Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia
Abstract
:1. Introduction
2. Plant Distribution
3. Chemical Constituents
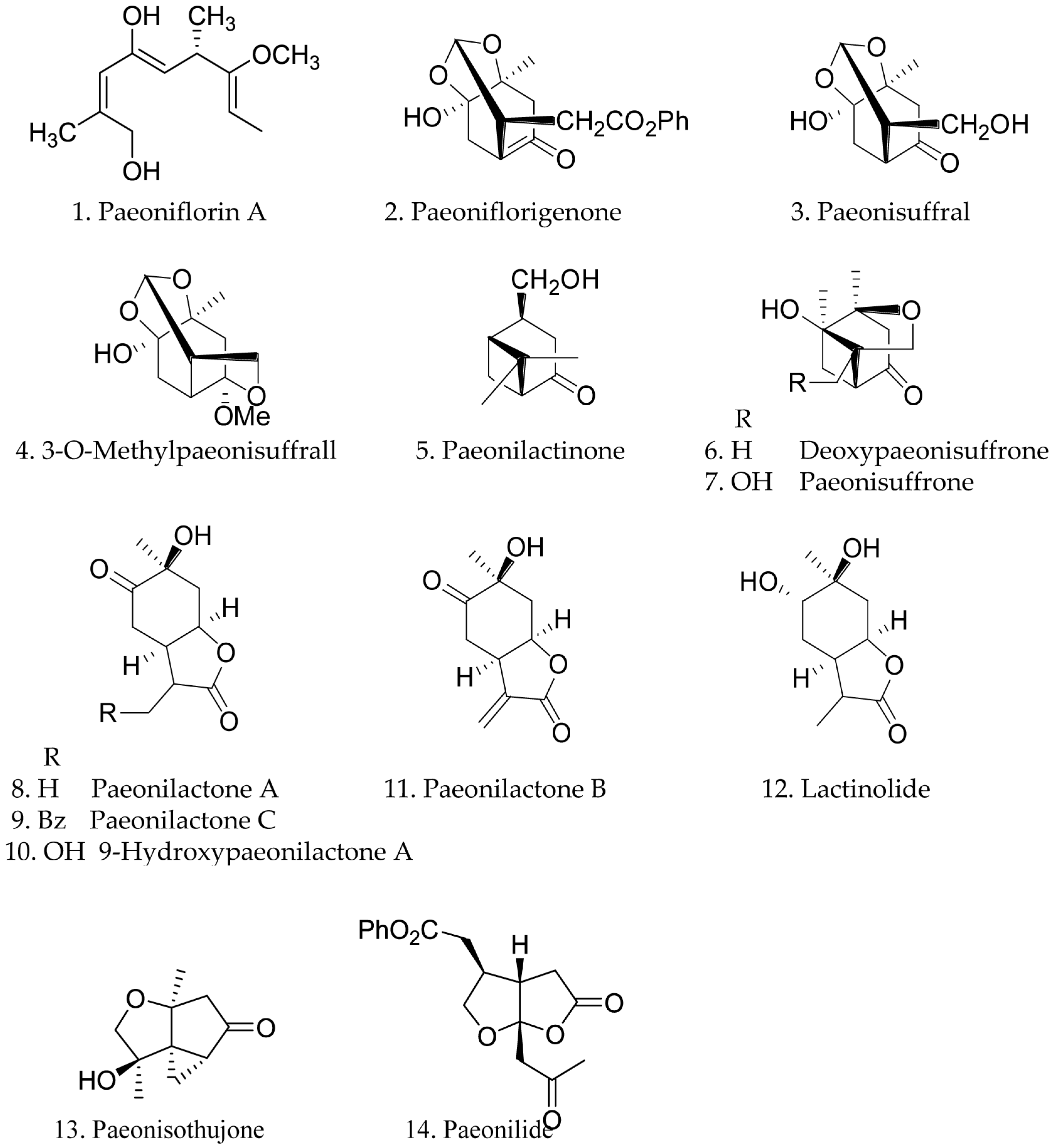
3.1. Monoterpenes
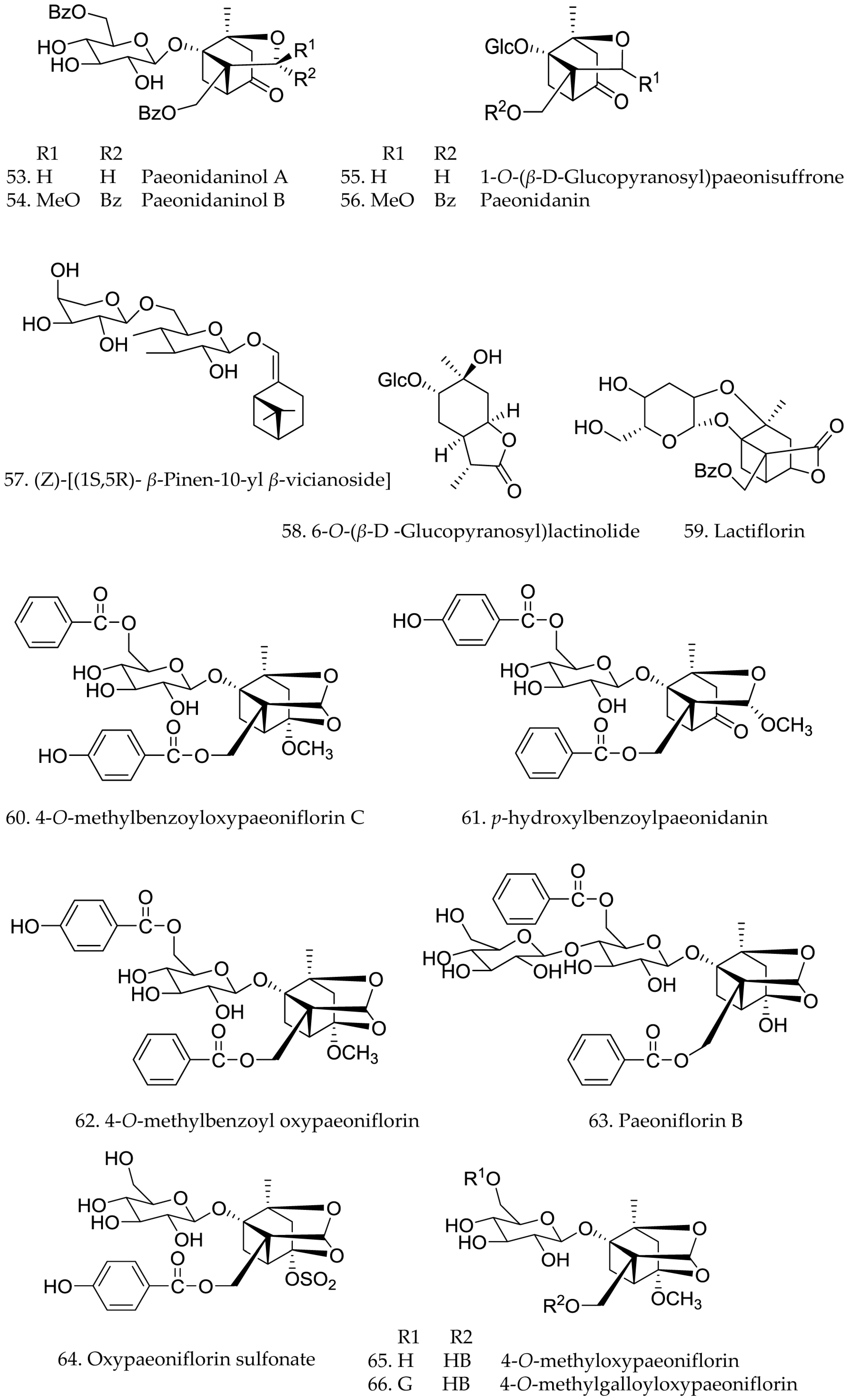
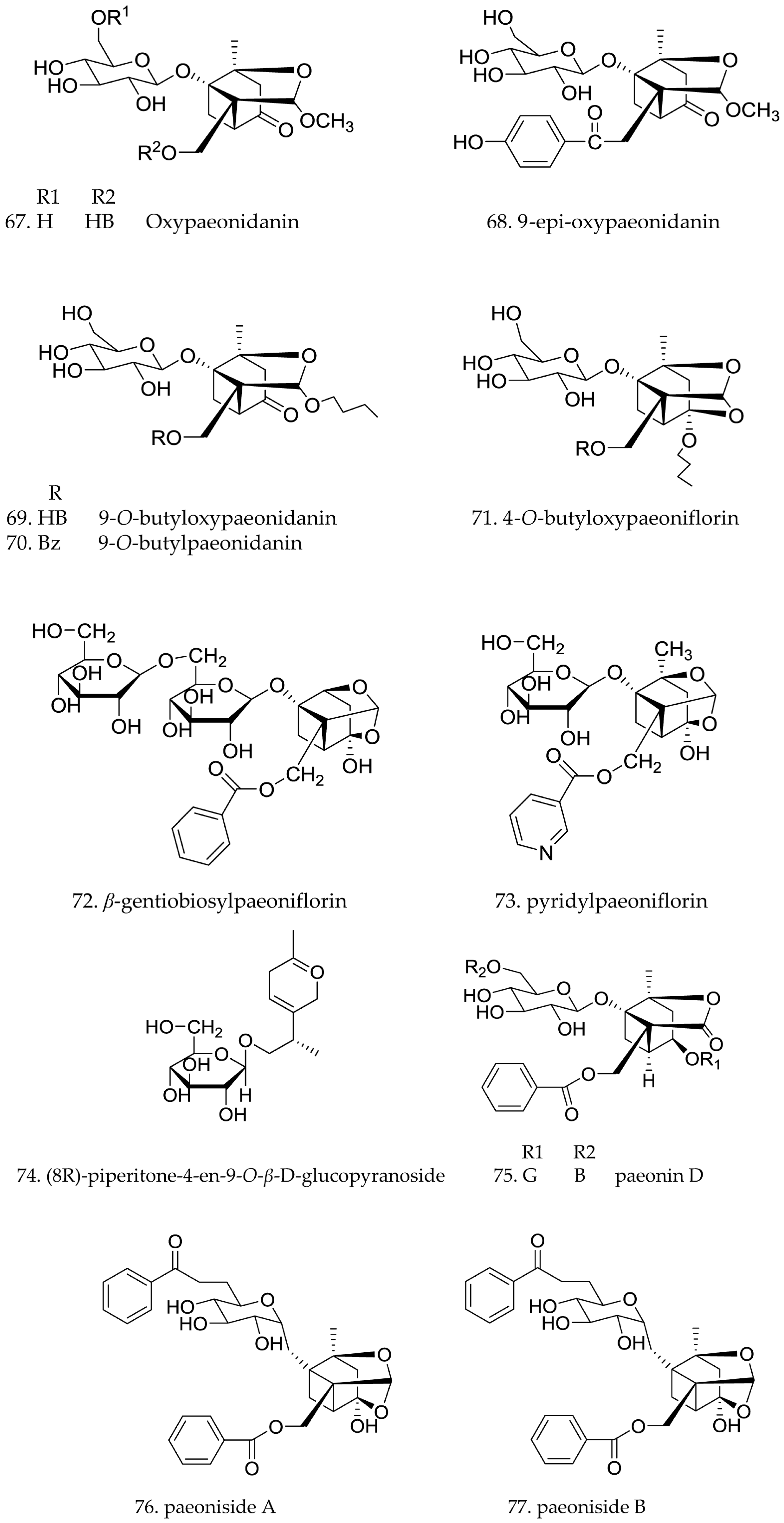
3.2. Monoterpene Glycosides
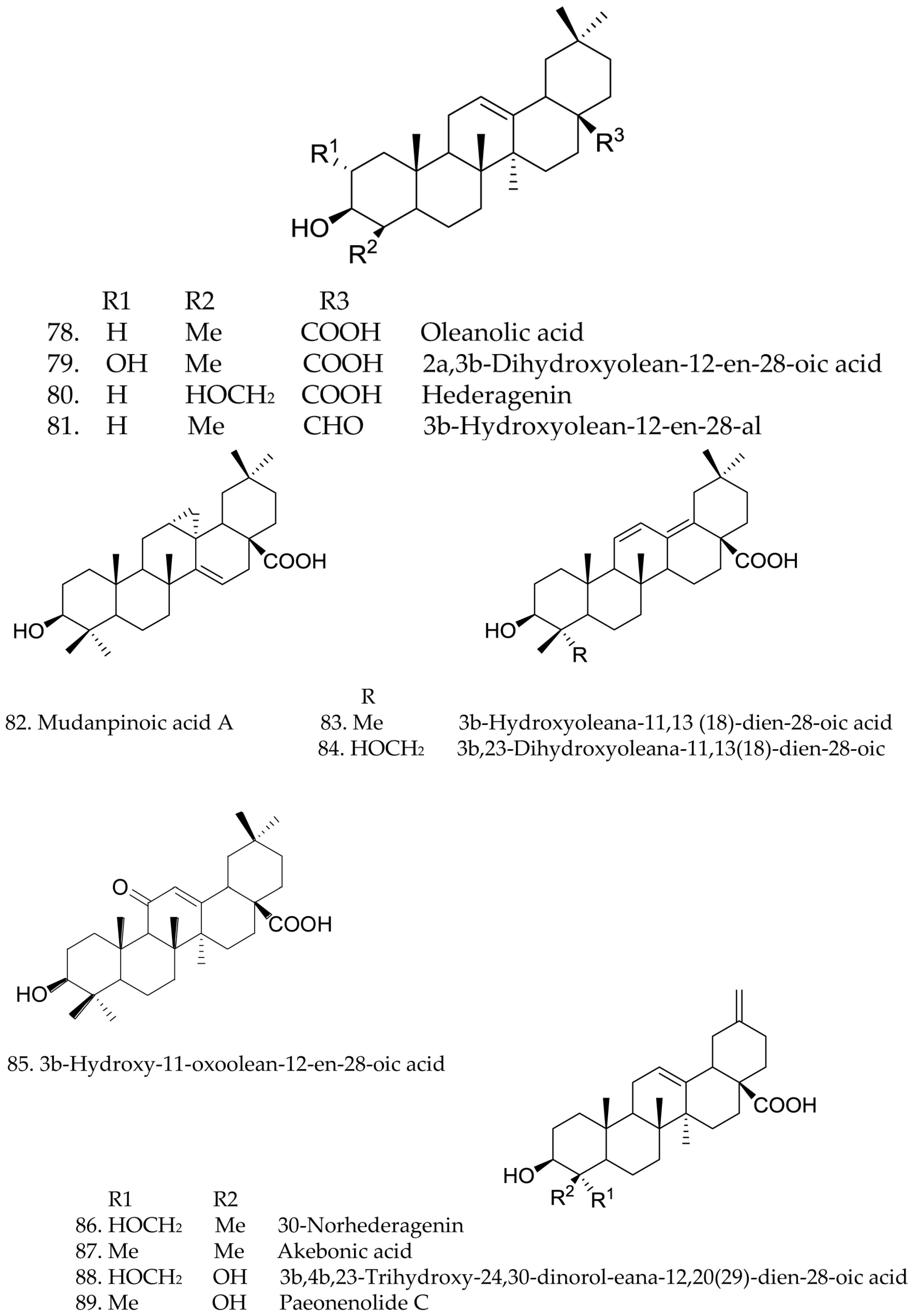
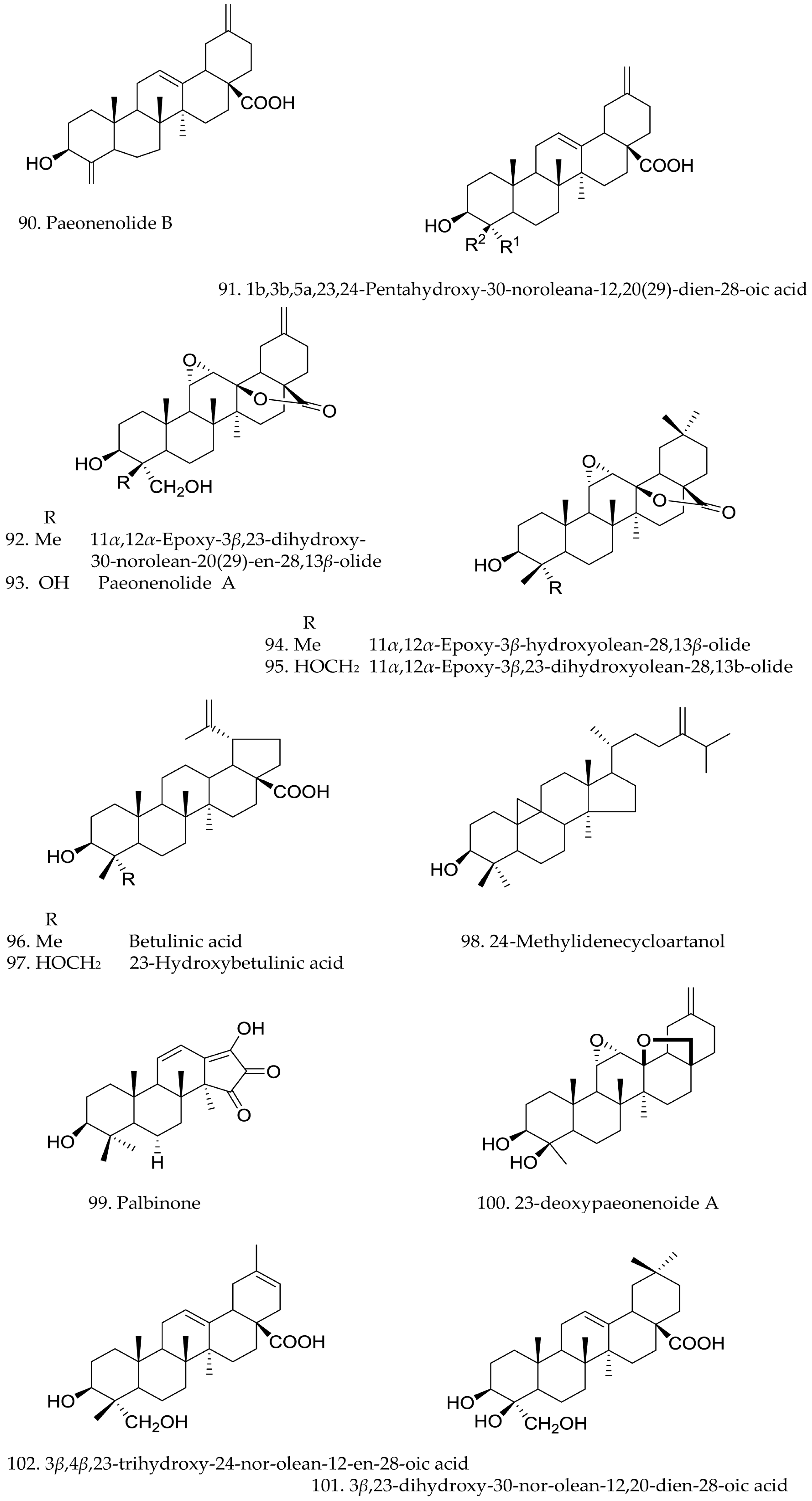
3.3. Triterpenes
4. Biological Activities
4.1. Inhibitors of Nitric Oxide Production
4.2. Anti-Tumor Activity
4.3. Anti-Inflammatory Effects
4.4. Anti-Oxidative Effects
4.5. Anti-Aggregatory and Anti-Coagulative Effects
4.6. Sedative and Analgesic
4.7. Other Activities
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Talmadge, J.E. Natural product derived immune-regulatory agents. Int. Immunopharmacol. 2016, 37, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Maarten, J.M.C.; James, W.B. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar]
- Chen, C.; Du, P.; Wang, J. Paeoniflorin ameliorates acute myocardial infarction of rats by inhibiting inflammation and inducible nitric oxide synthase signaling pathways. Mol. Med. Rep. 2015, 12, 3937–3943. [Google Scholar] [PubMed]
- Ding, L.; Zhao, F.; Chen, L.; Jiang, Z.; Liu, Y.; Li, Z.; Qiu, F.; Yao, X. New monoterpene glycosides from Paeonia suffruticosa andrews and their inhibition on NO production in LPS-induced RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2012, 22, 7243–7247. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xue, X.; Zhang, B.; Jiang, W.; Cao, H.; Wang, R.; Sun, D.; Guo, R. The protective effects of paeonol against epirubicin-induced hepatotoxicity in 4T1-tumor bearing mice via inhibition of the PI3K/Akt/NF-κB pathway. Chem. Biol. Interact. 2016, 244, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yazawa, K.; Kondo, S.; Mukudai, Y.; Sato, D.; Kurihara, Y.; Kamatani, T.; Shintani, S. The root bark of Paeonia moutan is a potential anticancer agent in human oral squamous cell carcinoma cells. Anticancer Res. 2012, 32, 2625–2630. [Google Scholar] [PubMed]
- Ma, Z.; Chu, L.; Liu, H.; Li, J.; Zhang, Y.; Liu, W.; Dai, J.; Yi, J.; Gao, Y. Paeoniflorin alleviates non-alcoholic steatohepatitis in rats: Involvement with the rock/NF-κB pathway. Int. Immunopharmacol. 2016, 38, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Huang, L. Anti-influenza virus activity and constituents. Characterization of Paeonia delavayi extracts. Molecules 2016, 21, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, L.; Yang, Z.; Wang, J.; Li, W.; Zhou, J.; Zhang, J. Hematopoietic effects of paeoniflorin and albiflorin on radiotherapy-induced myelosuppression mice. Evid. Complement. Altern. Med. eCAM 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.K.; Kim, J.M.; Koo, J.Y.; Kang, S.S.; Bae, K.; Kim, Y.S.; Chung, J.H.; Yun-Choi, H.S. Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa. Die Pharm. 2010, 65, 624–628. [Google Scholar]
- He, C.; Peng, B.; Dan, Y.; Peng, Y.; Xiao, P. Chemical taxonomy of tree peony species from China based on root cortex metabolic fingerprinting. Phytochemistry 2014, 107, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Jugran, A.K.; Chaudhary, W.Y.; Bahukhandi, A.; Bhatt, I.D.; Rawal, R.S.; Dhyani, P.P. Effect of processing and storage methods on the nutritional, anti-nutritional, and anti-oxidant properties of Paeonia emodi, wall. Ex. Royle. Appl. Biochem. Biotechnol. 2016, 180, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Kim, C.Y.; Kim, H.J.; Park, J.H.; Ahn, M.J. Differences in the chemical profiles and biological activities of Paeonia lactiflora and Paeonia obovata. J. Med. Food 2015, 18, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, X.; Wu, Y.; Shiraishi, F.; Kawahara, N.; Komatsu, K. Genetic and chemical characterization of white and red peony root derived from Paeonia lactiflora. J. Nat. Med. 2015, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chou, G.X.; Zhu, E.Y.; Wang, Z.T.; Bi, K.S. A new phenolic glycoside from the roots of Paeonia veitchii. J. Asian Nat. Prod. Res. 2006, 8, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L.; Zou, X.H.; Zhou, Z.Q.; Liu, J.; Xu, C.; Yu, J.; Wang, Q.; Zhang, D.M.; Wang, X.Q.; Ge, S.; et al. Multiple species of wild tree peonies gave rise to the “king of flowers”, Paeonia suffruticosa andrews. Proc. R. Soc. Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Gong, X.; Kuroda, C. Chemical diversity of iridal-type triterpenes in iris delavayi collected in Yunnan province of China. Nat. Prod. Commun. 2011, 6, 789–792. [Google Scholar] [PubMed]
- Horikoshi, T.; Homma, N.; Hatakeyama, Y.; Hemmi, S. Studies on the cultivation of medicinal plants. V. On the growth of Paeonia suffruticosa Andr. Especially multiplication and yield (author’s transl). Bull. Natl. Inst. Hyg. Sci. 1975, 109–113. [Google Scholar]
- Ding, L.; Jiang, Z.; Liu, Y.; Chen, L.; Zhao, Q.; Yao, X.; Zhao, F.; Qiu, F. Monoterpenoid inhibitors of no production from Paeonia suffruticosa. Fitoterapia 2012, 83, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.C.; Ding, W.; Wu, J.; Qian, D.W.; Li, Z.H.; Qian, Y.F.; Guo, J.M.; Duan, J.A. UPLC-Q-TOF/MS coupled with multivariate statistical analysis as a powerful technique for rapidly exploring potential chemical markers to differentiate between radix paeoniae alba and radix paeoniae rubra. Nat. Prod. Commun. 2013, 8, 487–491. [Google Scholar] [PubMed]
- Yoshikawa, M.; Ohta, T.; Kawaguchi, A.; Matsuda, H. Bioactive constituents of Chinese natural medicines. V. Radical scavenging effect of moutan cortex. (1): Absolute stereostructures of two monoterpenes, paeonisuffrone and paeonisuffral. Chem. Pharm. Bull. 2000, 48, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Saka, M.; Shimada, H.; Matsuda, H.; Yamahara, J.; Yoshikawa, M. New bioactive monoterpene glycosides from paeoniae radix. Chem. Pharm. Bull. 1996, 44, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Ma, Y.B.; Wu, D.G.; Lu, Y.; Shen, Z.Q.; Zheng, Q.T.; Chen, Z.H. Paeonilide, a novel anti-PAF-active monoterpenoid-derived metabolite from Paeonia delavayi. Biosci. Biotechnol. Biochem. 2000, 64, 1511–1514. [Google Scholar] [PubMed]
- Wang, S.J.; Yang, Y.C.; Li, S.; Shi, J.G. A new paeoniflorin derivative isolated from the root bark ethanol extract of Paeonia suffruticosa. China J. Chin. Mater. Med. 2005, 30, 759–761. [Google Scholar]
- Wu, S.H.; Chen, Y.W.; Yang, L.Y.; Li, S.L.; Li, Z.Y. Monoterpene glycosides from Paeonia delavayi. Fitoterapia 2007, 78, 76–78. [Google Scholar] [CrossRef] [PubMed]
- An, R.B.; Kim, H.C.; Lee, S.H.; Jeong, G.S.; Sohn, D.H.; Park, H.; Kwon, D.Y.; Lee, J.H.; Kim, Y.C. A new monoterpene glycoside and antibacterial monoterpene glycosides from Paeonia suffruticosa. Arch. Pharm. Res. 2006, 29, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, M.; Chen, Y.; Zhang, Y.; Zhao, X.; Zheng, X. Revealing metabolomic variations in cortex moutan from different root parts using HPLC-MS method. Phytochem. Anal. PCA 2015, 26, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Park, E.K.; Joo, J.H.; Lee, B.H.; Choi, B.W.; Jung, D.S.; Lee, N.H. A new antioxidant monoterpene glycoside, α-benzoyloxypaeoniflorin from Paeonia suffruticosa. Arch. Pharm. Res. 2001, 24, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Lin, H.C.; Chang, T.S. Tyrosinase inhibitors isolated from the roots of Paeonia suffruticosa. J. Cosmet. Sci. 2009, 60, 347–352. [Google Scholar] [PubMed]
- Furuya, R.; Hu, H.; Zhang, Z.; Shigemori, H. Suffruyabiosides A and B, two new monoterpene diglycosides from moutan cortex. Molecules 2012, 17, 4915–4923. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Cheng, Z.H.; Chen, D.F. Anticomplement monoterpenoid glucosides from the root bark of Paeonia suffruticosa. J. Nat. Prod. 2014, 77, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Nagai, F.; Seto, T.; Satoh, K.; Ushiyama, K.; Kano, I. The inhibition of phenylhydroquinone-induced oxidative DNA cleavage by constituents of moutan cortex and paeoniae radix. Biol. Pharm. Bull. 2000, 23, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ohta, T.; Kawaguchi, A.; Yoshikawa, M. Bioactive constituents of Chinese natural medicines. VI. Moutan cortex. (2): Structures and radical scavenging effects of suffruticosides A, B, C, D, and e and galloyl-oxypaeoniflorin. Chem. Pharm. Bull. 2001, 49, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Uchida, E.; Kawaguchi, A.; Kitagawa, I.; Yamahara, J. Galloyl-oxypaeoniflorin, suffruticosides A, B, C, and D, five new antioxidative glycosides, and suffruticoside E, A paeonol glycoside, from Chinese moutan cortex. Chem. Pharm. Bull. 1992, 40, 2248–2250. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xiao, W.; Li, M.; Peng, Y.; Xu, L.; Gu, J.; Xiao, P. Chemical constituents from seeds of Paeonia suffruticosa. China J. Chin. Mater. Med. 2010, 35, 1428–1431. [Google Scholar]
- Tanaka, T.; Kataoka, M.; Tsuboi, N.; Kouno, I. New monoterpene glycoside esters and phenolic constituents of paeoniae radix, and increase of water solubility of proanthocyanidins in the presence of paeoniflorin. Chem. Pharm. Bull. 2000, 48, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Luo, X.D.; Ma, Y.B.; Hao, X.J.; Wu, D.G. Monoterpenoid derivatives from Paeonia delavayi. J. Asian Nat. Prod. Res. 2002, 4, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wang, S.B.; Zhao, S.H.; Chen, X.J.; Tu, P.F. Three new monoterpene glycosides from the roots of Paeonia lactiflora. J. Asian Nat. Prod. Res. 2013, 15, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Washida, K.; Yamagaki, T.; Iwashita, T.; Nomoto, K. Two new galloylated monoterpene glycosides, 4-O-galloylalbiflorin and 4′-O-galloylpaeoniflorin, from the roots of Paeonia lactiflora (Paeoniae radix) grown and processed in Nara Prefecture, Japan. Chem. Pharm. Bull. 2009, 57, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Kiem, P.V.; Yen, P.H.; Nhiem, N.X.; Quang, T.H.; Cuong, N.X.; Minh, C.V. New monoterpene glycosides from Paeonia lactiflora. Fitoterapia 2008, 79, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Anis, I.; Malik, A.; Ahmed, Z.; Muhammad, P.; Nawaz, S.A.; Choudhary, M.I. Paeonins A and B, lipoxygenase inhibiting monoterpene galactosides from Paeonia emodi. Chem. Pharm. Bull. 2003, 51, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.H.; Kiem, P.V.; Nhiem, N.X.; Tung, N.H.; Quang, T.H.; Minh, C.V.; Kim, J.W.; Choi, E.M.; Kim, Y.H. A new monoterpene glycoside from the roots of Paeonia lactiflora increases the differentiation of osteoblastic MC3T3-E1 cells. Arch. Pharm. Res. 2007, 30, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.Y.; Li, S.Z.; McCabe, T.; Clardy, J. A new monoterpene glycoside of Paeonia lactiflora. Planta Med. 1984, 50, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ullah, F.; Sadiq, A.; Ayaz, M.; Imran, M.; Ali, I.; Zeb, A.; Shah, M.R. Chemical composition, antioxidant and anticholinesterase potentials of essential oil of Rumex hastatus D. Don collected from the North West of Pakistan. BMC Complement. Altern. Med. 2016, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Zhang, Y.C.; Peng, Y.; Yang, J.S.; Xiao, P.G. Monoterpene glycosides from the seeds of Paeonia suffruticosa protect HEK 293 cells from irradiation-induced DNA damage. Phytochem. Lett. 2012, 5, 128–133. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.M.; Li, T.; Zhang, Y.B.; Sze, S.C.; Wang, G.C.; Li, Y.L.; Ye, W.C. Monoterpene derivatives from the roots of Paeonia lactiflora and their anti-proliferative activity. Fitoterapia 2014, 98, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fang, Z.H. New monoterpene glycosides from the root cortex of Paeonia suffruticosa and their potential anti-inflammatory activity. Nat. Prod. Res. 2014, 28, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, D.D.; Yang, H.S.; Chen, Y.Y.; Wei, J.F.; Kang, W.Y.; Guo, X.C. Determination of oleanic acid and paeoniflorin in Paeonia lactiflora by ultrasound-assisted ionic liquid-reversed phase liquid chromatography. China J. Chin. Mater. Med. 2015, 40, 443–449. [Google Scholar]
- Gao, H.; Zhang, X.; Wang, N.L.; Liu, H.W.; Zhang, Q.H.; Song, S.S.; Yu, Y.; Yao, X.S. Triterpenoid saponins from Stauntonia chinensis. J. Asian Nat. Prod. Res. 2007, 9, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mencherini, T.; Picerno, P.; Festa, M.; Russo, P.; Capasso, A.; Aquino, R. Triterpenoid constituents from the roots of Paeonia rockii ssp. rockii. J. Nat. Prod. 2011, 74, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Ding, H.Y.; Wu, Y.C. Two novel compounds from Paeonia suffructicosa. J. Nat. Prod. 1998, 61, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Z.; Yang, L.; Yu, Y.; Yao, Z.-H.; Wang, N.-L.; Zhou, G.-X.; Ye, W.-C.; Yao, X.-S. Five new bidesmoside triterpenoid saponins from Stauntonia chinensis. Magn. Reson. Chem. MRC 2008, 46, 630–637. [Google Scholar]
- Kadota, S.; Terashima, S.; Basnet, P.; Kikuchi, T.; Namba, T. Palbinone, a novel terpenoid from Paeonia albiflora; potent inhibitory activity on 3α-hydroxysteroid dehydrogenase. Chem. Pharm. Bull. 1993, 41, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Makino, M.; Ohkoshi, E.; Uchiyama, T.; Fujimoto, Y. Terpenoids and phenethyl glucosides from Hyssopus cuspidatus (labiatae). Phytochemistry 2011, 72, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Tantry, M.A.; Dar, J.A.; Khuroo, M.A.; Shawl, A.S. Nortriterpenoids from the roots of Paeonia emodi. Phytochem. Lett. 2012, 5, 253–257. [Google Scholar] [CrossRef]
- Fu, Q.; Qiu, L.; Yuan, H.M.; Yu, T.; Zou, L. Paeonenoides D and E: Two new nortriterpenoids from Paeonia lactiflora and their inhibitory activities on NO production. Helv. Chim. Acta 2016, 99, 46–49. [Google Scholar] [CrossRef]
- Ambroz, M.; Hanusova, V.; Skarka, A.; Bousova, I.; Kralova, V.; Langhasova, L.; Skalova, L. Essential oil from Myrica rubra leaves potentiated antiproliferative and prooxidative effect of doxorubicin and its accumulation in intestinal cancer cells. Planta Med. 2016, 82, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; Giffune, T.R. The thermodynamics of protein interactions with essential first row transition metals. Biochim. Biophys. Acta 2016, 1860, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kawamoto, A.; Tamura, A.; Tatsumi, Y.; Fujii, T. Mechanism of antioxidant action of pueraria glycoside (PG)-1 (an isoflavonoid) and mangiferin (a xanthonoid). Chem. Pharm. Bull. 1992, 40, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, W.; Shi, Q.; Zhao, L.; Mei, F.; Li, C.; Zuo, T.; He, X. Paeoniflorin ameliorates acute necrotizing pancreatitis and pancreatitisinduced acute renal injury. Mol. Med. Rep. 2016, 14, 1123–1131. [Google Scholar] [PubMed]
- Riaz, N.; Malik, A.; Rehman, A.U.; Ahmed, Z.; Muhammad, P.; Nawaz, S.A.; Siddiqui, J.; Choudhary, M.I. Lipoxygenase inhibiting and antioxidant oligostilbene and monoterpene galactoside from Paeonia emodi. Phytochemistry 2004, 65, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.W.; Piao, M.J.; Kim, K.C.; Zheng, J.; Cha, J.W.; Hyun, J.W. 6′-O-galloylpaeoniflorin protects human keratinocytes against oxidative stress-induced cell damage. Biomol. Ther. 2013, 21, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kimura, I.; Takahashi, K.; Muroi, M.; Yoshizaki, M.; Kanaoka, M.; Kitagawa, I. Blocking effects of blended paeoniflorin or its related compounds with glycyrrhizin on neuromuscular junctions in frog and mouse. Jpn. J. Pharmacol. 1984, 36, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Sun, Y.; Luo, Y.J.; Zhao, X.; Chen, J.F.; Yanagawa, Y.; Qu, W.M.; Huang, Z.L. Paeoniflorin promotes non-rapid eye movement sleep via adenosine a1 receptors. J. Pharmacol. Exp. Ther. 2016, 356, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Lee, M.M.; Fang, H.L.; Yang, J.G.; Chen, Y.C.; Tsai, H.Y. Paeoniflorin inhibits excitatory amino acid agonist-and high-dose morphine-induced nociceptive behavior in mice via modulation of N-methyl-d-aspartate receptors. BMC Complement. Altern. Med. 2016, 16, 240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, M.K.; Lee, K.Y.; Sung, S.H.; Kim, J.; Kim, Y.C. Chemical constituents isolated from Paeonia lactiflora roots and their neuroprotective activity against oxidative stress in vitro. J. Enzym. Inhib. Med. Chem. 2009, 24, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Lai, C.W.; Cheng, J.T. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of Paeonia lactiflora. Planta Med. 1997, 63, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Dou, Y.; Guo, J.; Wu, X.; Dai, Y. Paeoniflorin of Paeonia lactiflora prevents renal interstitial fibrosis induced by unilateral ureteral obstruction in mice. Phytomedicine 2013, 20, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Harada, M. Pharmacological studies on herb paeony root. II. Anti-inflammatory effect, inhibitory effect on gastric juice secretion, preventive effect on stress ulcer, antidiuretic effect of paeoniflorin and combined effects with licorice component Fm 100. J. Pharm. Soc. Jpn. 1969, 89, 887–892. [Google Scholar]
- Nawaz, H.R.; Malik, A.; Khan, P.M.; Shujaat, S.; Rahman, A. A novel β-glucuronidase inhibiting triterpenoid from Paeonia emodi. Chem. Pharm. Bull. 2000, 48, 1771–1773. [Google Scholar] [CrossRef] [PubMed]
- Josef, J.H.; James, W.W. The Genus Paeonia; Timber Press: Oregon, OR, USA, 2004. [Google Scholar]
- Michio, T. Paeoniaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 10, pp. 265–269. [Google Scholar]



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.-D.; Jiang, L.-L.; Li, H.-Y.; Yan, P.-F.; Zhang, Y.-L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules 2016, 21, 1362. https://doi.org/10.3390/molecules21101362
Zhao D-D, Jiang L-L, Li H-Y, Yan P-F, Zhang Y-L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules. 2016; 21(10):1362. https://doi.org/10.3390/molecules21101362
Chicago/Turabian StyleZhao, Dan-Dan, Li-Li Jiang, Hong-Yi Li, Peng-Fei Yan, and Yan-Long Zhang. 2016. "Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia" Molecules 21, no. 10: 1362. https://doi.org/10.3390/molecules21101362
APA StyleZhao, D.-D., Jiang, L.-L., Li, H.-Y., Yan, P.-F., & Zhang, Y.-L. (2016). Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules, 21(10), 1362. https://doi.org/10.3390/molecules21101362





