The Stimulatory Effect of Strontium Ions on Phytoestrogens Content in Glycine max (L.) Merr
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Analysis
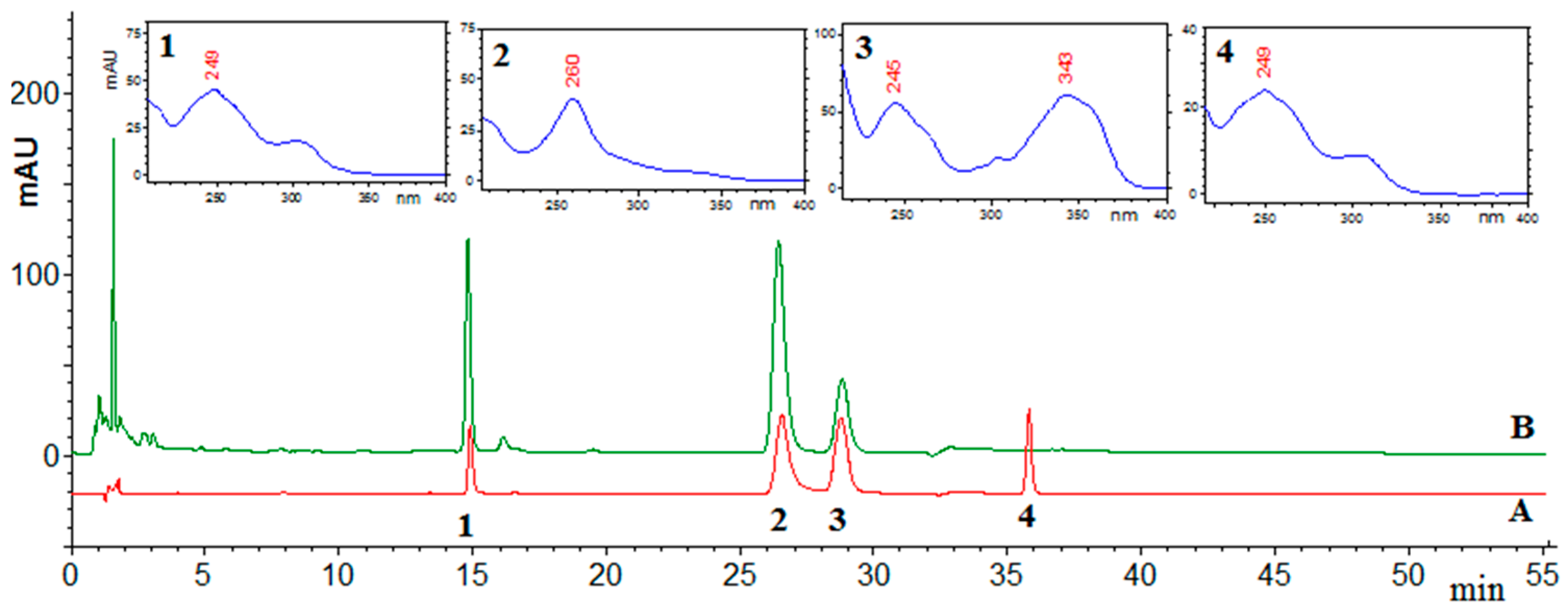
| Parameter | Daidzein | Genistein | Formononetin | Coumestrol |
|---|---|---|---|---|
| Concentration range (μg·mL−1) | 1.0–50.0 | 0.01–30.0 | 0.01–5.0 | 0.01–10.0 |
| Regression equation | y = 396815x + 104631 | y = 398812x − 155217 | y = 509672x − 245893 | y = 118314x − 67144 |
| Correlation coefficient R | 0.9998 | 0.9999 | 0.9999 | 0.9999 |
| Precision (* RSD %) | 0.5–1.1 | 0.6–1.2 | 0.6–1.0 | 0.5–1.3 |
| Accuracy (mean recovery %) | 98.72 | 98.91 | 97.58 | 98.11 |
| LOD (ng·mL−1) | 18.2 | 25.0 | 28.1 | 27.4 |
| LOQ (ng·mL−1) | 60.7 | 83.3 | 93.7 | 91.3 |
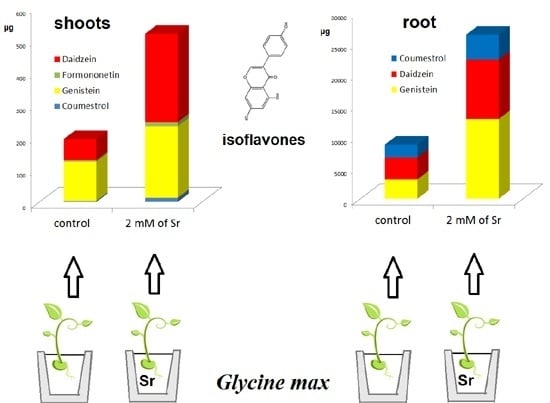
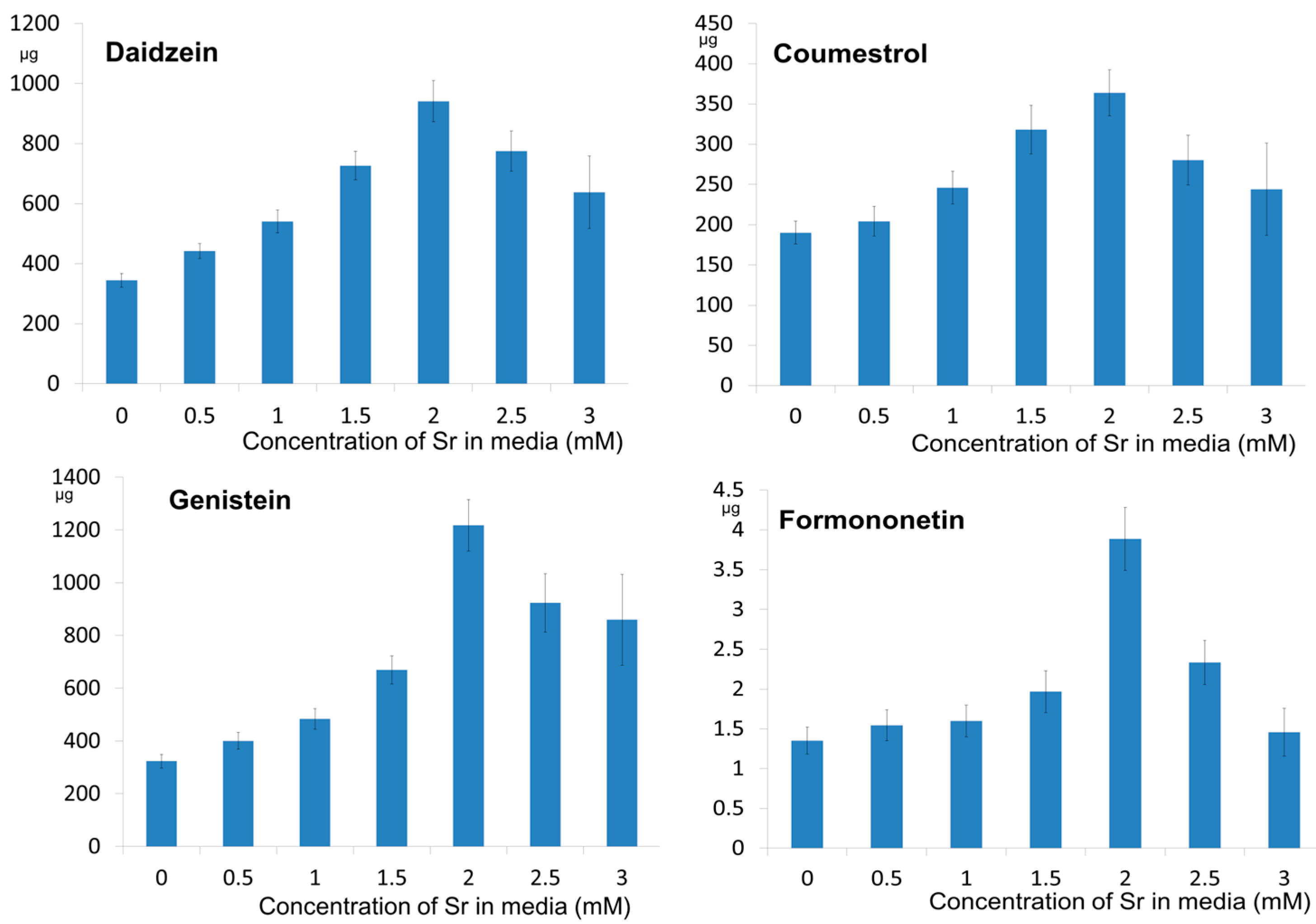
2.2. The Effect of Strontium Concentration on the Phytoestrogen Content
| Sr2+ (mM) | Daidzein | Coumestrol | Genistein | Formononetin | |||
|---|---|---|---|---|---|---|---|
| Roots (mg/g) | Shoots (µg/g) | Roots (mg/g) | Shoots (µg/g) | Roots (mg/g) | Shoots (µg/g) | Shoots (µg/g) | |
| 0 (control) | 3.53 ± 0.231 e | 65.5 ± 5.67 d | 2.06 ± 0.154 d | 3.25 ± 0.52 d | 3.11 ± 0.304 d | 121.2 ± 16.9 b | 4.33 ± 0.53 c |
| 0.5 | 4.40 ± 0.250 d | 118.2 ± 8.15 c | 2.21 ± 0.201 c,d | 3.40 ± 0.57 c,d | 3.93 ± 0.363 c | 121.8 ± 17.0 b | 4.85 ± 0.60 c |
| 1.0 | 5.10 ± 0.361 c | 141.9 ± 12.04 b | 2.53 ± 0.209 b,c | 3.57 ± 0.58 c,d | 4.56 ± 0.426 b,c | 126.6 ± 17.1 b | 4.93 ± 0.61 c |
| 1.5 | 5.96 ± 0.386 b | 150.4 ± 13.45 b | 2.80 ± 0.267 b | 4.61 ± 0.57 c | 5.311 ± 0.460 b | 193.8 ± 24.3 a | 5.49 ± 0.73 c |
| 2.0 | 9.54 ± 0.694 a | 272.1 ± 19.99 a | 4.00 ± 0.314 a | 12.76 ± 1.38 b | 12.79 ± 1.02 a | 220.1 ± 30.3 a | 12.82 ± 1.30 a |
| 2.5 | 9.48 ± 0.819 a | 257.3 ± 20.67 a | 3.76 ± 0.415 a | 8.16 ± 0.93 a | 11.94 ± 0.96 a | 138.9 ± 20.1 b | 8.13 ± 0.97 b |
| 3.0 | 7.98 ± 1.80 a | 233.3 ± 46.33 a | 3.36 ± 0.788 a | 7.23 ± 1.39 a | 11.42 ± 2.53 a | 136.8 ± 26.1 b | 5.32 ± 1.09 c |
| Sr2+ Concentration Range (mM) | Daidzein | Genistein | Coumestrol | Formononetin | |||
|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | Shoot | |
| 0–2.0 | 0.9815 | 0.9696 | 0.8996 | 0.8828 | 0.9784 | 0.8219 | 0.8352 |
| 2.0–3.0 | −0.9981 | −0.9991 | −0.9400 | −0.8992 | −0.9754 | −0.9421 | −0.9873 |
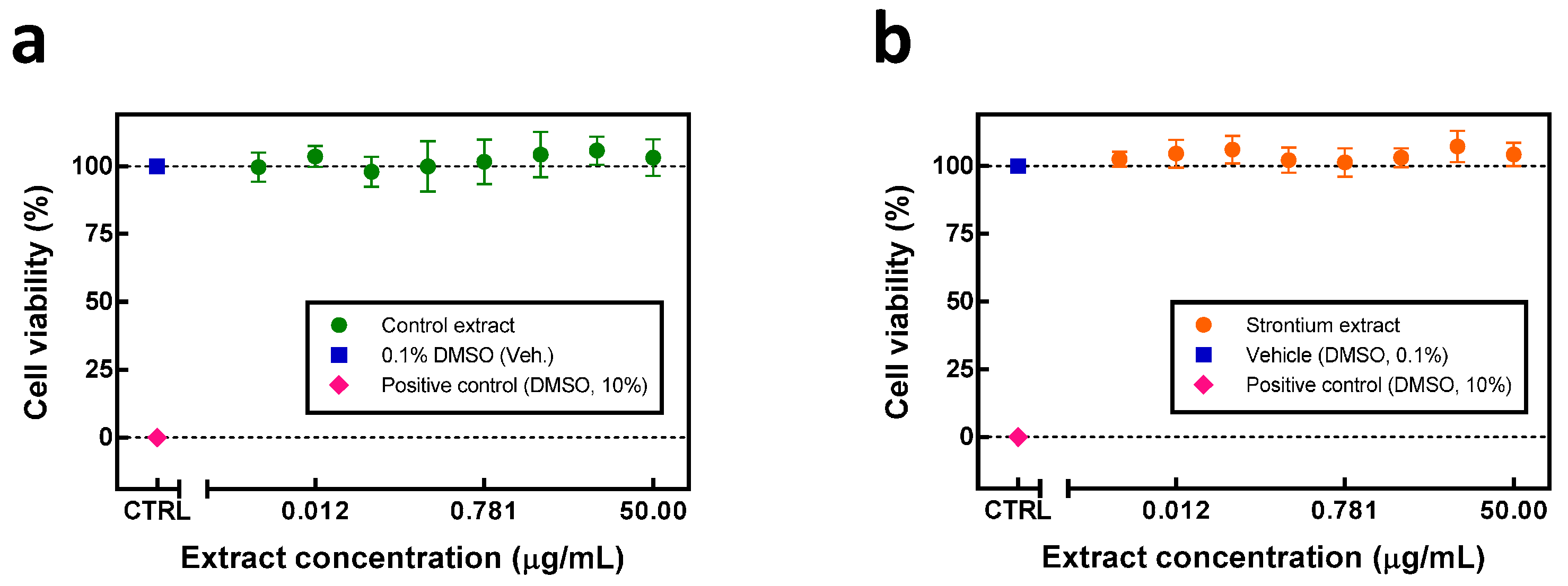
2.3. Cytotoxicity of Soybean Extract Obtained from Strontium-Stressed Plants
3. Materials and Methods
3.1. Materials and Reagents
3.2. Hydroponic Cultivation of Plant Material
3.3. Sample and Standard Preparation
3.4. HPLC Condition
3.5. Cell Culture
3.6. Cell Viability Assessment
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delmonte, P.; Perry, J.; Rader, J.I. Determination of isoflavones in dietary supplements containing soy, red clover and kudzu: Extraction followed by basic or acid hydrolysis. J. Chromatogr. A 2006, 1107, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Messina, V. The Role of Soy in Vegetarian Diets. Nutrients 2010, 2, 855–874. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Wójciak-Kosior, M.; Strzemski, M.; Rokicka, K.; Blicharski, T.; Kocjan, R. Analysis of compounds with phytoestrogenic activity in dietary supplements with use of HPTLC-densitometry method. Acta Pol. Pharm. 2014, 71, 265–269. [Google Scholar] [PubMed]
- Xiao, J.X.; Huang, G.Q.; Geng, X.; Qiu, H.W. Soy-derived isoflavones inhibit HeLa cell growth by inducing apoptosis. Plant Foods Hum. Nutr. 2011, 66, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Raynal, N.J.-M.; Momparler, L.; Charbonneau, M.; Momparler, R.L. Antileukemic activity of genistein, a major isoflavone present in soy products. J. Nat. Prod. 2008, 71, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Lepri, S.R.; Luiz, R.C.; Zanelatto, L.C.; da Silva, P.B.G.; Sartori, D.; Ribeiro, L.R.; Mantovani, M.S. Chemoprotective activity of the isoflavones, genistein and daidzein on mutagenicity induced by direct and indirect mutagens in cultured HTC cells. Cytotechnology 2013, 65, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, R.; Strzemski, M.; Sowa, I.; Polski, A.; Szwerc, W.; Świeboda, R.; Blicharski, T. Phytoestrogens—Classification, occurrence and significance in the prevention and treatment of osteoporosis. Ann. Sect. DDD 2011, 23, 195–203. [Google Scholar]
- Garcia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martinez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.D.; Díaz-Cruz, E.S.; Landini, S.; Kim, Y.W.; Brueggemeier, R.W. Evaluation of synthetic isoflavones on cell proliferation, estrogen receptor binding affinity, and apoptosis in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2008, 108, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Melby, M.K.; Nishi, N.; Omori, T.; Kurzer, M.S. Soy isoflavones for osteoporosis: An evidence-based approach. Maturitas 2011, 70, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; de Ponti, F. Phytoestrogens in Postmenopause: The State of the Art from a Chemical, Pharmacological and Regulatory Perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Stepan, J.J. Strontium ranelate: In search for the mechanism of action. J. Bone Miner. Metab. 2013, 31, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Wójciak-Kosior, M.; Strzemski, M.; Dresler, S.; Szwerc, W.; Blicharski, T.; Szymczak, G.; Kocjan, R. Biofortification of soy (Glycine max (L.) Merr.) with strontium ions. J. Agric. Food Chem. 2014, 62, 5248–5252. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Hanaka, A.; Bednarek, W.; Maksymiec, W. Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiol. Plant. 2014, 36, 1565–1575. [Google Scholar] [CrossRef]
- Movahhedy-Dehnavy, M.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A. Foliar application of zinc and manganese improves seed yield and quality of safflower (Carthamus tinctorius L.) grown under water deficit stress. Ind. Crops Prod. 2009, 30, 82–92. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Wang, Y.; Gao, Y.; Zhang, L. The response of ginseng grown on farmland to foliar-applied iron, zinc, manganese and copper. Ind. Crops Prod. 2013, 45, 388–394. [Google Scholar] [CrossRef]
- Murch, S.J.; Haq, K.; Rupasinghe, H.P.V.; Saxena, P.K. Nickel contamination affects growth and secondary metabolite composition of St. John’s wort (Hypericum perforatum L.). Environ. Exp. Bot. 2003, 49, 251–257. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, K.; Mukherjee, A.K. Impact of cadmium and lead on Catharanthus roseus—A phytoremediation study. J. Environ. Biol. 2007, 28, 655–662. [Google Scholar] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Rai, V.; Khatoon, S.; Bisht, S.S.; Mehrotra, S. Effect of cadmium on growth, ultramorphology of leaf and secondary metabolites of Phyllanthus amarus Schum and Thonn. Chemosphere 2005, 61, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Duncan, A.M.; Yang, R.; Marcone, M.F.; Rajcan, I.; Tsao, R. Systematic evaluation of pre-HPLC sample processing methods on total and individual isoflavones in soybeans and soy products. Food Res. Int. 2011, 44, 2425–2434. [Google Scholar] [CrossRef]
- Jiao, Z.; Si, X.; Zhang, Z.; Li, G.; Cai, Z. Compositional study of different soybean (Glycine max L.) varieties by 1H NMR spectroscopy, chromatographic and spectrometric techniques. Food Chem. 2012, 135, 285–291. [Google Scholar] [CrossRef]
- Algar, E.; Ramos-Solano, B.; García-Villaraco, A.; Saco Sierra, M.D.; Martín Gómez, M.S.; Gutiérrez-Mañero, F.J. Bacterial bioeffectors modify bioactive profile and increase isoflavone content in soybean sprouts (Glycine max var Osumi). Plant Foods Hum. Nutr. 2013, 68, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Nasim, S.A.; Dhir, B. Heavy metals alter the potency of medicinal plants. Rev. Environ. Contam. Toxicol. 2010, 203, 139–149. [Google Scholar] [PubMed]
- Chen, M.; Tang, Y.L.; Ao, J.; Wang, D. Effects of strontium on photosynthetic characteristics of oilseed rape seedlings. Russ. J. Plant Physiol. 2012, 59, 772–780. [Google Scholar] [CrossRef]
- Moyen, C.; Roblin, G. Uptake and translocation of strontium in hydroponically grown maize plants, and subsequent effects on tissue ion content, growth and chlorophyll a/b ratio: Comparison with Ca effects. Environ. Exp. Bot. 2010, 68, 247–257. [Google Scholar] [CrossRef]
- Wang, D.; Wen, F.; Xu, C.; Tang, Y.; Luo, X. The uptake of Cs and Sr from soil to radish (Raphanus sativus L.)—Potential for phytoextraction and remediation of contaminated soils. J. Environ. Radioact. 2012, 110, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of isoflavones are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójciak-Kosior, M.; Sowa, I.; Blicharski, T.; Strzemski, M.; Dresler, S.; Szymczak, G.; Wnorowski, A.; Kocjan, R.; Świeboda, R. The Stimulatory Effect of Strontium Ions on Phytoestrogens Content in Glycine max (L.) Merr. Molecules 2016, 21, 90. https://doi.org/10.3390/molecules21010090
Wójciak-Kosior M, Sowa I, Blicharski T, Strzemski M, Dresler S, Szymczak G, Wnorowski A, Kocjan R, Świeboda R. The Stimulatory Effect of Strontium Ions on Phytoestrogens Content in Glycine max (L.) Merr. Molecules. 2016; 21(1):90. https://doi.org/10.3390/molecules21010090
Chicago/Turabian StyleWójciak-Kosior, Magdalena, Ireneusz Sowa, Tomasz Blicharski, Maciej Strzemski, Sławomir Dresler, Grażyna Szymczak, Artur Wnorowski, Ryszard Kocjan, and Ryszard Świeboda. 2016. "The Stimulatory Effect of Strontium Ions on Phytoestrogens Content in Glycine max (L.) Merr" Molecules 21, no. 1: 90. https://doi.org/10.3390/molecules21010090
APA StyleWójciak-Kosior, M., Sowa, I., Blicharski, T., Strzemski, M., Dresler, S., Szymczak, G., Wnorowski, A., Kocjan, R., & Świeboda, R. (2016). The Stimulatory Effect of Strontium Ions on Phytoestrogens Content in Glycine max (L.) Merr. Molecules, 21(1), 90. https://doi.org/10.3390/molecules21010090