Anti-Inflammatory Effects of Aspalathus linearis and Cyclopia spp. Extracts in a UVB/Keratinocyte (HaCaT) Model Utilising Interleukin-1α Accumulation as Biomarker
Abstract
:1. Introduction
2. Results
2.1. Comparative Effects of Extracts in UVB Irradiated and Non-Irradiated HaCaTs
2.1.1. Cell Viability (Table 1)
2.1.2. Cell Proliferation (Table 1)
2.1.3. Modulation of UVB-Induced Apoptosis in Relation to Cell Viability (Table 2)
2.1.4. Modulation of UVB-Induced icIL-1α Accumulation
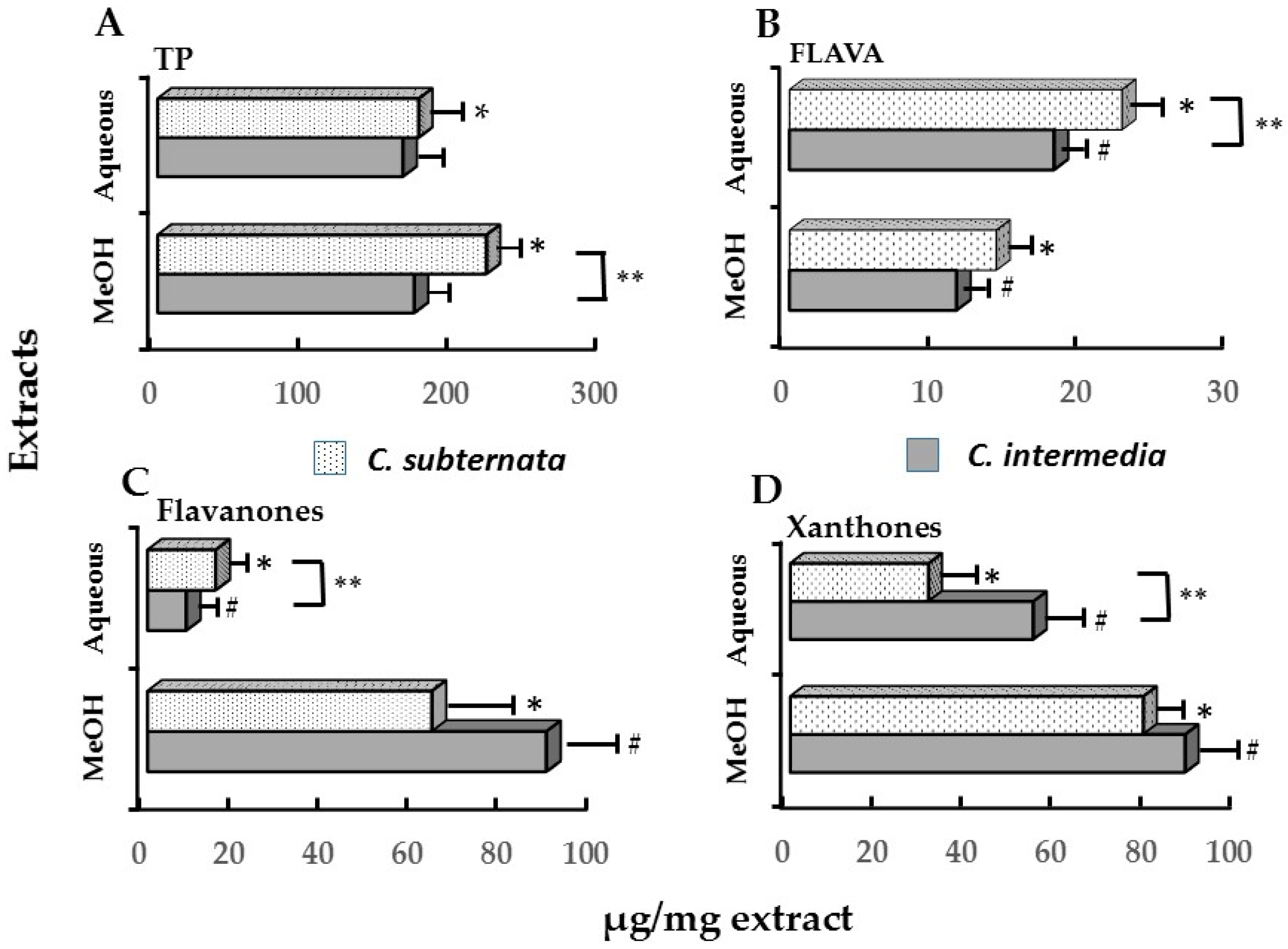
2.2. Relationships between Cell Viability, Apoptosis, Induction of icIL-1α and Major Polyphenolic Constituents
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extract Preparation
4.3. UVB/Keratinocyte Inflammatory Cell Model
4.4. Modulation of IL-1α Production and Different Cell Growth Parameters
4.5. Cell Growth Parameters
4.5.1. Cell Viability Assay
4.5.2. Cell Proliferation Assay
4.5.3. Modulation of Apoptosis
4.5.4. Modulation of IL-1α Accumulation
4.6. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Gruijl, F.R. Skin cancer and solar UV radiation. Eur. J. Cancer 1999, 35, 2003–2009. [Google Scholar] [CrossRef]
- Halliday, G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. 2005, 571, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Nishisgori, C. Current concept of photocarcinogenesis. Photochem. Photobiol. Sci. 2015, 14, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Soehnge, H.; Outiht, A.; Ananthaswamy, H.N. Mechanisms of induction of skin cancer induction by UV radiation. Front. Biosci. 1997, 2, 538–551. [Google Scholar]
- Melnikova, V.O.; Ananthaswamy, H.N. Cellular and molecular events leading to the development of skin cancer. Mutat. Res. 2005, 571, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Armstrong, A.W. Nonmelanoma skin cancer. Dermatol. Clin. 2012, 30, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P. Non-melanoma skin cancer: What drives tumor development and progression? Carcinogenesis. 2005, 26, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Halliday, G.M.; Damian, D.L. Non-melanoma skin cancer: Carcinogenesis and chemoprevention. Pathology 2013, 45, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R.; van kranen, H.J.; Mullenders, L.H. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J. Photochem. Photobiol. B 2001, 63, 19–27. [Google Scholar] [CrossRef]
- Kim, Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T. Mechanisms of UV-induced immunosuppression. Keio. J. Med. 2005, 54, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997, 8, 253–265. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Morello, A.P.; Mays, D.A. Early inflammatory processes in the skin. Curr. Mol. Med. 2013, 13, 1250–1269. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Carmi, Y.; Voronov, E.; Apte, R.N. Interleukin-1α. Semin. Immunol. 2013, 25, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Locniskar, M.F.; Fischer, S.M. Interleukin-1α mediates phorbol ester induced inflammation and epidermal hyperplasia. FASEB J. 1994, 8, 1081–1087. [Google Scholar] [PubMed]
- Rauschmayr, T.; Nakamura, K.; Sarkar, S.; Williams, I.R.; Kupper, T.S. Inflammatory and hyperproliferative skin disease in mice that express elevated levels of the IL-1 receptor (type I) on epidermal keratinocytes. Evidence that IL-1-inducible secondary cytokines produced by keratinocytesin vivo can cause skin disease. J. Clin. Invest. 1996, 98, 336–344. [Google Scholar]
- Li, X.; Eckard, J.; Shah, R.; Malluck, C.; Frenkel, K. Interleukin-1α up-regulationin vivo by a potent carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) and control of DMBA-induced inflammatory responses. Cancer Res. 2002, 62, 417–423. [Google Scholar] [PubMed]
- Lee, W.Y.; Butler, A.P.; Locniskar, M.F.; Fischer, S.M. Signal transduction pathway(s) involved in phorbol ester and autocrine induction of interleukin-1 alpha mRNA in murine keratinocytes. J. Biol. Chem. 1994, 269, 17971–17980. [Google Scholar] [PubMed]
- Hobbs, R.M.; Watt, F.M. Regulation of interleukin-1α expression by intergrins and epidermal growth factor receptor in keratinocytes from a mouse model of inflammatory skin disease. J. Biol. Chem. 2003, 278, 19798–19807. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Rider, P.; Carmi, Y.; Braiman, A.; Dotan, S.; White, M.R.; Voronov, E.; Martin, M.U.; Dinarello, C.A.; Apte, R.N. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. PNAS 2010, 107, 2574–2579. [Google Scholar] [CrossRef] [PubMed]
- Magcwebeba, T.U.; Riedel, S.; Swanevelder, S.; Swart, P.; de Beer, D.; Joubert, E.; Gelderblom, W.C.A. The potential role of polyphenols in the modulation of skin cell viability by Aspalathus linearis and Cyclopia spp. herbal tea extracts in vitro. J. Pharm. Pharmacol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Dossou, G.; Rougier, A.; Roguet, R. Measurement of inflammatory mediators produced by human keratinocytes in vitro: A predictive assessment of cutaneous irritation. Toxicol. In Vitro 1991, 5, 407–410. [Google Scholar] [CrossRef]
- Vazquez, R.; Nelson, M.R.; Guzman, J.J.; Corun, C.M.; Steinberg, M. Immortalised human keratinocytes: A model system to study the efficacy of therapeutic drugs in response to the chemical warfare agent sulphur mustard. Electron. J. Biotechnol. 2004, 7, 125–129. [Google Scholar] [CrossRef]
- Tebbe, B.; Wu, S.; Geilen, C.C.; Eberle, J.; Kodelja, V.; Orfanos, C.E. L-ascorbic acid inhibits UVA-induced lipid peroxidation and secretion of IL-1alpha and IL-6 in cultured human keratinocytes in vitro. J. Investig. Dermatol. 1997, 108, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Lulli, D.; Potapovich, A.I.; Fidanza, P.; Kostyuk, V.A.; Dellambra, E.; de Luca, C.; Maurelli, R.; Korkina, L.G. Differential modulation of stress-inflammation responses by plant polyphenols in cultured normal human keratinocytes and immortalized HaCaT cells. J. Dermatol. Sci. 2011, 63, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.; Potapovich, A.; de Luca, C. The promise of plant polyphenols as the golden standard skin anti-inflammatory agents. Curr. Drug Metab. 2010, 11, 415–424. [Google Scholar] [CrossRef]
- Mantena, S.K.; Katiyar, S.K. Grapeseed proanthocyanidins inhibit UV-radiation induced oxidative stress and activation of MAPK and NF-κB signalling in human keratinocytes. Free Rad. Biol. Med. 2006, 40, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; de Luca, C.; Pastore, S.; Korkina, L.G. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFκB and AhR and EGFR-ERK pathway. Toxicol. Appl. Pharmacol. 2011, 255, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Prasad, N.R. Sesamol modulates UVB induced apoptotic and inflammatory signalling in human skin dermal fibroblasts. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012, 2, 31–39. [Google Scholar]
- Katiyar, S.K.; Rupp, C.O.; Korman, N.J.; Agarwal, R.; Mukhtar, H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate and other skin tumor-promoter-caused induction of epidermal interleukin-1 mRNA and protein expression in SENCAR mice by green tea polyphenol. J. Investig. Dermatol. 1995, 105, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Mukhtar, H. Inhibition of phorbol ester tumor-promoter induced 12-O-tetradecanoylphorbol-13-acetate caused inflammatory response in SENCAR mouse skin by black tea polyphenols. Carcinogenesis 1997, 18, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Gelderblom, W.C.; Louw, A.; de Beer, D. South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoide—A review. J. Ethnopharmacol. 2008, 119, 376–412. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; de Beer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Joubert, E.; Joubert, M.E.; Bester, C.; de Beer, D.; de Lange, J.H. Honeybush (Cyclopia spp.): From local cottage industry to global markets—The catalytic and supporting role of research. S. Afr. J. Bot. 2011, 77, 887–907. [Google Scholar] [CrossRef]
- Marnewick, J.; Joubert, E.; Joseph, S.; Swanevelder, S.; Swart, P.; Gelderblom, W. Inhibition of tumour promotion in mouse skin by extracts of rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), unique South African herbal teas. Cancer Lett. 2005, 224, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Petrova, A.; Davids, L.M.; Rautenbach, F.; Marnewick, J.L. Photoprotection by honeybush extracts, hesperidin and mangiferin against UVB-induced skin damage in SKH-1 mice. J. Photochem. Photobiol. B 2011, 103, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Na, H.K.; Mossando, K.S.; Lee, J.Y.; Surh, Y.I. Inhibition of phorbol ester induced COX-2 expression by some edible African plants. Biofactors 2004, 21, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Kupper, T.S.; Chua, A.O.; Flood, P.; McGuire, J.; Gubler, U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J. Clin. Investig. 1987, 80, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, I.M.; Tomic-Canic, M.; Komine, M.; Blumenberg, M. Keratins and the keratinocytes activation cycle. J. Investig. Dermatol. 2001, 116, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Magcwebeba, T.; Riedel, S.; Swanevelder, S.; Bouic, P.; Swart, P.; Gelderblom, W. Interleukin-1α induction in human keratinocytes (HaCaT): An in vitro model for chemoprevention in skin. J. Skin Cancer 2012, 2012, 393681. [Google Scholar] [CrossRef] [PubMed]
- Gniadecki, R.; Thorn, T.; Vicanova, J.; Petersen, A.; Wulf, H.C. Role of mitochondria in ultraviolet-induced oxidative stress. J. Cell. Biochem. 2001, 80, 216–222. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Marques, M.P.M.; Diniz, C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.A., Jr.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing the NF-κB pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.; Arranz, J.A.; Yanez, M.; Ubeira, F.M.; Sanmartin, M.L.; Orallo, F. Expression profiles of genes involved in mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int. J. Immunopharmacol. 2004, 4, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; Delgado, R.; Lemus, Y.; Rodríguez, J.; García, D.; Núñez-Sellés, A.J. Protection against septic shock and suppression of tumor necrosis factor alpha and nitric oxide production on macrophages and microglia by a standard aqueous extract of Mangifera indica L. (VIMANG®) Role of mangiferin isolated from the extract. Pharmacol. Res. 2004, 50, 50165–50172. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-C.; Kao, S.-J.; Lin, C.-C.; Wang, S.-D.; Liu, C.-J.; Kao, S.-T. The immunomodulation of endotoxin-induced acute lung injury by hesperidinin vivo and in vitro. Life Sci. 2007, 80, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- OyetakinWhite, P.O.; Tribout, H.; Baron, E. Protective mechanisms of green tea polyphenols in skin. Oxid. Med. Cell. Longev. 2012, 2012, 560682. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-P.; Lou, Y.-R.; Xie, J.-G.; Peng, Q.-Y.; Liao, J.; Yang, C.S.; Huang, M.-T.; Conney, A.H. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 12455–12460. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-P.; Lou, Y.-R.; Peng, Q.-Y.; Xie, J.-G.; Huang, M.-T.; Conney, A.H. Stimulatory effect of topical application of caffeine on UVB-induced apoptosis in the epidermis of p53 and Bax knockout mice. Cancer Res. 2004, 64, 5020–5027. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Ming, M.; He, Y.-Y. Caffeine promotes Ultraviolet B-induced apoptosis in human keratinocytes without complete DNA repair. J. Biol. Chem. 2011, 286, 22825–22832. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.R.; Melton, T.; Dong, Z.; Bowden, G.T. Stabilization of quercetin paradoxically reduces its proapoptotic effect on UVB-irradiated human keratinocytes. Cancer Prev. Res. 2008, 1, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Perk, A.A.; Shatynska-Mytsyk, A.; Gerçek, Y.C.; Boztaş, K.; Yazgan, M.; Fayyaz, S.; Farooqi, A.A. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Chien, Y.S.; Chiu, T.H.; Huang, W.W.; Lu, C.C.; Chiang, J.H.; Yang, J.S. Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signalling pathway. Oncol. Rep. 2012, 28, 1883–1888. [Google Scholar]
- Yuan, L.; Wei, S.; Wang, J.; Liu, X. Iso-orientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38 Signaling pathways in HepG2 cancer cells. J. Agric. Food Chem. 2014, 62, 5390–5400. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, P.; Wenk, J.; Klotz, L.O.; Wlaschek, M.; Briviba, K.; Krieg, T.; Sies, H.; Scharffetter-Kochanek, K. Central role of ferrous/ferric iron in the ultraviolet B irradiation-mediated signaling pathway leading to increased interstitial collagenase (matrix-degrading metalloprotease (MMP)-1) and stromelysin-1 (MMP-3) mRNA levels in cultured human dermal fibroblasts. J. Biol. Chem. 1998, 273, 5279–5287. [Google Scholar] [PubMed]
- Joubert, E.; Winterton, P.; Britz, T.J.; Gelderblom, W.C. Antioxidant and pro-oxidant activities of aqueous extracts and crude polyphenolic fractions of rooibos (Aspalathus linearis). J. Agric. Food Chem. 2005, 53, 10260–10267. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-S.; Byun, H.-O.; Cho, H.; Kim, B.-K.; Yoon, G.Y. Complex II defect via down-regulation of iron-sulfur subunit induces mitochondrial dysfunction and cell cycle delay in iron chelation-induced senescence-associated growth arrest. J. Biol. Chem. 2003, 278, 51577–51586. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.O.; Kim, H.Y.; Lim, J.J.; Seo, Y.H.; Yoon, G. Mitochondrial dysfunction by complex II inhibition delays overall cell cycle progression via reactive oxygen species production. J. Cell. Biochem. 2008, 104, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Liao, J.; Li, C.; Chung, J.; Yurkow, E.J.; Ho, C.T.; Yang, C.S. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: Possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis 2000, 21, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Elbling, L.; Weiss, R.M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Micksche, M. Green tea extract and (−)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J. 2005, 19, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Lemarie, A.; Huc, L.; Pazarentzos, E.; Mahul-Mellier, A.L.; Grimm, S. Specific disintegration of complex II succinate:ubiquinone oxidoreductase links pH changes to oxidative stress for apoptosis induction. Cell Death Differ. 2011, 18, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Herman, R.; Bursch, W.; Kraupp-Grasl, B.; Oberhammer, F.; Wagner, A.; Jirtle, R. Cell proliferation and apoptosis in normal liver and preneoplastic foci. Environ. Health Perspect. 1993, 101, 87–90. [Google Scholar] [CrossRef]
- Chae, S.; Piao, M.J.; Kang, A.K.; Zhang, R.; Kim, K.C.; Youn, U.J.; Nam, K.-W.; Lee, J.H.; Hyun, J.W. Inhibition of matrix metalloproteinase-1 induced oxidative stress from Anemerhena asphodeloides. Biosci. Biotechnol. Biochem. 2011, 75, 2321–2325. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Nemati, A. Radioprotective effects of hesperidin against gamma irradiation in mouse bone marrow cells. Br. J. Radiol. 2006, 79, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Richards, E.S.; Merwe, J.D.; de Beer, D.; Manley, M.; Gelderblom, W.C. Effect of species variation and processing on phenolic composition and in vitro antioxidant activity of aqueous extracts of Cyclopia spp. (honeybush tea). J. Agric. Food Chem. 2008, 56, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.M.; Kim, J.; Chung, H.Y.; Choi, J.S. Isolation of luteolin 7-O-rutinoside and esculetin with potential antioxidant activity from the aerial parts of Artemisia montana. Arch. Pharm. Res. 2000, 23, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.S.; Sreedevi, M.V.; Nageshwar Rao, B. Cytoprotective and antigenotoxic potential of mangiferin, a glucosylxanthone against cadmium chloride induced toxicity in HepG2 cells. Food Chem. Toxicol. 2009, 47, 592–600. [Google Scholar]
- Im, A.-R.; Yeon, S.H.; Lee, J.S.; Um, K.A.; Ahn, Y.-J.; Chae, S. Protective effect of fermented Cyclopia intermedia against UVB-induced damage in HaCaT human keratinocytes. BMC Complenment. Altern. Med. 2016, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
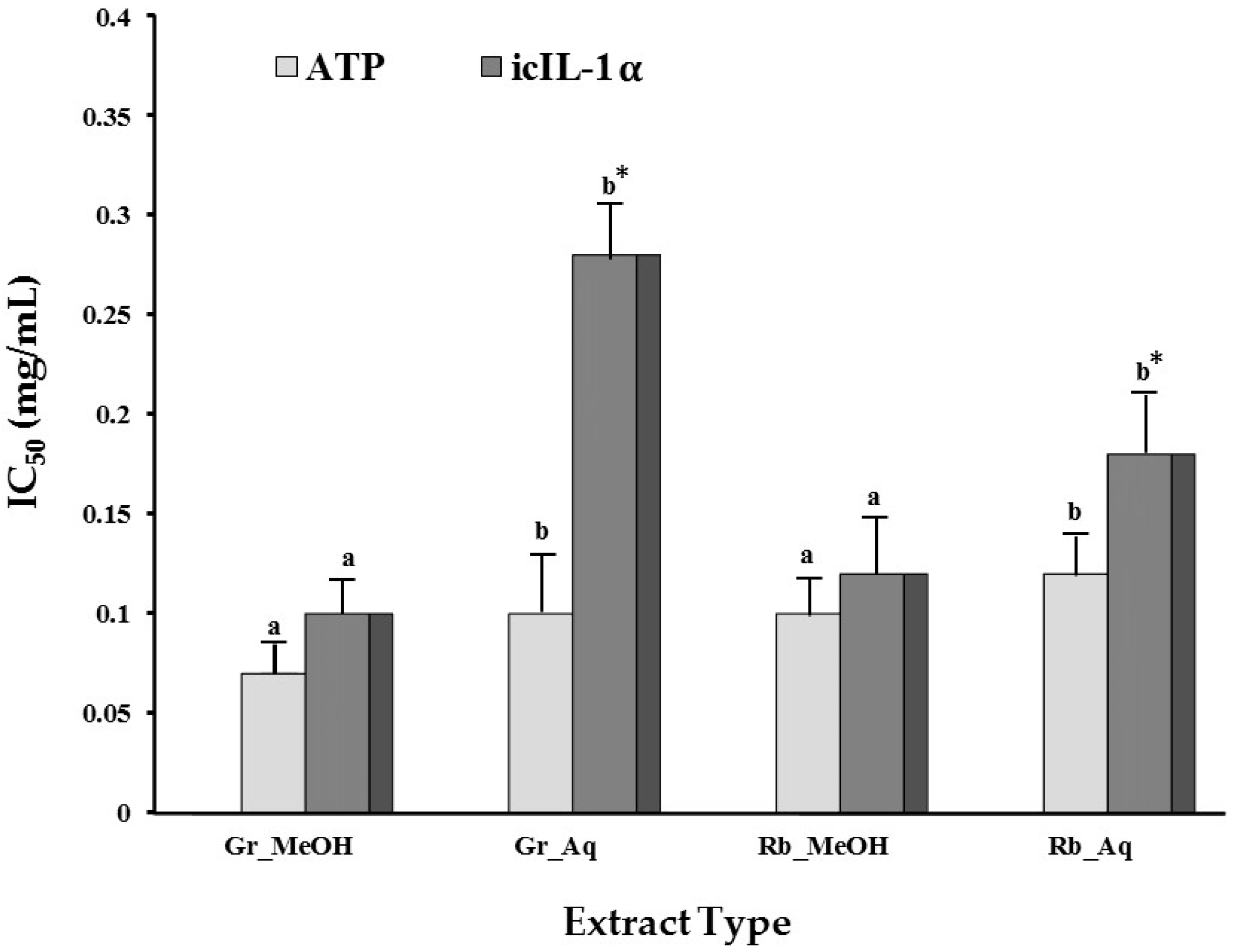
| Cell Viability (ATP IC50 (mg/mL)) | |||||
| Extract | UVB Irradiation | Green Tea | Rooibos | C. intermedia | C. subternata |
| Methanol | (−) | 0.08 ± 0.01 cA * | 0.15 ± 0.02 cA | 1.25 ± 0.21 b | 1.65 ± 0.14 a |
| (+) | 0.06 ± 0.01 aB * | 0.10 ± 0.01 aB * | >1.46 | >1.80 | |
| Aqueous | (−) | 0.14 ± 0.01 cA | 0.13 ± 0.01 cA | 0.49 ± 0 04 bA * | 0.89 ± 0.12 aA * |
| (+) | 0.14 ± 0.02 cA | 0.13 ± 0.01 cA | 0.41 ± 0.10 bA | 0.72 ± 0.11 aA | |
| Cell proliferation (BrdU IC50 (mg/mL)) | |||||
| Methanol | (−) | 0.06 ± 0.02 bA | 0.06 ± 0.01 bA | 0.37 ± 0.08 a | 0.27 ± 0.03 a |
| (+) | 0.09 ± 0.01 aA | 0.08 ± 0.01 aA | >0.71 | >1.80 | |
| Aqueous | (−) | 0.07 ± 0.01 bA | 0.08 ± 0.01 bA | 0.33 ± 0.07 aA | 0.31 ± 0.04 aA |
| (+) | 0.10 ± 0.02 cA | 0.10 ± 0.01 cA | 0.38 ± 0.05 bB | 0.53 ± 0.07 aB | |
| Extracts | Unit Measurement | Controls | Methanol Extracts (mg/mL) | Aqueous Extracts (mg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Green tea | (−) UVB | (+) UVB | 0.11 | 0.05 | 0.03 | 0.43 | 0.22 | 0.11 | |
| Casp-3 fold increase | 1.00 ± 0.19 d | 3.89 ± 0.68 c | 8.16 ± 0.74 a | 7.82 ± 0.76 a | 6.10 ± 0.21 b | 6.51 ± 0.77 a | 6.54 ± 0.48 a | 6.47 ± 0.81 a | |
| % ATP production | 100.00 ± 3.97 a | 74.63 ± 4.79 b | 27.74 ± 2.35 d | 49.16 ± 5.51 c | 69.47 ± 5.05 b | 8.90 ± 3.08 d | 37.98 ± 4.83 c | ± 7.36 c | |
| Rooibos | 0.19 | 0.10 | 0.05 | 0.55 | 0.28 | 0.14 | |||
| Casp-3 fold increase | 1.00 ± 0.14 e | 3.59 ± 0.62 d | 10.12 ± 0.88 a | 9.03 ± 0.66 b | 7.44 ± 0.49 c | 5.14 ± 0.50 a | 4.61 ± 0.36 b | 3.56 ± 0.19 d | |
| % ATP production | 100.00 ± 3.97 a | 74.63 ± 4.79 b | 28.43 ± 3.41 d | 48.18 ± 3.32 c | 66.74 ± 4.02 b | 15.94 ± 2.34 d | 29.56 ± 3.36 c | 48.94 ± 5.55 b | |
| C. intermedia | (−) UVB | (+) UVB | 0.73 | 0.37 | 0.18 | 0.79 | 0.39 | 0.20 | |
| Casp-3 fold increase | 1.00 ± 0.16 c | 3.24 ± 0.44 a | 1.13 ± 0.16 c | 1.98 ± 0.12 b | 2.80 ± 0.29 a | 5.38 ± 0.47 b | 4.76 ± 0.05 b | 3.17 ± 0.42 a | |
| % ATP production | 100.00 ± 3.80 a | 88.54 ± 8.84 b | 69.76 ± 2.92 c | 87.27 ± 5.25 b | 88.09 ± 3.59 b | 34.87 ± 2.39 d | 52.70 ± 5.46 b | 70.22 ± 6.27 c | |
| C. subternata | 0.71 | 0.36 | 0.18 | 0.75 | 0.36 | 0.18 | |||
| Casp-3 fold increase | 1.00 ± 0.13 c | 3.44 ± 0.26 a | 2.44 ± 0.26 b | 2.87 ± 0.63 b | 3.77 ± 0.27 a | 5.87 ± 0.46 b | 4.50 ± 0.45 b | 4.00 ± 0.36 a | |
| % ATP production | 100.00 ± 3.87 a | 92.37 ± 3.88 b | 85.12 ± 4.03 b | 94.50 ± 2.33 b | 93.78 ± 4.12 b | 56.94 ± 4.29 c | 82.76 ± 3.67 b | 93.46 ± 4.98 b | |
| Extract Type | (−) UVB | (+) UVB | Concentration (mg/mL) | |||
|---|---|---|---|---|---|---|
| 0.107 | 0.054 | 0.027 | 0.013 | |||
| Gr_Methanol | 2.57 ± 0.85a | 3.62 ± 0.31a | 1.35 ± 0.04b | 1.53 ± 0.15b | 1.84 ± 0.31b | 2.27 ± 0.57a |
| Gr_Aqueous | 2.92 ± 0.85a | 3.84 ± 0.56a | 2.01 ± 0.17b | 1.31 ± 0.20b | 1.15 ± 0.07b | 1.62 ± 0.15b |
| RB_Methanol | 1.62 ± 0.36a | 1.71 ± 0.24a | 1.43 ± 0.10a | 1.49 ± 0.36a | 1.62 ± 0.21a | 2.21 ± 0.18a |
| RB_Aqueous | 1.62 ± 0.36a | 1.71 ± 0.24a | 1.31 ± 0.29a | 1.26 ± 0.21a | 1.67 ± 0.49a | 1.20 ± 0.10a |
| Parameter | Controls | Methanol Extracts (mg/mL) | Aqueous Extracts (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. intermedia | ||||||||||
| (−) UVB | (+) UVB | 0.73 | 0.37 | 0.18 | 0.09 | 0.79 | 0.39 | 0.20 | 0.10 | |
| icIL-1α (pg/mL) | 8.35 ± 1.0 d | 28.14 ± 2.41 b | 40.88 ± 2.78 a | 30.77 ± 2.34 b | 23.4 ± 2.52 b | 22.6 ± 2.24 b | 17.93 ± 7.45 c | 22.11 ± 7.35 b | 28.95 ± 7.43 b | 29.52 ± 7.63 b |
| exIL-1α (pg/mL) | nd | 1.41 ± 0.20 b | 1.73 ± 0.21 b | 1.75 ± 0.43 b | 1.93 ± 0.73 b | 2.42 ± 0.40 a | 2.63 ± 0.11 a | 1.39 ± 0.25 b | 1.35 ± 0.12 b | 1.46 ± 0.10 b |
| % ATP production | 100.0 ± 5.0 a | 83.96 ± 3.65 b | 82.29 ± 7.43 b | 88.63 ± 5.90 b | 81.91 ± 5.07 b | 84.31 ± 2.79 b | 35.15 ± 7.13 e | 55.64 ± 8.02 d | 68.25 ± 7.62 c | 74.82 ± 10.54 b |
| C. subternata | ||||||||||
| (−) UVB | (+) UVB | 0.71 | 0.36 | 0.18 | 0.09 | 0.75 | 0.36 | 0.19 | 0.09 | |
| icIL-1α (pg/mL) | 15.53 ± 3.51 d | 36.10 ± 5.73 b | 51.94 ± 4.2 a | 43.55 ± 9.16 b | 40.83 ± 8.24 b | 36.23 ± 7.87 b | 56.53 ± 4.14 a | 45.32 ± 3.97 c | 48.02 ± 5.63 c | 39.91 ± 9.92 bc |
| exIL-1α (pg/mL) | 2.72 ± 0.71 b | 3.72 ± 0.22 a | 2.40 ± 0.19 b | 2.62 ± 0.42 b | 2.65 ± 0.19 b | 2.72 ± 0.28 b | 1.22 ± 0.07 c | 1.31 ± 0.13 c | 1.84 ± 0.20 b | 1.74 ± 0.21 b |
| % ATP production | 100.00 ± 4.12 a | 89.60 ± 4.41 b | 82.07 ± 3.02 c | 86.28 ± 2.54 b | 88.01 ± 7.66 b | 93.48 ± 5.92 b | 42.02 ± 6.94 e | 62.22 ± 7.00 d | 77.33 ± 9.51 c | 79.37 ± 8.52 c |
| Extract | icIL-1α_ATP | Casp-3_F_ATP | Casp-3_F _icIL-1α |
|---|---|---|---|
| Green Tea | |||
| Methanol | 0.618 (<0.0001) | −0.895 (<0.0001) | −0.863 (<0.0001) |
| Aqueous | 0.754 (<0.0001) | −0.880 (<0.0001) | −0.833 (<0.0001) |
| Rooibos | |||
| Methanol | 0.742 (<0.0001) | −0.878 (<0.0001) | −0.899 (<0.0001) |
| Aqueous | 0.796 (<0.0001) | −0.848 (<0.0001) | −0.876 (<0.0001) |
| C. intermedia | |||
| Methanol | −0.494 (0.0005) | ||
| Aqueous | 0.720 (<0.0001) | −0.820 (<0.0001) | −0.555 (<0.0001) |
| C. subternata | |||
| Methanol | −0.309 (0.0017) | −0.597 (<0.0001) | |
| Aqueous | −0.229 (0.023) | −0.822 (<0.0001) | 0.517 (0.0001) |
| Polyphenols ** | icIL_1α | Cell Viability (% ATP) | |||
|---|---|---|---|---|---|
| Methanol | Aqueous | Methanol | Aqueous | ||
| Green tea | 0.08 mg/mL * | 0.28 mg/mL * | 0.06 mg/mL * | 0.14 mg/mL * | |
| TP | 28.06 ± 2.62 a | 41.86 ± 5.62 b | 15.03 ± 0.99 a | 35.07 ± 2.30 b | |
| FLAVA | 10.58 ± 0.30 a | 20.18 ± 0.81 b | 7.94 ± 0.22 a | 10.86 ± 043 b | |
| EGCG | 8.95 ± 0.24 a | 29.10 ± 0.39 b | 6.72 ± 0.18 a | 6.45 ± 0.21 a | |
| ECG | 1.63 ± 0.03 a | 1.94 ± 0.11 b | 1.22 ± 0.02 a | 1.05 ± 0.06 a | |
| EGC | 3.38 ± 0.14 a | 8.932 ± 0.83 b | 2.54 ± 0.11 a | 4.48 ± 0.45 b | |
| EC | 1.19 ± 0.08 a | 2.93 ± 0.30 b | 0.90 ± 0.06 a | 1.58 ± 0.16 b | |
| Catechin | 0.11 ± 0.01 a | 0.29 ± 0.01 b | 0.08 ± 0.01 a | 0.16 ± 0.01 b | |
| Total flavanols | 15.26 ± 0.50 a | 25.47 ± 0.73 b | 11.45 ± 0.37 a | 13.72 ± 0.39 b | |
| Rooibos | 0.14 mg/mL * | 0.21 mg/mL * | 0.10 mg/mL * | 0.13 mg/mL * | |
| TP | 49.11 ± 4.82 a | 52.62 ± 5.97 a | 35.08 ± 3.44 a | 32.57 ± 3.70 a | |
| FLAVA | 3.26 ± 0.20 a | 3.24 ± 0.27 a | 2.71 ± 0.16 a | 2.34 ± 0.20 a | |
| Aspalathin | 17.39 ± 0.20 a | 17.61 ± 0.44 a | 12.44 ± 0.14 a | 10.90 ± 0.27 b | |
| Nothofagin | 3.86 ± 0.05 a | 3.50 ± 0.06 b | 2.76 ± 0.04 a | 2.17 ± 0.04 b | |
| Total DHC | 21.25 ± 0.23 a | 21.12 ± 0.38 a | 15.18 ± 0.17 a | 13.07 ± 0.24 b | |
| Iso-orientin | 2.21 ± 0.01 a | 2.30 ± 0.41 a | 1.58 ± 0.01 a | 1.42 ± 0.25 a | |
| Orientin | 1.62 ± 0.01 a | 1.86 ± 0.30 a | 1.16 ± 0.01 a | 1.15 ± 0.19 a | |
| Vitexin | 0.22 ± 0.0 a | 0.25 ± 0.0 a | 0.16 ± 0.0 a | 0.16 ± 0.0 a | |
| Isovitexin | 0.32 ± 0.0 a | 0.32 ±0.03 a | 0.26 ± 0.0 a | 0.20 ± 0.02 a | |
| L7Glc | 0.25 ± 0.13 a | 0.09 ± 0.02 a | 0.18 ± 0.01 a | 0.06 ± 0.01 b | |
| Total flavones | 4.67 ± 0.01 a | 4.83 ± 0.62 a | 3.34 ± 0.01 a | 2.99 ± 0.45 a | |
| Rutin | 0.60 ± 0.0 a | 0.76 ± 0.0 b | 0.43 ± 0.0 a | 0.47 ± 0.0 a | |
| Hyperoside | 0.49 ± 0.01 a | 0.31 ± 0.16 a | 0.35 ± 0.01 a | 0.19 ± 0.10 b | |
| Isoquercitrin | 0.63 ± 0.0 a | 0.42 ± 0.21 a | 0.45 ± 0.0 a | 0.26 ± 0.12 b | |
| QROB | 1.62 ± 0.0 a | 1.58 ± 0.0 a | 1.16 ± 0.01 a | 0.98 ± 0.12 a | |
| Total flavanols | 3.35 ± 0.01 a | 3.06 ± 0.35 a | 2.39 ± 0.01 a | 1.89 ± 0.22 b | |
| PPAG | 0.54 ± 0.03 a | 0.89 ± 0.02 b | 0.39 ± 0.02 a | 0.55 ± 0.01 b | |
| Polyphenols ** | C. intermedia | C. subternata # | ||
|---|---|---|---|---|
| IC50 % ATP (0.41 mg/mL) * | IC50 icIL-1α (0.61 mg/mL) * | IC50 % ATP (0.72 mg/mL) * | ||
| TP | 67.45 ± 4.63 a | 133.25 ± 10.86 | 126.00 ± 17.35 b | |
| FLAVA | 7.30 ± 0.37 a | 6.89 ± 0.55 | 16.49 ± 0.65 b | |
| Xanthones | Mangiferin | 16.30 ± 0.16 a | 24.26 ± 0.25 | 15.98 ± 2.15 a |
| Isomangiferin | 5.85 ± 0.24 a | 8.70 ± 0.24 | 6.26 ± 1.05 a | |
| Total | 22.16 ± 0.31 a | 32.96 ± 0.46 | 22.10 ± 3.20 a | |
| Flavanones | Eriocitrin | 0.51 ± 0.02 a | 0.76 ± 0.04 | 2.35 ± 0.18 b |
| Hesperidin | 3.00 ± 0.23 a | 4.47 ± 0.34 | 5.75 ± 0.15 b | |
| Eriodictyol-glucoside | 2.79 ± 0.14 | |||
| Total | 3.51 ± 0.25 a | 5.23 ± 0.38 | 10.89 ± 0.17 b | |
| Flavones | Luteolin | 0.09 ± 0.01 a | 0.14 ± 0.01 | 0.09 ± 0.04 a |
| Scolymoside | 2.90 ± 0.09 | |||
| Total | 0.09 ± 0.01 a | 0.14 ± 0.01 | 3.00 ± 0.06 b | |
| DHC | Phloretin-3′,5′-di-C-glucoside | 0.28 ± 0.01 a | 0.41 ± 0.01 | 9.02 ± 1.02 b |
| Benzophenone | Iriflophenone-3-C-glucoside | 2.18 ± 0.05 a | 2.21 ± 0.07 | 6.71 ± 0.11 b |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magcwebeba, T.; Swart, P.; Swanevelder, S.; Joubert, E.; Gelderblom, W. Anti-Inflammatory Effects of Aspalathus linearis and Cyclopia spp. Extracts in a UVB/Keratinocyte (HaCaT) Model Utilising Interleukin-1α Accumulation as Biomarker. Molecules 2016, 21, 1323. https://doi.org/10.3390/molecules21101323
Magcwebeba T, Swart P, Swanevelder S, Joubert E, Gelderblom W. Anti-Inflammatory Effects of Aspalathus linearis and Cyclopia spp. Extracts in a UVB/Keratinocyte (HaCaT) Model Utilising Interleukin-1α Accumulation as Biomarker. Molecules. 2016; 21(10):1323. https://doi.org/10.3390/molecules21101323
Chicago/Turabian StyleMagcwebeba, Tandeka, Pieter Swart, Sonja Swanevelder, Elizabeth Joubert, and Wentzel Gelderblom. 2016. "Anti-Inflammatory Effects of Aspalathus linearis and Cyclopia spp. Extracts in a UVB/Keratinocyte (HaCaT) Model Utilising Interleukin-1α Accumulation as Biomarker" Molecules 21, no. 10: 1323. https://doi.org/10.3390/molecules21101323
APA StyleMagcwebeba, T., Swart, P., Swanevelder, S., Joubert, E., & Gelderblom, W. (2016). Anti-Inflammatory Effects of Aspalathus linearis and Cyclopia spp. Extracts in a UVB/Keratinocyte (HaCaT) Model Utilising Interleukin-1α Accumulation as Biomarker. Molecules, 21(10), 1323. https://doi.org/10.3390/molecules21101323






