiel, M.; Lefterov, I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J. Biol. Chem. 2005, 280, 43224–43235. [Google Scholar] [CrossRef] [PubMed]
- Reinke, A.A.; Gestwicki, J.E. Structure-activity relationships of amyloid β-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Westerman, M.A.; Cooper-Blacketer, D.; Mariash, A.; Kotilinek, L.; Kawarabayashi, T.; Younkin, L.H.; Carlson, G.A.; Younkin, S.G.; Ashe, K.H. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci. 2002, 22, 1858–1867. [Google Scholar] [PubMed]
- Hsia, A.Y.; Masliah, E.; McConlogue, L.; Yu, G.Q.; Tatsuno, G.; Hu, K.; Kholodenko, D.; Malenka, R.C.; Nicoll, R.A.; Mucke, L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. USA 1999, 96, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Moosmann, B.; Behl, C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin. Investig. Drugs 2002, 11, 1407–1435. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.R.; Streit, W.J. Microglia in the aging brain. J. Neuropathol. Exp. Neurol. 2006, 65, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001, 2, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Sardiello, M.; Palmieri, M.; di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S. A gene network regulating lysosomal biogenesis and function. Science 2009, 325, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Valenza, M.; Cattaneo, E. Cholesterol dysfunction in neurodegenerative diseases: Is Huntington’s disease in the list? Prog. Neurobiol. 2006, 80, 165–176. [Google Scholar] [CrossRef] [PubMed]
 | |||
|---|---|---|---|
| Abbreviation | n | Substituent (R) | Number of R Group by CD |
| α-CD | 6 | (−) | 0 |
| β-CD | 7 | (−) | 0 |
| γ-CD | 8 | (−) | 0 |
| HPαCD | 6 | -CH2-CHOH-CH3 | 3.6 |
| RAMEα | 6 | -CH3 | 10.8 |
| HPβCD | 7 | -CH2-CHOH-CH3 | 5.6 |
| KLEPTOSE® CRYSMEB | 7 | -CH3 | 4 |
| Methyl-β-CD | 7 | -CH3 | 1.6 |
| RAMEβ | 7 | -CH3 | 12.6 |
| SBE7-β-CD | 7 | -(CH2)4-SO3Na | 7 |
| TRIMETHYL-β-CD | 7 | -CH3 | 21 |
| HPγCD | 8 | -CH2-CHOH-CH3 | 4.8 |
| RAMEγ | 8 | -CH3 | 14.4 |
| Disease | CD | Therapeutical Effect(s) of CDs | Reference |
|---|---|---|---|
| Atherosclerosis | β-CD | Cholesterol depletion | [12] |
| Enhances cholesterol efflux from cell membranes | [52], alone or associated with chitosan and/or CEEP | ||
| Selective binding to cholesterol crystals allowing early detection of plaques | [51], carboxy-methyl-β-CD conjugated with superparamagnetic ion oxyde nanoparticles | ||
| RAMEβ | Cholesterol depletion | [12] | |
| KLEPTOSE® CRYSMEβ | Reduced atherosclerotic plaque size in vivo and T cell content | [11] | |
| Cholesterol depletion | [11,12] | ||
| Methyl-β-CD | Cholesterol depletion | [12] | |
| HPβCD | Cholesterol depletion | [13,53,54,55,56] | |
| Reduces inflammation and lowers atherosclerotic lesion formation, reduces amounts of cholesterol crystals in atherosclerotic plaques | [13] | ||
| Niemann–Pick type C | β-CD | Reduced intracellular cholesterol pool | [57], conjugated to octa-arginine |
| HPβCD | Cholesterol depletion, reverses defective lysosomal transport, delays demyelination, recovers neuronal function, increased life expectation in animal models, reduces hepatic cholesterol level and liver dysfunction. Do not cross the mature BBB, ototoxicity | [54,58,59,60,61,62,63,64,65,66,67], associated with allopregnanolone | |
| Completed phase I clinical trial | [68], associated with itraconazole | ||
| Alzheimer’s disease | β-CD | Binds Aβ peptide, reduces Aβ neurotoxicity | [69,70,71] |
| Nasal delivery system for brain targeting | [72], coupled with alginate or chitosan | ||
| Nasal delivery system for brain targeting | [73], associated with tacrine/albumin | ||
| Oral delivery system for brain targeting | [74], associated with chitosan and phospholipids | ||
| HPβCD | Cholesterol depletion, improves spatial learning and memory deficits in animal model, decreases Aβ plaque deposition | [75] | |
| Nasal delivery system for brain targeting | [72], coupled with alginate or chitosan | ||
| Nasal delivery system for brain targeting | [73], associated with tacrine/albumin | ||
| Cryoprotective in drug delivery complex | [76], coupled with curcumin | ||
| Improved brain delivery and neurogenic drug efficacy | [77], associated with allopregnanolone | ||
| Nasal delivery system for brain targeting, | [78], coupled with chitosan or sodium alginate | ||
| per-6-alkylamino-β-CD | Binds Aβ peptide, reduces Aβ neurotoxicity | [79] | |
| SBE7-β-CD | Nasal delivery system for brain targeting, decreases Aβ-induced neurotoxicity in rat hippocampus | [73], associated with tacrine/albumin | |
| Improved brain delivery and neurogenic drug efficacy | [77], coupled with allopregnanolone | ||
| Parkinson’s disease | β-CD | Nasal delivery system for l-DOPA into the brain | [80] |
| Increases curcumin potency in inhibiting α-synuclein aggregation | [81], associated with curcumin | ||
| Dissolves preformed α-synuclein aggregates | [82], associated with curcumin | ||
| HPβCD | Activates the transcription factor EB, which is involved in the autophagy-lysosomal degradation pathway | [83] | |
| Nasal delivery system for l-DOPA into the brain | [84], coupled with L-DPOPA and maleic acid | ||
| Enhances drug delivery across the BBB | [85], conjugated with dopamine receptor agonist | ||
| methyl-β-CD | Reduces α-synuclein protein aggregation | [86] | |
| SBE7-β-CD | Nasal delivery system for l-DOPA into the brain | [87], incorporated in nanoparticles with l-DOPA | |
| Huntington’s disease | β-CD | Reduces lipid content in cell membrane | [88] |
| Enhances siRNA delivery to the brain | [89], coupled to htt siRNA | ||
| HPβCD | Enhances drug delivery to the brain | [90], associated with SAHA |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules 2016, 21, 1748. https://doi.org/10.3390/molecules21121748
Coisne C, Tilloy S, Monflier E, Wils D, Fenart L, Gosselet F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules. 2016; 21(12):1748. https://doi.org/10.3390/molecules21121748
Chicago/Turabian StyleCoisne, Caroline, Sébastien Tilloy, Eric Monflier, Daniel Wils, Laurence Fenart, and Fabien Gosselet. 2016. "Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases" Molecules 21, no. 12: 1748. https://doi.org/10.3390/molecules21121748
APA StyleCoisne, C., Tilloy, S., Monflier, E., Wils, D., Fenart, L., & Gosselet, F. (2016). Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules, 21(12), 1748. https://doi.org/10.3390/molecules21121748






